Development of a Novel Primer–TaqMan Probe Set for Diagnosis and Quantification of Meloidogyne enterolobii in Soil Using qPCR and Droplet Digital PCR Assays
Abstract
:1. Introduction
2. Results
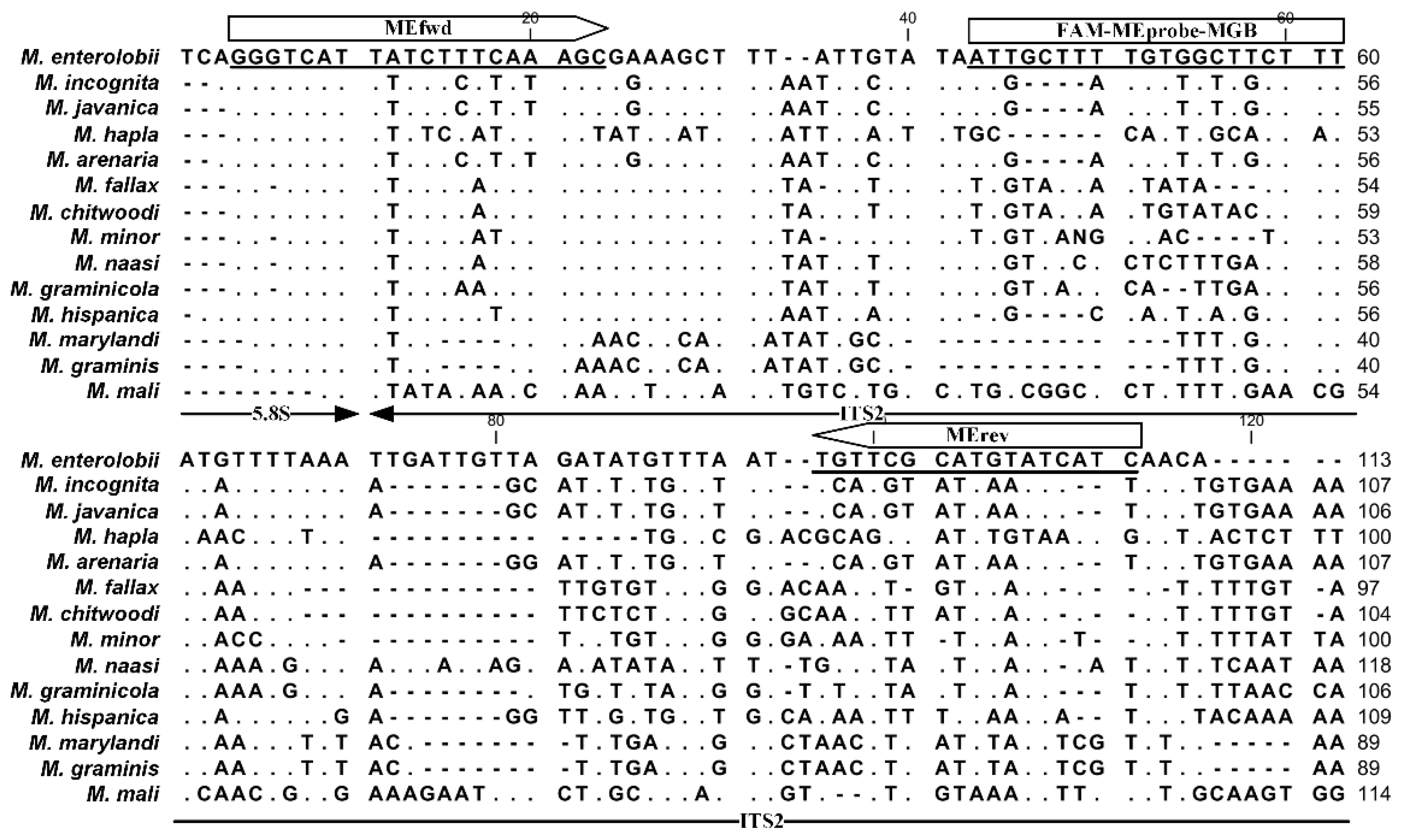
2.1. Species-Specific Primers and TaqMan Probe Designing for M. enterolobii Identification
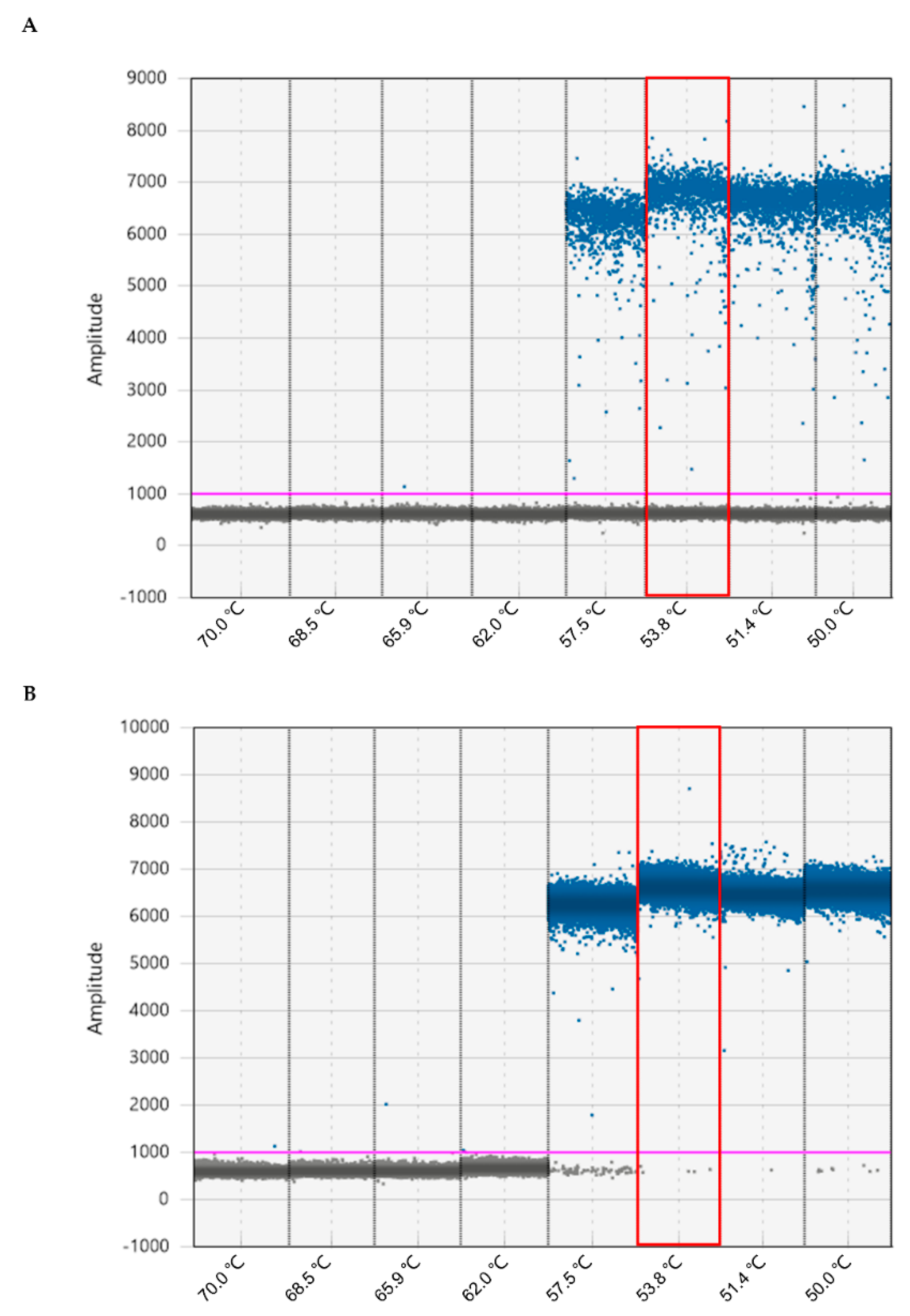
2.2. Optimization of Annealing Temperature for ddPCR Assay in M. enterolobii Identification
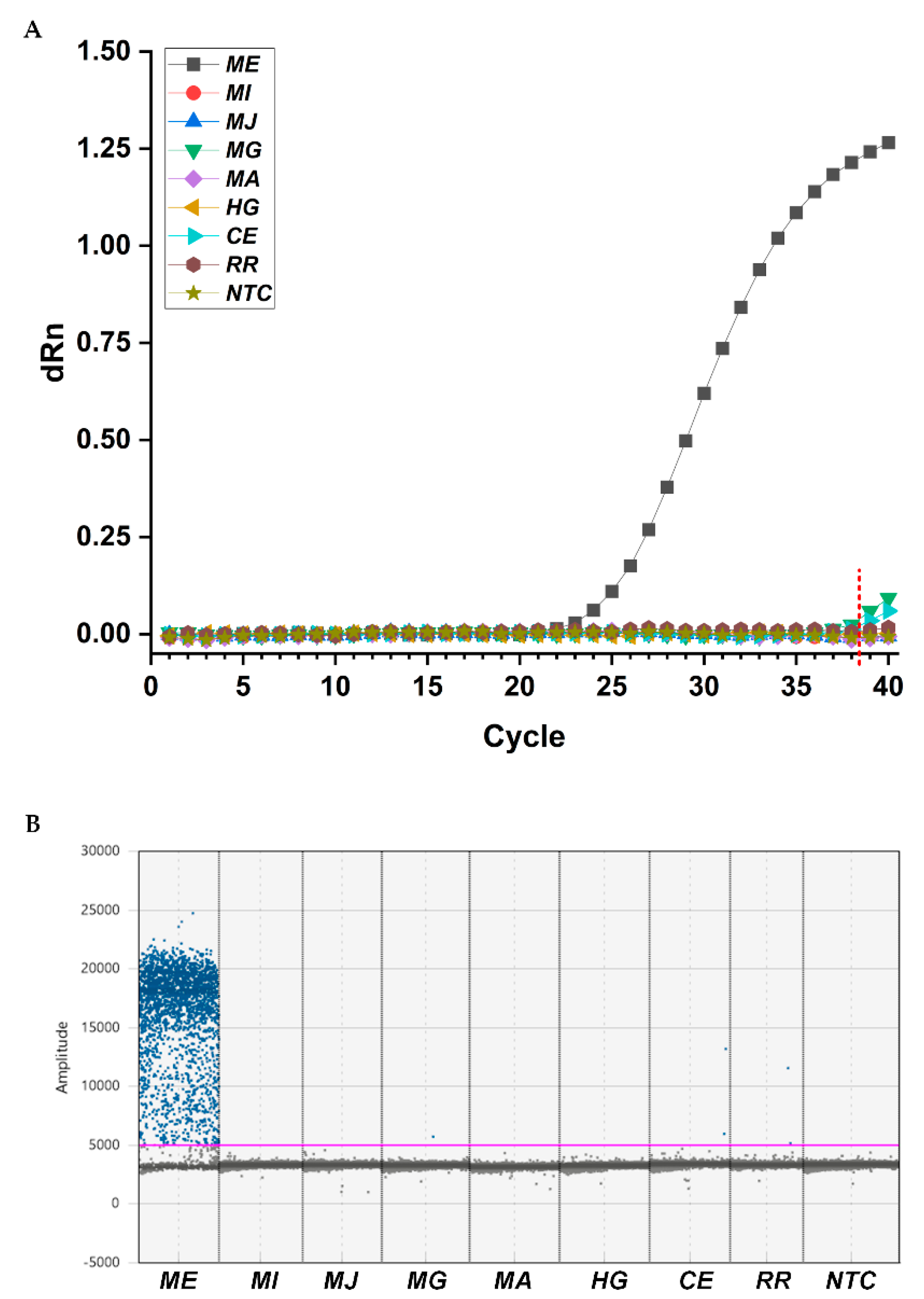
2.3. Primers and Probe Specificity Tests Analyzed by qPCR and ddPCR
2.4. Quantitative Linearity to Quantify the gDNA Dilutions and Eggs in Soil of M. enterolobii by qPCR and ddPCR
2.5. Comparison of the Minimum Detection Limit between qPCR and ddPCR Platforms
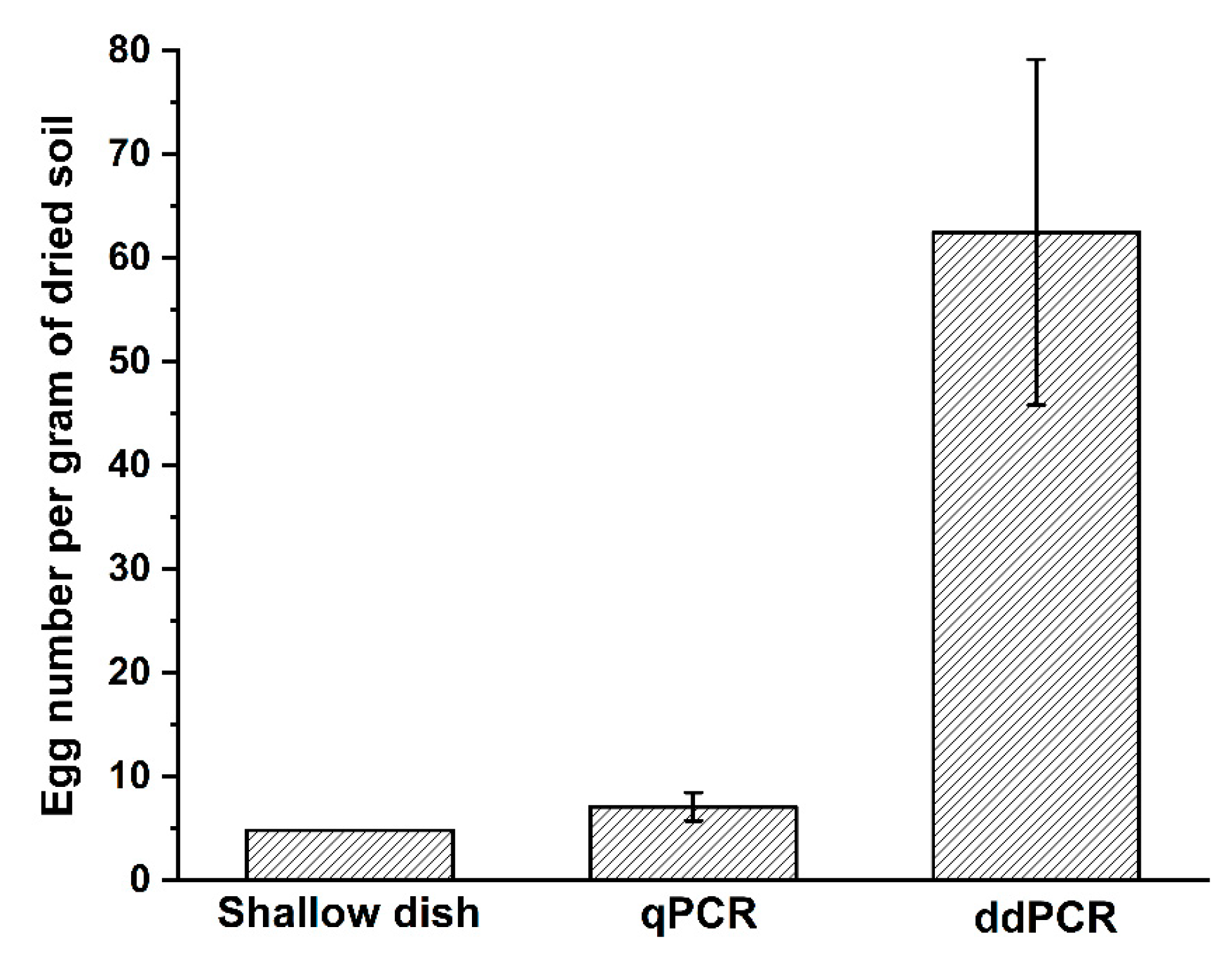
2.6. Assessments of Field Samples Quantified Using Shallow Dish, qPCR, and ddPCR Methods
3. Discussion
4. Materials and Methods
4.1. Preparation and Identification of Nematodes Populations
4.2. DNA Extraction of Nematodes from Pure Culture and Soil
4.3. Desigh of M. enterolobii-Specific Primers and TaqMan Probe
4.4. TaqMan qPCR Assays
4.5. ddPCR Assays
4.6. Specificity Tests for Designed Primers and TaqMan Probe
4.7. Standard Curve Determination of Genomic DNA and Total DNA from Soil Containing Nematode Eggs
4.8. Minimum Threshold Detection for qPCR and ddPCR Assays
4.9. Quantification of M. enterolobii Eggs in Field Samples
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghareeb, R.Y.; Hafez, E.E.; Ibrahim, D.S.S. Current management strategies for phytoparasitic nematodes. In Management of Phytonematodes: Recent Advances and Future Challenges; Ansari, R.A., Rizvi, R., Mahmood, I., Eds.; Springer: Singapore, 2020; pp. 339–352. [Google Scholar]
- Bui, H.X.; Desaeger, J.A. Efficacy of nonfumigant nematicides against Meloidogyne javanica as affected by soil temperature under pasteurized and natural soil conditions. Pest Manag. Sci. 2021, 77, 3179–3186. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M.; Ahmad, E.M.; Martínez-Medina, A.; Aly, M.A.M. Effective approaches to study the plant-root knot nematode interaction. Plant Physiol. Biochem. 2019, 141, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Grundler, F.M. Parasitic nematodes manipulate plant development to establish feeding sites. Curr. Opin. Microbiol. 2018, 46, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Eisenback, J.D. Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpod tree in China. J. Nematol. 1983, 15, 381–391. [Google Scholar] [PubMed]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Bogale, M.; Baniya, A.; DiGennaro, P. Nematode identification techniques and recent advances. Plants 2020, 9, 1260. [Google Scholar] [CrossRef]
- Rashidifard, M.; Fourie, H.; Daneel, M.S.; Marais, M. Morphological and morphometrical identification of Meloidogyne populations from various crop production areas in South Africa with emphasis on M. enterolobii. Zootaxa 2019, 4658, zootaxa.4658.2.3. [Google Scholar] [CrossRef]
- Janssen, T.; Karssen, G.; Verhaeven, M.; Coyne, D.; Bert, W. Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. Sci. Rep. 2016, 6, 22591. [Google Scholar] [CrossRef]
- Fargette, M.; Phillips, M.S.; Blok, V.C.; Waugh, R.; Trudgill, D.L. An RFLP study of relationships between species, populations and resistance-breaking lines of tropical species of Meloidogyne. Fundam. Appl. Nematol. 1996, 19, 193–200. [Google Scholar]
- Almeida, M.R.; Carneiro, R.; dos Santos, M.; Tigano, M.; Gomes, A.C.; Mota, F.C. Diversity of Meloidogyne arenaria using morphological, cytological and molecular approaches. Nematology 2008, 10, 819–834. [Google Scholar] [CrossRef]
- Blok, V.C.; Phillips, M.S.; Fargette, M. Comparison of sequences from the ribosomal DNA intergenic region of Meloidogyne mayaguensis and other major tropical root-knot nematodes. J. Nematol. 1997, 29, 16–22. [Google Scholar]
- Semblat, J.P.; Wajnberg, E.; Dalmasso, A.; Abad, P.; Castagnone-Sereno, P. High-resolution DNA fingerprinting of parthenogenetic root-knot nematodes using AFLP analysis. Mol. Ecol. 1998, 7, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Fargette, M.; Lollier, V.; Phillips, M.; Blok, V.; Frutos, R. AFLP analysis of the genetic diversity of Meloidogyne chitwoodi and M. fallax, major agricultural pests. Comptes Rendus Biol. 2005, 328, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, P.; Meng, Q.; Long, H. Characterisation of Meloidogyne species from China using isozyme phenotypes and amplified mitochondrial DNA restriction fragment length polymorphism. Eur. J. Plant Pathol. 2004, 110, 209–315. [Google Scholar] [CrossRef]
- Blok, V.C.; Wishart, J.; Fargette, M.; Berthier, K.; Phillips, M. Mitochondrial DNA differences distinguishing Meloidogyne mayaguensis from the major species of tropical root-knot nematodes. Nematology 2002, 4, 773–781. [Google Scholar] [CrossRef]
- Schwarz, T.; Li, C.; Ye, W.; Davis, E. Distribution of Meloidogyne enterolobii in eastern North Carolina and comparison of four isolates. Plant Health Prog. 2020, 21, 91–96. [Google Scholar] [CrossRef]
- Randig, O.; Deau, F.; dos Santos, M.F.A.; Tigano, M.S.; Carneiro, R.M.D.G.; Castagnone-Sereno, P. A novel species-specific satellite DNA family in the invasive root-knot nematode Meloidogyne mayaguensis and its potential use for diagnostics. Eur. J. Plant Pathol. 2009, 125, 485–495. [Google Scholar] [CrossRef]
- Tigano, M.; De Siqueira, K.; Castagnone-Sereno, P.; Mulet, K.; Queiroz, P.; Dos Santos, M.; Teixeira, C.; Almeida, M.; Silva, J.; Carneiro, R. Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava-damaging species. Plant Pathol. 2010, 59, 1054–1061. [Google Scholar] [CrossRef]
- Niu, J.H.; Jian, H.; Guo, Q.X.; Chen, C.L.; Wang, X.Y.; Liu, Q.; Guo, Y.D. Evaluation of loop-mediated isothermal amplification (LAMP) assays based on 5S rDNA-IGS2 regions for detecting Meloidogyne enterolobii. Plant Pathol. 2012, 61, 809–819. [Google Scholar] [CrossRef]
- Holland, P.M.; Abramson, R.D.; Watson, R.; Gelfand, D.H. Detection of specific polymerase chain reaction product by utilizing the 5’----3’exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 1991, 88, 7276–7280. [Google Scholar] [CrossRef]
- Bell, A.S.; Ranford-Cartwright, L.C. Real-time quantitative PCR in parasitology. Trends Parasitol. 2002, 18, 338–342. [Google Scholar] [CrossRef]
- Sapkota, R.; Skantar, A.M.; Nicolaisen, M. A TaqMan real-time PCR assay for detection of Meloidogyne hapla in root galls and in soil. Nematology 2016, 18, 147–154. [Google Scholar] [CrossRef]
- Zijlstra, C.; Van Hoof, R.A. A multiplex real-time polymerase chain reaction (TaqMan) assay for the simultaneous detection of Meloidogyne chitwoodi and M. fallax. Phytopathology 2006, 96, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, M.; Kox, L.; Waeyenberge, L.; Viaene, N.; Zijlstra, C. A real-time PCR assay to identify Meloidogyne minor. J. Phytopathol. 2011, 159, 80–84. [Google Scholar] [CrossRef]
- Kiewnick, S.; Frey, J.E.; Braun-Kiewnick, A. Development and validation of LNA-based quantitative real-time PCR assays for detection and identification of the root-knot nematode Meloidogyne enterolobii in complex DNA backgrounds. Phytopathology 2015, 105, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.F.; Foy, C.A.; Benes, V.; Emslie, K.; Garson, J.A.; Haynes, R.; Hellemans, J.; Kubista, M.; Mueller, R.D.; Nolan, T.; et al. The digital MIQE guidelines: Minimum information for publication of quantitative digital PCR experiments. Clin. Chem. 2013, 59, 892–902. [Google Scholar] [CrossRef]
- Li, H.; Bai, R.; Zhao, Z.; Tao, L.; Ma, M.; Ji, Z.; Jian, M.; Ding, Z.; Dai, X.; Bao, F.; et al. Application of droplet digital PCR to detect the pathogens of infectious diseases. Biosci. Rep. 2018, 38, BSR20181170. [Google Scholar] [CrossRef]
- Morcia, C.; Ghizzoni, R.; Delogu, C.; Andreani, L.; Carnevali, P.; Terzi, V. Digital PCR: What relevance to plant studies? Biology 2020, 9, 433. [Google Scholar] [CrossRef]
- Rani, A.; Donovan, N.; Mantri, N. The future of plant pathogen diagnostics in a nursery production system. Biosens. Bioelectron. 2019, 145, 111631. [Google Scholar] [CrossRef]
- Cirillo, P.D.R.; Margiotti, K.; Mesoraca, A.; Giorlandino, C. Quantification of circulating microRNAs by droplet digital PCR for cancer detection. BMC Res. Notes 2020, 13, 351. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, P.; Xin, T.; Lou, Q.; Li, R.; Fu, W.; Ma, T.; Song, J. Droplet digital PCR for the identification of plant-derived adulterants in highly processed products. Phytomedicine 2022, 105, 154376. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. Quantification using real-time PCR technology: Applications and limitations. Trends Mol. Med. 2002, 8, 257–260. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Li, H.; Chen, X. Extraction efficiency of soil nematodes by different methods. Chin. J. Ecol. 2004, 23, 149–151. [Google Scholar]
- Perry, R.N.; Moens, M. Survival of parasitic nematodes outside the host. In Molecular and Physiological Basis of Nematode Survival; Perry, R.N., Wharton, D.A., Eds.; CABI: Cambridge, MA, USA, 2011; p. 4. [Google Scholar]
- Gaur, H.; Beane, J.; Perry, R. The influence of root diffusate, host age and water regimes on hatching of the root-knot nematode, Meloidogyne triticoryzae. Nematology 2000, 2, 191–199. [Google Scholar] [CrossRef]
- Wesemael, W.; Perry, R.; Moens, M. The influence of root diffusate and host age on hatching of the root-knot nematodes, Meloidogyne chitwoodi and M. fallax. Nematology 2006, 8, 895–902. [Google Scholar] [CrossRef]
- Onstad, D.W. Calculation of economic-injury levels and economic thresholds for pest management. J. Econ. Entomol. 1987, 80, 297–303. [Google Scholar] [CrossRef]
- Long, H.; Liu, H.; Xu, J.H. Development of a PCR diagnostic for the root-knot nematode Meloidogyne enterolobii. Acta Phytopathol. Sin. 2006, 36, 109–115. [Google Scholar]
- Meng, Q.P.; Long, H.; Xu, J.H. PCR assays for rapid and sensitive identification of three major root-knot nematodes, Meloidogyne incognita, M. javanica and M. arenaria. Acta Phytopathol. Sin. 2004, 34, 204–210. [Google Scholar]
- Guo, J.R.; Schnieder, F.; Verreet, J.A. Presymptomatic and quantitative detection of Mycosphaerella graminicola development in wheat using a real-time PCR assay. FEMS Microbiol. Lett. 2006, 262, 223–229. [Google Scholar] [CrossRef]
- Ou, S.; Peng, D.; Liu, X.; Li, Y.; Moens, M. Identification of Heterodera glycines using PCR with sequence characterised amplified region (SCAR) primers. Nematology 2008, 10, 397–403. [Google Scholar]
- Sayler, R.J.; Walker, C.; Goggin, F.; Agudelo, P.; Kirkpatrick, T. Conventional PCR detection and real-time PCR quantification of reniform nematodes. Plant Dis. 2012, 96, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.R.B. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.K. Methods for Soil Agrochemistry Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 127–332. [Google Scholar]

| qPCR (Ct) | ddPCR (Copies·μL−1) | qPCR (Ct) | ddPCR (Copies·μL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| gDNA (pg) | Mean ± SD | RSD (%) | Mean ± SD | RSD (%) | Number of Eggs in Soil | Mean ± SD | RSD (%) | Mean ± SD | RSD (%) |
| 1 | 32.877 ± 1.972 | 5.997 | 0.261 ± 0.028 | 10.728 | 1 | 36.839 ± 1.694 | 4.598 | 0.284 ± 0.011 | 3.873 |
| 5 | 30.361 ± 1.187 | 3.910 | 5.510 ± 0.210 | 3.811 | 5 | 31.580 ± 1.216 | 3.851 | 5.827 ± 0.363 | 6.230 |
| 10 | 29.489 ± 1.282 | 4.349 | 28.300 ± 1.707 | 6.032 | 10 | 30.050 ± 0.771 | 2.566 | 22.488 ± 1.252 | 5.567 |
| 50 | 27.157 ± 1.040 | 3.830 | 129.767 ± 8.619 | 6.642 | 25 | 28.945 ± 0.909 | 3.140 | 46.633 ± 2.439 | 5.230 |
| 100 | 26.527 ± 0.412 | 1.553 | 358.556 ± 2.169 | 0.605 | 50 | 26.603 ± 1.001 | 3.763 | 88.433 ± 6.436 | 7.278 |
| 500 | 23.634 ± 0.513 | 2.172 | 1925.167 ± 159.269 | 8.273 | 100 | 25.836 ± 0.389 | 1.506 | 207.333 ± 21.362 | 10.303 |
| 1000 | 22.651 ± 0.573 | 2.530 | 2485.778 ± 222.958 | 8.969 | 200 | 24.450 ±0.347 | 1.419 | 408.556 ± 6.345 | 1.553 |
| 5000 | 19.856 ± 0.656 | 3.306 | 9242.889 ± 599.605 | 6.487 | |||||
| Eggs in Soil | qPCR (Ct) | ddPCR (Copies·μL−1) | DNA Concentration (fg·μL−1) | qPCR (Ct) | ddPCR (Copies·μL−1) |
|---|---|---|---|---|---|
| 1/30 | 36.814 | 0.393 | 1000 | 32.901 | 0.283 |
| 1/150 | 37.748 | 0.256 | 100 | 37.195 | 0.164 |
| 1/300 | / 1 | 0.103 | 10 | / | 0.055 |
| 1/3000 | / | / | 1 | / | / |
| Species | Origin | Host |
|---|---|---|
| M. enterolobii | Wenchang, Hainan | Pepper |
| M. incognita | Zhengzhou, Henan | Tobacco |
| M. graminicola | Haikou, Hainan | Rice |
| M. arenaria | Langfang, Hebei | Tomato |
| M. javanica | Chengmai, Hainan | Tomato |
| C. elegans | Haikou, Hainan | / |
| H. glycines | Langfang, Hebei | Soybean |
| R. reniformis | Chengmai, Hainan | Banana |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Long, H.; Feng, T.; Pei, Y.; Sun, Y.; Zhang, X. Development of a Novel Primer–TaqMan Probe Set for Diagnosis and Quantification of Meloidogyne enterolobii in Soil Using qPCR and Droplet Digital PCR Assays. Int. J. Mol. Sci. 2022, 23, 11185. https://doi.org/10.3390/ijms231911185
Chen Y, Long H, Feng T, Pei Y, Sun Y, Zhang X. Development of a Novel Primer–TaqMan Probe Set for Diagnosis and Quantification of Meloidogyne enterolobii in Soil Using qPCR and Droplet Digital PCR Assays. International Journal of Molecular Sciences. 2022; 23(19):11185. https://doi.org/10.3390/ijms231911185
Chicago/Turabian StyleChen, Yuan, Haibo Long, Tuizi Feng, Yueling Pei, Yanfang Sun, and Xinchun Zhang. 2022. "Development of a Novel Primer–TaqMan Probe Set for Diagnosis and Quantification of Meloidogyne enterolobii in Soil Using qPCR and Droplet Digital PCR Assays" International Journal of Molecular Sciences 23, no. 19: 11185. https://doi.org/10.3390/ijms231911185





