Role of the Ghrelin System in Colitis and Hepatitis as Risk Factors for Inflammatory-Related Cancers
Abstract
1. Introduction
2. The Possible Mechanisms of Cancer-Related Inflammation in Colon and Liver
3. The Ghrelin System—General Overview
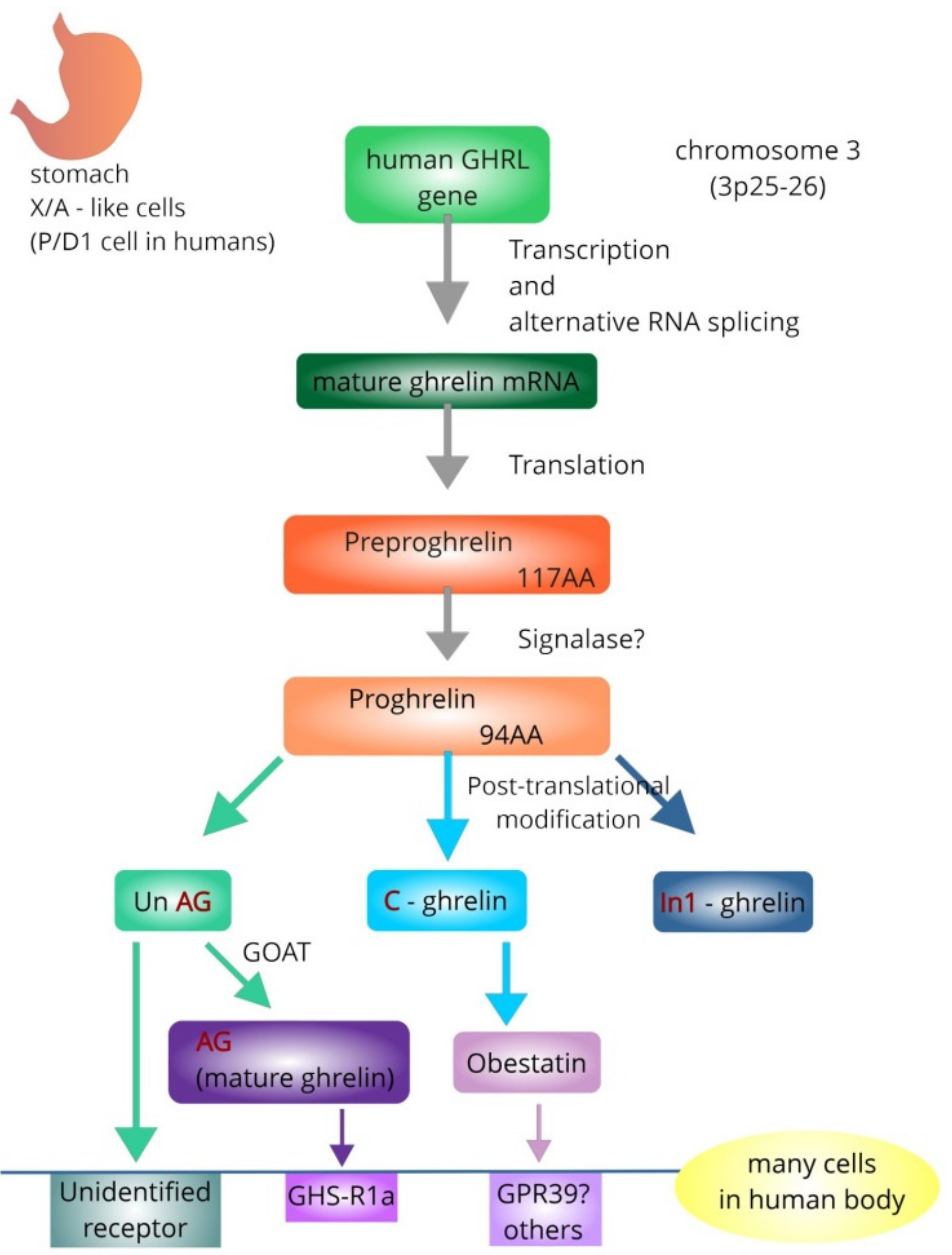
4. The Effects of Ghrelin System on the Intestinal Inflammation
4.1. Clinical Studies
4.2. Animal Models of Colon Inflammation
4.3. In Vitro Models of Colon Inflammation
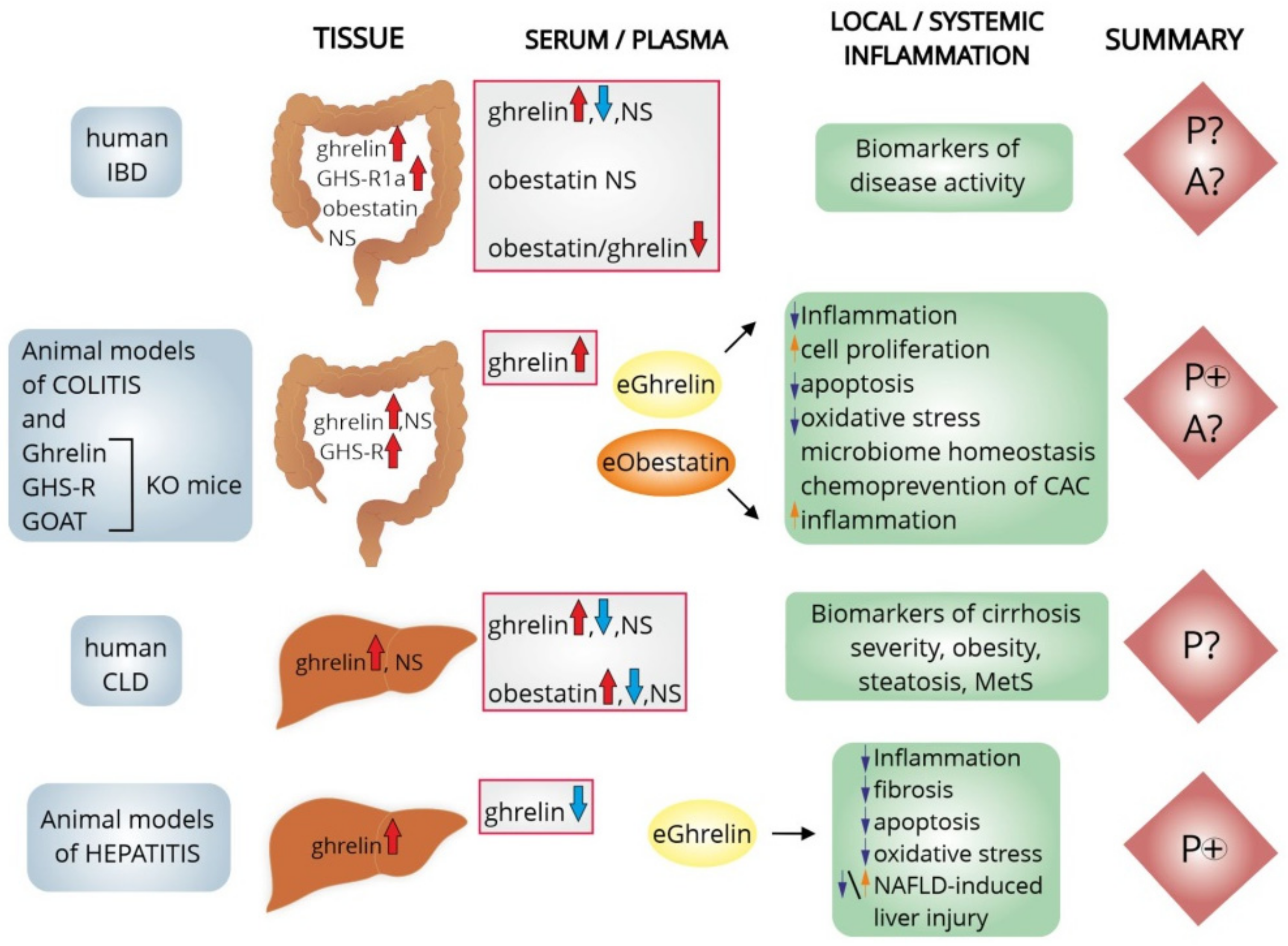
5. The Effects of Ghrelin System on the Hepatic Inflammation
5.1. Clinical Studies
5.2. Genetic Study
5.3. Animal Models of Liver Inflammation
5.4. In vitro Models of Liver Inflammation
6. Ghrelin System in the Treatment of Inflammatory Bowel and Liver Diseases
7. Final Remarks and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | serine/threonine-protein kinase or protein kinase B (PKB) |
| APC | adenomatous polyposis coli |
| BMI | body mass index |
| cAMP | cyclic adenosine monophosphate |
| CAT | catalase |
| CI | confidence interval |
| CRC | colorectal cancer |
| CRI | cancer-related inflammation |
| COX-2 | cyclooxygenase-2 |
| DSS | dextran sodium sulphate |
| eNOS | endothelial nitric oxide synthase |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinase |
| GH | growth hormone |
| GHS-R | ghrelin receptor |
| GLP-1 | glucagon-like peptide-1 |
| GOAT | ghrelin-O-acyl-transferase |
| GSH | glutathion |
| HR | hazard ratio |
| IBD | inflammatory bowel diseases |
| IGF-1, -2 | insulin-like growth factor 1, -2 |
| IR | insulin resistance |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinases |
| MAPK | a mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MetS | metabolic syndrome |
| MnSOD | manganese superoxide dysmutase |
| MPO | myeloperoxidase |
| mTOR | the mammalian target of rapamycin; protein kinase from PI3K family |
| NAFLD | nonalcoholic fatty liver diseases |
| NASH | nonalcoholic steatohepatitis |
| NF-κB | nuclear factor-kappa B |
| OR | odds ratio |
| PGE2 | prostaglandin E2 |
| PI3K | phosphoinositide 3-kinase |
| ROS | reactive oxygen species |
| SNPs | single nucleotide polymorphisms |
| STZ | streptozocin |
| TGF-β | transforming-growth factor beta |
| TLRs | toll-like receptors |
| TNBS | trinitrobenzene sulphonic acid |
| UPR | unfolded protein response |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Morlote, D. Molecular Pathology of Colorectal Cancer. Adv. Anat. Pathol. 2020, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.J.; Wickremesekera, S.K.; Peng, L.; Tan, S.T.; Itinteang, T. Cancer stem cells in colorectal cancer: A review. J. Clin. Pathol. 2018, 71, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Remo, A.; Fassan, M.; Vanoli, A.; Bonetti, L.R.; Barresi, V.; Tatangelo, F.; Gafà, R.; Giordano, G.; Pancione, M.; Grillo, F.; et al. Morphology and Molecular Features of Rare Colorectal Carcinoma Histotypes. Cancers 2019, 11, 1036. [Google Scholar] [CrossRef]
- Sharma, R. Descriptive epidemiology of incidence and mortality of primary liver cancer in 185 countries: Evidence from GLOBOCAN 2018. Jpn. J. Clin. Oncol. 2020, 50, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Stojsavljević, S.; Gomerčić Palčić, M.; Virović Jukić, L.; Smirčić Duvnjak, L.; Duvnjak, M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 18070–18091. [Google Scholar] [CrossRef]
- Quiñones, M.; Fernø, J.; Al-Massadi, O. Ghrelin and liver disease. Rev. Endocr. Metab. Disord. 2020, 21, 45–56. [Google Scholar] [CrossRef]
- Kořínková, L.; Pražienková, V.; Černá, L.; Karnošová, A.; Železná, B.; Kuneš, J.; Maletínská, L. Pathophysiology of NAFLD and NASH in Experimental Models: The Role of Food Intake Regulating Peptides. Front. Endocrinol. 2020, 11, 597583. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Karin, M. Tumor-Elicited Inflammation and Colorectal Cancer. Adv. Cancer Res. 2015, 128, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Ramachandran, I.K.; Babu, K.N.; Krishnamoorthy, S.; Guruswamy, A.; Queimado, L.; Chaudhuri, G.; Ramachandran, I. Role of Inflammation in the Development of Colorectal Cancer. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Ramachandran, I.; Krishnamoorthy, S.; Sambandam, Y.; Ramalingam, S.; Queimado, L.; Chaudhuri, G.; Ramachandran, I.K. Regulation of MicroRNAs in Inflammation-Associated Colorectal Cancer: A Mechanistic Approach. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Keenan, B.P.; Fong, L.; Kelley, R.K. Immunotherapy in hepatocellular carcinoma: The complex interface between inflammation, fibrosis, and the immune response. J. Immunother. Cancer 2019, 7, 267. [Google Scholar] [CrossRef]
- Golonka, R.M.; Vijay-Kumar, M. Atypical immunometabolism and metabolic reprogramming in liver cancer: Deciphering the role of gut microbiome. Adv. Cancer Res. 2021, 149, 171–255. [Google Scholar] [CrossRef]
- Pancione, M.; Giordano, G.; Remo, A.; Febbraro, A.; Sabatino, L.; Manfrin, E.; Ceccarelli, M.; Colantuoni, V. Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J. Immunol. Res. 2014, 2014, 686879. [Google Scholar] [CrossRef]
- Hao, X.; Sun, G.; Zhang, Y.; Kong, X.; Rong, D.; Song, J.; Tang, W.; Wang, X. Targeting Immune Cells in the Tumor Microenvironment of HCC: New Opportunities and Challenges. Front. Cell Dev. Biol. 2021, 9, 775462. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rincón-Fernández, D.; Villa-Osaba, A.; Hormaechea-Agulla, D.; Ibáñez-Costa, A.; Martínez-Fuentes, A.J.; Gracia-Navarro, F.; Castaño, J.P.; Luque, R.M. Ghrelin gene products, receptors, and GOAT enzyme: Biological and pathophysiological insight. J. Endocrinol. 2013, 220, R1–R24. [Google Scholar] [CrossRef]
- Gortan Cappellari, G.; Barazzoni, R. Ghrelin forms in the modulation of energy balance and metabolism. Eat Weight Disord. 2019, 24, 997–1013. [Google Scholar] [CrossRef]
- Davis, T.R.; Pierce, M.R.; Novak, S.X.; Hougland, J.L. Ghrelin octanoylation by ghrelin O-acyltransferase: Protein acylation impacting metabolic and neuroendocrine signalling. Open Biol. 2021, 11, 210080. [Google Scholar] [CrossRef] [PubMed]
- Ginter, G.; Ceranowicz, P.; Warzecha, Z. Protective and Healing Effects of Ghrelin and Risk of Cancer in the Digestive System. Int. J. Mol. Sci. 2021, 22, 10571. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Córdoba-Chacón, J.; Hergueta-Redondo, M.; Martínez-Fuentes, A.J.; Kineman, R.D.; Moreno-Bueno, G.; Luque, R.M.; Castaño, J.P. A novel human ghrelin variant (In1-ghrelin) and ghrelin-O-acyltransferase are overexpressed in breast cancer: Potential pathophysiological relevance. PLoS ONE 2011, 6, e23302. [Google Scholar] [CrossRef]
- Delhanty, P.J.; Neggers, S.J.; van der Lely, A.J. Should we consider des-acyl ghrelin as a separate hormone and if so, what does it do? Front. Horm. Res. 2014, 42, 163–174. [Google Scholar] [CrossRef]
- Luque, R.M.; Sampedro-Nuñez, M.; Gahete, M.D.; Ramos-Levi, A.; Ibáñez-Costa, A.; Rivero-Cortés, E.; Serrano-Somavilla, A.; Adrados, M.; Culler, M.D.; Castaño, J.P.; et al. In1-ghrelin, a splice variant of ghrelin gene, is associated with the evolution and aggressiveness of human neuroendocrine tumors: Evidence from clinical, cellular and molecular parameters. Oncotarget 2015, 6, 19619–19633. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, T.; Wang, G.; Li, Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci. Rep. 2018, 38, BSR20181061. [Google Scholar] [CrossRef]
- Soleyman-Jahi, S.; Sadeghi, F.; Pastaki Khoshbin, A.; Khani, L.; Roosta, V.; Zendehdel, K. Attribution of Ghrelin to Cancer; Attempts to Unravel an Apparent Controversy. Front. Oncol. 2019, 9, 1014. [Google Scholar] [CrossRef]
- Fang, C.; Xu, H.; Guo, S.; Mertens-Talcott, S.U.; Sun, Y. Ghrelin Signaling in Immunometabolism and Inflamm-Aging. Adv. Exp. Med. Biol. 2018, 1090, 165–182. [Google Scholar] [CrossRef]
- Villarreal, D.; Pradhan, G.; Zhou, Y.; Xue, B.; Sun, Y. Diverse and Complementary Effects of Ghrelin and Obestatin. Biomolecules 2022, 12, 517. [Google Scholar] [CrossRef]
- Aydin, S.; Erman, F.; Kilic, N.; Sahpaz, F. Des-acylated ghrelin, rather than acylated ghrelin, might be more valuable in inflammatory bowel diseases. Dig. Dis. Sci. 2008, 53, 2583. [Google Scholar] [CrossRef]
- El-Salhy, M. Ghrelin in gastrointestinal diseases and disorders: A possible role in the pathophysiology and clinical implications (review). Int. J. Mol. Med. 2009, 24, 727–732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mathur, N.; Mehdi, S.F.; Anipindi, M.; Aziz, M.; Khan, S.A.; Kondakindi, H.; Lowell, B.; Wang, P.; Roth, J. Ghrelin as an Anti-Sepsis Peptide: Review. Front. Immunol. 2021, 11, 610363. [Google Scholar] [CrossRef] [PubMed]
- Karaskova, E.; Velganova-Veghova, M.; Geryk, M.; Foltenova, H.; Kucerova, V.; Karasek, D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 4226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hai, J.; Li, L.; Chen, X.; Peng, H.; Cao, M.; Zhang, Q. Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 2013, 43, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Tziomalos, K.; Athyros, V.G. The adipokines in the pathogenesis and treatment of nonalcoholic fatty liver disease. Hippokratia 2016, 20, 259–263. [Google Scholar]
- Yin, Y.; Wang, Q.; Qi, M.; Zhang, C.; Li, Z.; Zhang, W. Ghrelin ameliorates nonalcoholic steatohepatitis induced by chronic low-grade inflammation via blockade of Kupffer cell M1 polarization. J. Cell. Physiol. 2021, 236, 5121–5133. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Qu, Y.; Zhao, J.; Tong, L.; Ye, S.; Qin, Y. Ghrelin ameliorates transformation of hepatic ischemia-reperfusion injury to liver fibrosis by blocking Smad and ERK signalling pathways, and promoting anti-inflammation and anti-oxidation effects. Transpl. Immunol. 2022, 73, 101597. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecień, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. 2009, 60, 41–47. [Google Scholar]
- Li, Z.; Xu, G.; Qin, Y.; Zhang, C.; Tang, H.; Yin, Y.; Xiang, X.; Li, Y.; Zhao, J.; Mulholland, M.; et al. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARγ signaling pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 13163–13168. [Google Scholar] [CrossRef]
- Barazzoni, R.; Semolic, A.; Cattin, M.R.; Zanetti, M.; Guarnieri, G. Acylated ghrelin limits fat accumulation and improves redox state and inflammation markers in the liver of high-fat-fed rats. Obesity 2014, 22, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Chaves, J.F.; Sancho-Bru, P.; Ramalho, F.; Ramalho, L.N.; Mansego, M.L.; Ivorra, C.; Dominguez, M.; Conde, L.; Millán, C.; et al. Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology 2010, 51, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Herszényi, L.; Barabás, L.; Miheller, P.; Tulassay, Z. Colorectal cancer in patients with inflammatory bowel disease: The true impact of the risk. Dig. Dis. 2015, 33, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Azer, S.A. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur. J. Gastroenterol. Hepatol. 2013, 25, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Q.; Xing, B.; Luo, N.; Gao, R.; Yu, K.; Hu, X.; Bu, Z.; Peng, J.; Ren, X.; et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell. 2022, 40, 424–437. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Q.; Liang, N.; Xue, H.; Yang, T.; Chen, X.; Qiu, Z.; Zeng, C.; Sun, T.; Yuan, W.; et al. Oncogenic driver genes and tumor microenvironment determine the type of liver cancer. Cell Death Dis. 2020, 11, 313. [Google Scholar] [CrossRef]
- Koike, K. The Way to Decoding Pathogenesis and Conquering of National Afflictions, Viral Hepatitis and Liver Cancer. JMA J. 2021, 4, 332–338. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef]
- Kim, E.R.; Chang, D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014, 20, 9872–9881. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.S.; Kumar, V.; Al-Abbasi, F.A.; Kamal, M.A.; Anwar, F. Risk of colorectal cancer in inflammatory bowel diseases. Semin. Cancer Biol. 2020, 64, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, M.W.; van Oijen, M.G.; van der Heijden, G.J.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef]
- Neri, B.; Scribano, M.L.; Armuzzi, A.; Castiglione, F.; D’Incà, R.; Orlando, A.; Festa, S.; Riegler, G.; Fries, W.; Meucci, G.; et al. Incident Colorectal Cancer in Inflammatory Bowel Disease. Cancers 2022, 14, 721. [Google Scholar] [CrossRef]
- Najafimehr, H.; Aghdaei, H.A.; Pourhoseingholi, M.A.; Shalmani, H.M.; Vahedian-Azimi, A.; Kroh, M.; Zali, M.R.; Sahebkar, A. A Systematic Review and Meta-Analysis on the Association between Inflammatory Bowel Disease Family History and Colorectal Cancer. Gastroenterol. Res. Pract. 2021, 2021, 4874459. [Google Scholar] [CrossRef]
- Wijnands, A.M.; de Jong, M.E.; Lutgens, M.W.M.D.; Hoentjen, F.; Elias, S.G.; Oldenburg, B. Dutch Initiative on Crohn and Colitis (ICC). Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology 2021, 160, 1584–1598. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Sukowati, C.H.; El-Khobar, K.E.; Ie, S.I.; Anfuso, B.; Muljono, D.H.; Tiribelli, C. Significance of hepatitis virus infection in the oncogenic initiation of hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 1497–1512. [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef] [PubMed]
- D’souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Kangawa, K. Purification of rat and human ghrelins. Methods Enzymol. 2012, 514, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.B.; Leite-Moreira, A.F. Ghrelin, des-acyl ghrelin and obestatin: Three pieces of the same puzzle. Peptides 2008, 29, 1255–1270. [Google Scholar] [CrossRef]
- Seim, I.; Amorim, L.; Walpole, C.; Carter, S.; Chopin, L.K.; Herington, A.C. Ghrelin gene-related peptides: Multifunctional endocrine/autocrine modulators in health and disease. Clin. Exp. Pharmacol. Physiol. 2010, 37, 125–131. [Google Scholar] [CrossRef]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef]
- McKee, K.K.; Palyha, O.C.; Feighner, S.D.; Hreniuk, D.L.; Tan, C.P.; Phillips, M.S.; Smith, R.G.; Van der Ploeg, L.H.; Howard, A.D. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol. Endocrinol. 1997, 11, 415–423. [Google Scholar] [CrossRef]
- Petersenn, S.; Rasch, A.C.; Penshorn, M.; Beil, F.U.; Schulte, H.M. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology 2001, 142, 2649–2659. [Google Scholar] [CrossRef]
- Camiña, J.P. Cell biology of the ghrelin receptor. J. Neuroendocrinol. 2006, 18, 65–76. [Google Scholar] [CrossRef]
- Germain, N.; Cuenco, J.; Ling, Y.; Minnion, J.S.; Bageacu, S.; Grouselle, D.; Estour, B.; Galusca, B. Ghrelin acylation by ghrelin- O-acyltransferase can occur in healthy part of oncological liver in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G366–G371. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Lu, X.; Jin, N.; Shi, J. Knockdown of ghrelin-O-acyltransferase attenuates colitis through the modulation of inflammatory factors and tight junction proteins in the intestinal epithelium. Cell Biol. Int. 2020, 44, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, M.A.; Feighner, S.D.; Pong, S.S.; McKee, K.K.; Hreniuk, D.L.; Silva, M.V.; Warren, V.A.; Howard, A.D.; Van Der Ploeg, L.H.; Heck, J.V. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: Minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J. Med. Chem. 2000, 43, 4370–4376. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hosoda, H.; Kitajima, Y.; Morozumi, N.; Minamitake, Y.; Tanaka, S.; Matsuo, H.; Kojima, M.; Hayashi, Y.; Kangawa, K. Structure-activity relationship of ghrelin: Pharmacological study of ghrelin peptides. Biochem. Biophys. Res. Commun. 2001, 287, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Ariyasu, H.; Takaya, K.; Tagami, T.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Suda, M.; Koh, T.; Natsui, K.; Toyooka, S.; et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4753–4758. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Leproult, R.; Scherberg, N.; Van Cauter, E. Twenty-four-hour profiles of acylated and total ghrelin: Relationship with glucose levels and impact of time of day and sleep. J. Clin. Endocrinol. Metab. 2011, 96, 486–493. [Google Scholar] [CrossRef]
- Yang, H.; Youm, Y.H.; Nakata, C.; Dixit, V.D. Chronic caloric restriction induces forestomach hypertrophy with enhanced ghrelin levels during aging. Peptides 2007, 28, 1931–1936. [Google Scholar] [CrossRef]
- Fontana, L. Neuroendocrine factors in the regulation of inflammation: Excessive adiposity and calorie restriction. Exp. Gerontol. 2009, 44, 41–45. [Google Scholar] [CrossRef]
- Dixit, V.D.; Taub, D.D. Ghrelin and immunity: A young player in an old field. Exp. Gerontol. 2005, 40, 900–910. [Google Scholar] [CrossRef]
- Ertosun, M.G.; Kocak, G.; Ozes, O.N. The regulation of circadian clock by tumor necrosis factor alpha. Cytokine Growth Factor Rev. 2019, 46, 10–16. [Google Scholar] [CrossRef]
- De Vriese, C.; Gregoire, F.; Lema-Kisoka, R.; Waelbroeck, M.; Robberecht, P.; Delporte, C. Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology 2004, 145, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Ghrelin and des-acyl ghrelin: Two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000, 279, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Moncada, R.; Valentí, V.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G.; Rodríguez, A. Acylated and desacyl ghrelin are associated with hepatic lipogenesis, β-oxidation and autophagy: Role in NAFLD amelioration after sleeve gastrectomy in obese rats. Sci. Rep. 2016, 6, 39942. [Google Scholar] [CrossRef]
- Ezquerro, S.; Frühbeck, G.; Rodríguez, A. Ghrelin and autophagy. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 402–408. [Google Scholar] [CrossRef]
- Ezquerro, S.; Mocha, F.; Frühbeck, G.; Guzmán-Ruiz, R.; Valentí, V.; Mugueta, C.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C.; et al. Ghrelin Reduces TNF-α-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J. Clin. Endocrinol. Metab. 2019, 104, 21–37. [Google Scholar] [CrossRef]
- Ezquerro, S.; Becerril, S.; Tuero, C.; Méndez-Giménez, L.; Mocha, F.; Moncada, R.; Valentí, V.; Cienfuegos, J.A.; Catalán, V.; Gómez-Ambrosi, J.; et al. Role of ghrelin isoforms in the mitigation of hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress after bariatric surgery in rats. Int. J. Obes. 2020, 44, 475–487. [Google Scholar] [CrossRef]
- Zizzari, P.; Longchamps, R.; Epelbaum, J.; Bluet-Pajot, M.T. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology 2007, 148, 1648–1653. [Google Scholar] [CrossRef]
- Seim, I.; Walpole, C.; Amorim, L.; Josh, P.; Herington, A.; Chopin, L. The expanding roles of the ghrelin-gene derived peptide obestatin in health and disease. Mol. Cell. Endocrinol. 2011, 340, 111–117. [Google Scholar] [CrossRef]
- Lauwers, E.; Landuyt, B.; Arckens, L.; Schoofs, L.; Luyten, W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem. Biophys. Res. Commun. 2006, 351, 21–25. [Google Scholar] [CrossRef]
- Laitakari, A.; Liu, L.; Frimurer, T.M.; Holst, B. The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target. Int. J. Mol. Sci. 2021, 22, 3872. [Google Scholar] [CrossRef] [PubMed]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, M.; Tsolakis, A.V.; Magnusson, L.; Janson, E.T.; Saras, J. Distribution of obestatin and ghrelin in human tissues: Immunoreactive cells in the gastrointestinal tract, pancreas, and mammary glands. J. Histochem. Cytochem. 2008, 56, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sakai, T. Characteristic features of ghrelin cells in the gastrointestinal tract and the regulation of stomach ghrelin expression and production. World J. Gastroenterol. 2008, 14, 6306–6311. [Google Scholar] [CrossRef]
- Ueberberg, B.; Unger, N.; Saeger, W.; Mann, K.; Petersenn, S. Expression of ghrelin and its receptor in human tissues. Horm. Metab. Res. 2009, 41, 814–821. [Google Scholar] [CrossRef]
- Waseem, T.; Ahmad, F.; Azam, M.; Qureshi, M.A. Role of ghrelin axis in colorectal cancer: A novel association. Peptides 2008, 29, 1369–1376. [Google Scholar] [CrossRef]
- Lim, C.T.; Kola, B.; Grossman, A.; Korbonits, M. The expression of ghrelin O-acyltransferase (GOAT) in human tissues. Endocr. J. 2011, 58, 707–710. [Google Scholar] [CrossRef]
- Liu, A.; Huang, C.; Xu, J.; Cai, X. Lentivirus-mediated shRNA interference of ghrelin receptor blocks proliferation in the colorectal cancer cells. Cancer Med. 2016, 9, 2417–2426. [Google Scholar] [CrossRef]
- Stojsavljevic-Shapeski, S.; Virovic-Jukic, L.; Tomas, D.; Duvnjak, M.; Tomasic, V.; Hrabar, D.; Kralj, D.; Budimir, I.; Barsic, N.; Ljubicic, N. Expression of adipokine ghrelin and ghrelin receptor in human colorectal adenoma and correlation with the grade of dysplasia. World J. Gastrointest. Surg. 2021, 13, 1708–1720. [Google Scholar] [CrossRef]
- Grönberg, M.; Amini, R.M.; Stridsberg, M.; Janson, E.T.; Saras, J. Neuroendocrine markers are expressed in human mammary glands. Regul. Pept. 2010, 160, 68–74. [Google Scholar] [CrossRef]
- Dixit, V.D.; Schaffer, E.M.; Pyle, R.S.; Collins, G.D.; Sakthivel, S.K.; Palaniappan, R.; Lillard, J.W., Jr.; Taub, D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Investig. 2004, 114, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Saito, T.; Yagyu, T.; Jiang, B.H.; Kitagawa, K.; Inagaki, C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J. Clin. Endocrinol. Metab. 2001, 86, 4284–4291. [Google Scholar] [CrossRef] [PubMed]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Baatar, D.; Patel, K.; Taub, D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011, 340, 44–58. [Google Scholar] [CrossRef]
- Taub, D.D. Novel connections between the neuroendocrine and immune systems: The ghrelin immunoregulatory network. Vitam. Horm. 2008, 77, 325–346. [Google Scholar] [CrossRef]
- Waseem, T.; Duxbury, M.; Ashley, S.W.; Robinson, M.K. Ghrelin promotes intestinal epithelial cell proliferation through PI3K/Akt pathway and EGFR trans-activation both converging to ERK 1/2 phosphorylation. Peptides 2014, 52, 113–121. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Kuśnierz-Cabala, B.; Bonior, J.; Jaworek, J.; Ambroży, T.; Gil, K.; Olszanecki, R.; et al. Essential Role of Growth Hormone and IGF-1 in Therapeutic Effect of Ghrelin in the Course of Acetic Acid-Induced Colitis. Int. J. Mol. Sci. 2017, 18, 1118. [Google Scholar] [CrossRef]
- Guneli, E.; Onal, A.; Ates, M.; Bagriyanik, H.A.; Resmi, H.; Orhan, C.E.; Kolatan, H.E.; Gumustekin, M. Effects of repeated administered ghrelin on chronic constriction injury of the sciatic nerve in rats. Neurosci. Lett. 2010, 479, 226–230. [Google Scholar] [CrossRef]
- Madison, L.D.; Scarlett, J.M.; Levasseur, P.; Zhu, X.; Newcomb, K.; Batra, A.; Bowe, D.; Marks, D.L. Prostacyclin signaling regulates circulating ghrelin during acute inflammation. J. Endocrinol. 2008, 196, 263–273. [Google Scholar] [CrossRef]
- Xing, Y.X.; Yang, L.; Kuang, H.Y.; Gao, X.Y.; Liu, H.L. Function of obestatin in the digestive system. Nutrition 2017, 34, 21–28. [Google Scholar] [CrossRef]
- Eissa, N.; Ghia, J.E. Immunomodulatory effect of ghrelin in the intestinal mucosa. Neurogastroenterol. Motil. 2015, 11, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Solomon, T.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 5068–5085. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M. Faces of ghrelin—Research for the 21st century. Neurogastroenterol. Motil. 2009, 21, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Prodam, F.; Filigheddu, N. Ghrelin gene products in acute and chronic inflammation. Arch. Immunol. Ther. Exp. 2014, 62, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Pamukcu, O.; Kumral, Z.N.; Ercan, F.; Yegen, B.C.; Ertem, D. Anti-inflammatory effect of obestatin and ghrelin in dextran sulfate sodium-induced colitis in rats. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Kuśnierz-Cabala, B.; Konturek, P.; Ambroży, T.; Dembiński, A. Obestatin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 2834386. [Google Scholar] [CrossRef]
- Konarska, K.; Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Chmura, A.; Kuśnierz-Cabala, B.; Gałązka, K.; Kowalczyk, P.; Miskiewicz, A.; Konturek, T.J.; et al. Treatment with Obestatin-A Ghrelin Gene-Encoded Peptide-Reduces the Severity of Experimental Colitis Evoked by Trinitrobenzene Sulfonic Acid. Int. J. Mol. Sci. 2018, 19, 1643. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Gałązka, K.; Bonior, J.; Jaworek, J.; Konturek, P.C.; Gil, K.; Dembiński, A. Pretreatment with obestatin inhibits the development of acetic acid-induced colitis in rats. Arch. Med. Sci. 2018, 14, 920–929. [Google Scholar] [CrossRef]
- Tiaka, E.K.; Manolakis, A.C.; Kapsoritakis, A.N.; Potamianos, S.P. Unraveling the link between leptin, ghrelin and different types of colitis. Ann. Gastroenterol. 2011, 24, 20–28. [Google Scholar]
- Karmiris, K.; Koutroubakis, I.E.; Kouroumalis, E.A. Leptin, adiponectin, resistin, and ghrelin—Implications for inflammatory bowel disease. Mol. Nutr. Food Res. 2008, 52, 855–866. [Google Scholar] [CrossRef]
- Hosomi, S.; Oshitani, N.; Kamata, N.; Sogawa, M.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Watanabe, T.; Fujiwara, Y.; Maeda, K.; et al. Phenotypical and functional study of ghrelin and its receptor in the pathogenesis of Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Peracchi, M.; Bardella, M.T.; Caprioli, F.; Massironi, S.; Conte, D.; Valenti, L.; Ronchi, C.; Beck-Peccoz, P.; Arosio, M.; Piodi, L. Circulating ghrelin levels in patients with inflammatory bowel disease. Gut 2006, 55, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Ates, Y.; Degertekin, B.; Erdil, A.; Yaman, H.; Dagalp, K. Serum ghrelin levels in inflammatory bowel disease with relation to disease activity and nutritional status. Dig. Dis. Sci. 2008, 53, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, E.; Zisimopoulos, A.; Liratzopoulos, N.; Katsos, I.; Manolas, K.; Kouklakis, G. Obestatin/ghrelin ratio: A new activity index in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.W.; Leslie, F.C.; McLaughlin, J.T. Crohn’s disease affecting the small bowel is associated with reduced appetite and elevated levels of circulating gut peptides. Clin. Nutr. 2013, 32, 404–411. [Google Scholar] [CrossRef]
- Cekic, C.; Arabul, M.; Alper, E.; Pakoz, Z.B.; Saritas, E.; Yuksel; Ünsal, B. Evaluation of the relationship between serum ghrelin, C-reactive protein and interleukin-6 levels, and disease activity in inflammatory bowel diseases. Hepatogastroenterology 2014, 61, 1196–1200. [Google Scholar]
- Jung, J.Y.; Jeong, J.B.; Kim, J.W.; Kim, S.H.; Koh, S.J.; Kim, B.G.; Lee, K.L. Circulating ghrelin levels and obestatin/ghrelin ratio as a marker of activity in ulcerative colitis. Intest. Res. 2015, 13, 68–73. [Google Scholar] [CrossRef][Green Version]
- Ghomraoui, F.A.; Alotaibi, S.T.; Alharthi, M.A.; Asiri, S.S.; Almadi, M.A.; Alharbi, O.R.; Azzam, N.A.; Aljebreen, A.M.; Saeed, M.; Hajkhder, B.; et al. Plasma ghrelin and leptin in patients with inflammatory bowel disease and its association with nutritional status. Saudi J. Gastroenterol. 2017, 23, 199–205. [Google Scholar] [CrossRef]
- Trejo-Vazquez, F.; Garza-Veloz, I.; Villela-Ramirez, G.A.; Ortiz-Castro, Y.; Mauricio-Saucedo, P.; Cardenas-Vargas, E.; Diaz-Baez, M.; Cid-Baez, M.A.; Castañeda-Miranda, R.; Ortiz-Rodriguez, J.M.; et al. Positive association between leptin serum levels and disease activity on endoscopy in inflammatory bowel disease: A case-control study. Exp. Ther. Med. 2018, 15, 3336–3344. [Google Scholar] [CrossRef]
- Hosoda, H.; Kangawa, K. Standard sample collections for blood ghrelin measurements. Methods Enzymol. 2012, 514, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Deschaine, S.L.; Leggio, L. Understanding plasma treatment effect on human acyl-ghrelin concentrations. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. Role of the Ghrelin System in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 5380. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Cross, A.J.; Dawsey, S.M.; Stanczyk, F.Z.; Kamangar, F.; Weinstein, S.J.; Taylor, P.R.; Männistö, S.; Albanes, D.; Abnet, C.C.; et al. Serum ghrelin is associated with risk of colorectal adenocarcinomas in the ATBC study. Gut 2018, 67, 1646–1651. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Snider, A.J.; Bialkowska, A.B.; Ghaleb, A.M.; Yang, V.W.; Obeid, L.M.; Hannun, Y.A. Murine Model for Colitis-Associated Cancer of the Colon. Methods Mol. Biol. 2016, 1438, 245–254. [Google Scholar] [CrossRef]
- Symonds, E.L.; Riedel, C.U.; O’Mahony, D.; Lapthorne, S.; O’Mahony, L.; Shanahan, F. Involvement of T helper type 17 and regulatory T cell activity in Citrobacter rodentium invasion and inflammatory damage. Clin. Exp. Immunol. 2009, 157, 148–154. [Google Scholar] [CrossRef]
- Zhao, D.; Zhan, Y.; Zeng, H.; Moyer, M.P.; Mantzoros, C.S.; Pothoulakis, C. Ghrelin stimulates interleukin-8 gene expression through protein kinase C-mediated NF-kappaB pathway in human colonic epithelial cells. J. Cell. Biochem. 2006, 97, 1317–1327. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Wang, W.G.; Li, Q.; Tang, M.; Li, J.; Wu, W.T.; Wan, Y.H.; Wang, Z.G.; Bao, S.S.; Fei, J. Growth hormone secretagogue receptor is important in the development of experimental colitis. Cell Biosci. 2015, 5, 12. [Google Scholar] [CrossRef]
- Noh, J.Y.; Wu, C.S.; DeLuca, J.A.A.; Devaraj, S.; Jayaraman, A.; Alaniz, R.C.; Tan, X.D.; Allred, C.D.; Sun, Y. Novel Role of Ghrelin Receptor in Gut Dysbiosis and Experimental Colitis in Aging. Int. J. Mol. Sci. 2022, 23, 2219. [Google Scholar] [CrossRef] [PubMed]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. 2015, 66, 875–885. [Google Scholar] [PubMed]
- Cheng, J.; Zhang, L.; Dai, W.; Mao, Y.; Li, S.; Wang, J.; Li, H.; Guo, C.; Fan, X. Ghrelin ameliorates intestinal barrier dysfunction in experimental colitis by inhibiting the activation of nuclear factor-kappa B. Biochem. Biophys. Res. Commun. 2015, 458, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Pihut, M.; et al. The Influence of Ghrelin on the Development of Dextran Sodium Sulfate-Induced Colitis in Rats. Biomed. Res. Int. 2015, 2015, 718314. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous Ghrelin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Shen, J.; Wang, S.; Guo, C.; Fan, X. Ghrelin Inhibits Intestinal Epithelial Cell Apoptosis Through the Unfolded Protein Response Pathway in Ulcerative Colitis. Front. Pharmacol. 2021, 12, 661853. [Google Scholar] [CrossRef]
- De Smet, B.; Thijs, T.; Moechars, D.; Colsoul, B.; Polders, L.; Ver Donck, L.; Coulie, B.; Peeters, T.L.; Depoortere, I. Endogenous and exogenous ghrelin enhance the colonic and gastric manifestations of dextran sodium sulphate-induced colitis in mice. Neurogastroenterol. Motil. 2009, 1, 59–70. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kanemaru, A.; Fukushima, T.; Yamamoto, K.; Tanaka, H.; Haruyama, Y.; Itoh, H.; Matsumoto, N.; Kangawa, K.; Nakazato, M.; et al. Ghrelin administration suppresses inflammation-associated colorectal carcinogenesis in mice. Cancer Sci. 2015, 106, 1130–1136. [Google Scholar] [CrossRef]
- Di Giovangiulio, M.; Stakenborg, N.; Bosmans, G.; Meroni, E.; Farro, G.; Gomez-Pinilla, P.J.; Depoortere, I.; Boeckxstaens, G.E.; Matteoli, G. Ghrelin receptor modulates T helper cells during intestinal inflammation. Neurogastroenterol. Motil. 2015, 27, 1542–1552. [Google Scholar] [CrossRef]
- Bułdak, R.J.; Pilc-Gumuła, K.; Bułdak, Ł.; Witkowska, D.; Kukla, M.; Polaniak, R.; Zwirska-Korczala, K. Effects of ghrelin, leptin and melatonin on the levels of reactive oxygen species, antioxidant enzyme activity and viability of the HCT 116 human colorectal carcinoma cell line. Mol. Med. Rep. 2015, 12, 2275–2282. [Google Scholar] [CrossRef]
- Zhao, D. Protein kinase Cdelta-mediated CREB activation regulates ghrelin-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colonic epithelial cells. J. Cell. Biochem. 2007, 102, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Streba, L.A.; Vere, C.C.; Rogoveanu, I.; Streba, C.T. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: An open question. World J. Gastroenterol. 2015, 21, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, A.; Mottershead, M.; Syn, W.K.; Jones, R.; Smith, S.; Nwokolo, C.U. Ciprofloxacin suppresses bacterial overöowth, increases fasting insulin but does not correct low acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2005, 22, 291–299. [Google Scholar] [CrossRef]
- Yalniz, M.; Bahcecioglu, I.H.; Ataseven, H.; Ustundag, B.; Ilhan, F.; Poyrazoglu, O.K.; Erensoy, A. Serum adipokine and ghrelin levels in nonalcoholic steatohepatitis. Mediat. Inflamm. 2006, 2006, 34295. [Google Scholar] [CrossRef] [PubMed]
- Estep, M.; Abawi, M.; Jarrar, M.; Wang, L.; Stepanova, M.; Elariny, H.; Moazez, A.; Goodman, Z.; Chandhoke, V.; Baranova, A.; et al. Association of obestatin, ghrelin, and inflammatory cytokines in obese patients with non-alcoholic fatty liver disease. Obes. Surg. 2011, 21, 1750–1757. [Google Scholar] [CrossRef]
- Machado, M.V.; Coutinho, J.; Carepa, F.; Costa, A.; Proença, H.; Cortez-Pinto, H. How adiponectin, leptin, and ghrelin orchestrate together and correlate with the severity of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1166–1172. [Google Scholar] [CrossRef]
- Okamatsu, Y.; Matsuda, K.; Hiramoto, I.; Tani, H.; Kimura, K.; Yada, Y.; Kakuma, T.; Higuchi, S.; Kojima, M.; Matsuishi, T. Ghrelin and leptin modulate immunity and liver function in overweight children. Pediatr. Int. 2009, 51, 9–13. [Google Scholar] [CrossRef]
- Tacke, F.; Brabant, G.; Kruck, E.; Horn, R.; Schöffski, P.; Hecker, H.; Manns, M.P.; Trautwein, C. Ghrelin in chronic liver disease. J. Hepatol. 2003, 38, 447–454. [Google Scholar] [CrossRef]
- Elbadri, A.; Esmat, S.; Abosaif, N.; Morsi, A.; Shaker, O. Study of serum ghrelin changes and its correlation with malnutrition in liver cirrhosis in Egypt. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 638–643. [Google Scholar] [CrossRef]
- Dornelles, C.T.; Goldani, H.A.; Wilasco, M.I.; Maurer, R.L.; Kieling, C.O.; Porowski, M.; Ferreira, C.T.; Santos, J.L.; Vieira, S.M.; Silveira, T.R. Ghrelin, leptin and insulin in cirrhotic children and adolescents: Relationship with cirrhosis severity and nutritional status. Regul. Pept. 2013, 180, 26–32. [Google Scholar] [CrossRef]
- Elaghori, A.; Salem, P.E.S.; Azzam, E.; Abu Elfotoh, N. Ghrelin Level in Patients With Liver Cirrhosis. Acta Endocrinol. 2019, 5, 62–68. [Google Scholar] [CrossRef]
- Goodyear, S.J.; Mottershead, M.; Sung, E.Z.; Wong, L.S.; McTernan, P.G.; Kumar, S.; Nwokolo, C.U. Dysregulation of plasma ghrelin in alcoholic cirrhosis. Clin. Endocrinol. 2010, 73, 323–329. [Google Scholar] [CrossRef]
- Cortez, A.P.B.; Mattar, R.H.D.M.; de Azevedo, R.A.; Fisberg, M.; Lederman, H.M.; de Morais, M.B. Adiponectin is Increased in Pediatric Patients With Autoimmune Hepatitis Independent of Body Weight. J. Pediatr. Gastroenterol. Nutr. 2020, 71, e118–e123. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nagao, Y.; Sata, M. Independent factors associated with altered plasma active ghrelin levels in HCV-infected patients. Liver Int. 2013, 33, 1510–1516. [Google Scholar] [CrossRef]
- Zhang, X.; Zhai, L.; Rong, C.; Qin, X.; Li, S. Association of Ghrelin Gene Polymorphisms and Serum Ghrelin Levels with the Risk of Hepatitis B Virus-Related Liver Diseases in a Chinese Population. PLoS ONE 2015, 10, e0143069. [Google Scholar] [CrossRef]
- Hamdy, M.; Kassim, S.K.; Khairy, E.; Maher, M.; Mansour, K.A.; Albreedy, A.M. Ghrelin gene polymorphism as a genetic biomarker for prediction of therapy induced clearance in Egyptian chronic HCV patients. Gene 2018, 649, 74–79. [Google Scholar] [CrossRef]
- Watanabe, H.; Saito, T.; Karasawa, T.; Kudo, S.; Nakano, K.; Ito, J.I.; Sugahara, K.; Saito, K.; Togashi, H.; Kawata, S. Reduction of serum ghrelin concentration during interferon-alpha therapy in patients with chronic hepatitis C. Hepatol. Res. 2005, 33, 14–18. [Google Scholar] [CrossRef]
- Pavlidis, C.; Panoutsopoulos, G.I.; Tiniakos, D.; Koutsounas, S.; Vlachogiannakos, J.; Zouboulis-Vafiadis, I. Serum leptin and ghrelin in chronic hepatitis C patients with steatosis. World J. Gastroenterol. 2011, 17, 5097–5104. [Google Scholar] [CrossRef]
- Uribe, M.; Zamora-Valdés, D.; Moreno-Portillo, M.; Bermejo-Martínez, L.; Pichardo-Bahena, R.; Baptista-González, H.A.; Ponciano-Rodríguez, G.; Uribe, M.H.; Medina-Santillán, R.; Méndez-Sánchez, N. Hepatic expression of ghrelin and adiponectin and their receptors in patients with nonalcoholic fatty liver disease. Ann. Hepatol. 2008, 7, 67–71. [Google Scholar] [CrossRef]
- Gutierrez-Grobe, Y.; Villalobos-Blasquez, I.; Sánchez-Lara, K.; Villa, A.R.; Ponciano-Rodríguez, G.; Ramos, M.H.; Chavez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. High ghrelin and obestatin levels and low risk of developing fatty liver. Ann. Hepatol. 2010, 9, 52–57. [Google Scholar] [CrossRef]
- Aktas, B.; Yilmaz, Y.; Eren, F.; Yonal, O.; Kurt, R.; Alahdab, Y.O.; Celikel, C.A.; Ozdogan, O.; Imeryuz, N.; Kalayci, C.; et al. Serum levels of vaspin, obestatin, and apelin-36 in patients with nonalcoholic fatty liver disease. Metabolism 2011, 60, 544–549. [Google Scholar] [CrossRef]
- Ataseven, H.; Bahcecioglu, I.H.; Kuzu, N.; Yalniz, M.; Celebi, S.; Erensoy, A.; Ustundag, B. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediat. Inflamm. 2006, 2006, 78380. [Google Scholar] [CrossRef]
- Naguib, R.; Fayed, A.; Elkemary, E.; Naguib, H. Serum Ghrelin Concentration in Patients With Primary Biliary Cirrhosis (PBC). Cureus 2021, 13, e20288. [Google Scholar] [CrossRef]
- Tóth, G.; Rauh, M.; Nyul, Z.; Sulyok, E.; Rascher, W. Serum ghrelin, adipokine and insulin levels in children with acute hepatitis. Eur. J. Gastroenterol. Hepatol. 2009, 21, 739–743. [Google Scholar] [CrossRef]
- Motawi, T.K.; Shaker, O.G.; Ismail, M.F.; Sayed, N.H. Genetic variants associated with the progression of hepatocellular carcinoma in hepatitis C Egyptian patients. Gene 2013, 527, 516–520. [Google Scholar] [CrossRef]
- Golestan Jahromi, M.; Nabavizadeh, F.; Vahedian, J.; Nahrevanian, H.; Dehpour, A.R.; Zare-Mehrjardi, A. Protective effect of ghrelin on acetaminophen-induced liver injury in rat. Peptides 2010, 31, 2114–2117. [Google Scholar] [CrossRef]
- Cetin, E.; Kanbur, M.; Cetin, N.; Eraslan, G.; Atasever, A. Hepatoprotective effect of ghrelin on carbon tetrachloride-induced acute liver injury in rats. Regul. Pept. 2011, 171, 1–5. [Google Scholar] [CrossRef]
- Kabil, N.N.; Seddiek, H.A.; Yassin, N.A.; Gamal-Eldin, M.M. Effect of ghrelin on chronic liver injury and fibrogenesis in male rats: Possible role of nitric oxide. Peptides 2014, 52, 90–97. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, J.; Yu, F.; Cheng, J.; Li, H.; Guo, C.; Fan, X. Ghrelin reduces liver impairment in a model of concanavalin A-induced acute hepatitis in mice. Drug Des. Devel. Ther. 2015, 9, 5385–5396. [Google Scholar] [CrossRef]
- Ercan, S.; Kencebay, C.; Basaranlar, G.; Ozcan, F.; Derin, N.; Aslan, M. Induction of omega 6 inflammatory pathway by sodium metabisulfite in rat liver and its attenuation by ghrelin. Lipids Health Dis. 2015, 14, 7. [Google Scholar] [CrossRef][Green Version]
- Mao, Y.; Cheng, J.; Yu, F.; Li, H.; Guo, C.; Fan, X. Ghrelin Attenuated Lipotoxicity via Autophagy Induction and Nuclear Factor-κB Inhibition. Cell. Physiol. Biochem. 2015, 37, 563–576. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Li, Z.; Gong, Y. The role of acylated ghrelin and unacylated ghrelin in the blood and hypothalamus and their interaction with nonalcoholic fatty liver disease. Iran. J. Basic Med. Sci. 2020, 23, 1191–1196. [Google Scholar] [CrossRef]
- Nagoya, T.; Kamimura, K.; Inoue, R.; Ko, M.; Owaki, T.; Niwa, Y.; Sakai, N.; Setsu, T.; Sakamaki, A.; Yokoo, T.; et al. Ghrelin-insulin-like growth factor-1 axis is activated via autonomic neural circuits in the non-alcoholic fatty liver disease. Neurogastroenterol. Motil. 2020, 32, e13799. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Ande, S.R.; Nguyen, K.H.; Grégoire Nyomba, B.L.; Mishra, S. Prohibitin-induced, obesity-associated insulin resistance and accompanying low-grade inflammation causes NASH and HCC. Sci. Rep. 2016, 6, 23608. [Google Scholar] [CrossRef]
- Alharbi, S. Exogenous administration of unacylated ghrelin attenuates hepatic steatosis in high-fat diet-fed rats by modulating glucose homeostasis, lipogenesis, oxidative stress, and endoplasmic reticulum stress. Biomed. Pharmacother. 2022, 151, 113095. [Google Scholar] [CrossRef]
- Guillory, B.; Jawanmardi, N.; Iakova, P.; Anderson, B.; Zang, P.; Timchenko, N.A.; Garcia, J.M. Ghrelin deletion protects against age-associated hepatic steatosis by downregulating the C/EBPα-p300/DGAT1 pathway. Aging Cell. 2018, 17, e12688. [Google Scholar] [CrossRef]
- Dallak, M.A. Acylated ghrelin induces but deacylated ghrelin prevents hepatic steatosis and insulin resistance in lean rats: Effects on DAG/PKC/JNK pathway. Biomed. Pharmacother. 2018, 105, 299–311. [Google Scholar] [CrossRef]
- El-Gohary, O.A. Obestatin improves hepatic injury induced by ischemia/reperfusion in rats: Role of nitric oxide. Gen. Physiol. Biophys. 2017, 36, 109–115. [Google Scholar] [CrossRef]
- Khaleel, E.F.; Abdel-Aleem, G.A. Obestatin protects and reverses nonalcoholic fatty liver disease and its associated insulin resistance in rats via inhibition of food intake, enhancing hepatic adiponectin signaling, and blocking ghrelin acylation. Arch. Physiol. Biochem. 2019, 125, 64–78. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, S.; Yu, F.; Li, H.; Guo, C.; Fan, X. Ghrelin Attenuates Liver Fibrosis through Regulation of TGF-β1 Expression and Autophagy. Int. J. Mol. Sci. 2015, 16, 21911–21930. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Sui, J.; Luo, G. Robust Human and Murine Hepatocyte Culture Models of Hepatitis B Virus Infection and Replication. J. Virol. 2018, 92, e01255-18. [Google Scholar] [CrossRef] [PubMed]
- Wakita, T. Cell Culture Systems of HCV Using JFH-1 and Other Strains. Cold Spring Harb. Perspect. Med. 2019, 9, a036806. [Google Scholar] [CrossRef] [PubMed]
- Allweiss, L.; Strick-Marchand, H. In-vitro and in-vivo models for hepatitis B cure research. Curr. Opin. HIV AIDS 2020, 15, 173–179. [Google Scholar] [CrossRef]
- Aardoom, M.A.; Veereman, G.; de Ridder, L. A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019, 20, 2529. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Parra-Holguín, N.N. Emerging therapeutic options in inflammatory bowel disease. World J. Gastroenterol. 2021, 27, 8242–8261. [Google Scholar] [CrossRef]
- Li, M.; Weigmann, B. A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System. Metabolites 2022, 12, 31. [Google Scholar] [CrossRef]
- Deboer, M.D. Use of ghrelin as a treatment for inflammatory bowel disease: Mechanistic considerations. Int. J. Pept. 2011, 2011, 189242. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Sun, D.; Myasnikov, A.; Damian, M.; Baneres, J.L.; Sun, J.; Zhang, C. Structural basis of human ghrelin receptor signaling by ghrelin and the synthetic agonist ibutamoren. Nat. Commun. 2021, 12, 6410. [Google Scholar] [CrossRef]
- Strasser, F. Clinical application of ghrelin. Curr. Pharm. Des. 2012, 18, 4800–4812. [Google Scholar] [CrossRef]
- Usai-Satta, P.; Lai, M.; Oppia, F.; Cabras, F. Effects of Prokinetics on the Digestive Tract. Curr. Rev. Clin. Exp. Pharmacol. 2022, 7, 161–165. [Google Scholar] [CrossRef] [PubMed]
| Model of the Study | Material and Methods | Tissue Expression | The Main Effects of Ghrelin/Obestatin | Mechanisms of Action/Pathways; Role in CRC Development | Ref. | |
|---|---|---|---|---|---|---|
| Ghrelin/Obestatin | GHS-R | |||||
| Animal | mice; TNBS/DSS colitis; eG 1 nmol/mouse/d | nt | nt | (i) ↓ acute and chronic inflammatory response; (ii) ↓ of both inflammatory and Th1-driven autoimmune response; (iii) prevents recurrence of the disease; (iv) ↓ NF-κB | Anti-inflammatory; NF-κB; role in cancer—nd | [38] |
| rats; TNBS colitis and C; eG 20 µg/kg | ↑ mRNA (max at day 7th) | nt | (i) ↑ healing of colonic lesions with ↑ mRNA (iNOS, PGE2) and protein (COX-2); (ii) ↑ of neuropeptides (e.g., CGRP) from sensory nerves | Anti-inflammatory; role in cancer—nd | [39] | |
| mice; C. rodentium-colitis; 7-plex base kit; RT-PCR; no eG treatment | ↑ mRNA at the peak and late stage | nt | (i) in the distal colon: ↑ TNF-α and FoxP3 throughout the study; (ii) ↑ IL-6 and IL-17 during the peak and late stages of infection | Anti-inflammatory—probable; Th cell pathways; G can be involved with clearance of infection; role in cancer—nd | [138] | |
| rats; DSS colitis; eG 20 µg/kg | nt | nt | (i) ↓ severity of chronic colitis, less effective in the acute form; (ii) ↓ lipid peroxidation and Th1 response; (iii) in chronic colitis: ↓ of pro- and ↑ of anti-inflammatory cytokines (TGF-β) | Anti-inflammatory; role in cancer—nd | [115] | |
| rats; DSS colitis; eO 50 µg/kg | nt | nt | (i) ↓ disease activity in acute and in chronic colitis; (ii) ↓ MDA; (iii) ↑ GSH; (iv) in acute colitis: ↓ IL-1β and TNF-α in colon; (v) in chronic colitis: ↓ IL-1β, IFN-γ, TNF-α with ↑ IL-10 and TGF-β in colon | Anti-inflammatory; role in cancer—nd | ||
| rats; AA colitis and C; eG 4–16 nmol/kg/dose | nt | nt | (i) ↓ the area and grade of mucosal damage; (ii) ↓ IL-1β and MDA in mucosa; (iii) ↓ MPO activity | Anti-inflammatory; role in cancer—nd | [142] | |
| rats; DSS colitis and C; eG 8 nmol/kg/dose | nt | nt | (i) ↓ of mucosal damage; (ii) ↓ IL-1β and MDA in mucosa; (iii) reversed ↓ in BW gain | Anti-inflammatory; role in cancer—nd | [144] | |
| mice; AOM/DSS colitis; (Apc(Min/+) model; eG; RT-PCR, IHC | nt | nt | (i) ↓ in tumor incidence in AOM/DSS colitis; (ii) no tumor-promoting effect in either model; (iii) loss of G did not affect the incidence of intestinal tumor formation in either model | The chemopreventive effect of inflammation-associated colorectal carcinogenesis | [148] | |
| mice; DSS colitis; eG 125 or 250 μg/kg | nt | nt | (i) ↓ the disease activity index, histological score, and MPO activities; (ii) ↑ in TJ structural integrity and cytokine secretion; (iii) ↓ NF-κB, inhibitory κB-α, MLCK, and pMLC2 activation | Anti-inflammatory; GHS-R1a; NF-κB; role in cancer—nd | [143] | |
| mice; T cell transfer model of chronic colitis; Rag(−/−) mice; eG 0–100 ng/mL; FC | nt | nt | (i) the lack of G signaling in Th cells resulted in a ↑ severity of colitis with ↑ colonic inflammation dependent on a pathological ↑ of CD4 T cells in lamina propria; (ii) ↓ proliferation and ↑ apoptosis of Th cells; (iii) specific effect on Th cells | Anti-inflammatory; role in cancer—nd | [149] | |
| rats; AA-induced colitis and C; eAG 8 nmol/kg/dose | nt | nt | (i) ↓ histological colonic damage and ↑ spontaneous colonic regeneration; (ii) ↑ DNA synthesis in mucosa; (iii) ↑ of blood flow in mucosa; (iv) ↓ IL-1β, TNF-α, and MPO activity in mucosa | Anti-inflammatory; role in cancer—nd | [145] | |
| rats; AA colitis and C; rat O 8 nmol/kg; 2×/day; 7 and 14 days | nt | nt | (1) ↑ healing of colonic lesions; (ii) ↓ MPO and IL-1β in mucosa; (iii) reversed the colitis-evoked decrease in blood flow and DNA synthesis (↑ cell proliferation in mucosa) | Anti-inflammatory; role in cancer—nd | [116] | |
| rats; AA colitis and C; eAG 8 nmol/kg/dose | nt | nt | (i) ↓ damage of mucosa only in pituitary-intact rat, correlated with ↑ serum levels of GH and IGF-1; (ii) ↑ blood flow and ↑ cell proliferation; (iii) ↓ IL-1β, MDA and MPO activity in mucosa | Anti-inflammatory; GH and IGF-1; role in cancer—nd | [107] | |
| rats; AA colitis; eO 4, 8 or 16 nmol/kg/dose; 2×/day | nt | nt | (1) ↓ the area of colonic damage; (ii) ↑ blood flow and DNA synthesis in mucosa; (iii) dose-dependent ↓ IL-1β and MPO in mucosa | Anti-inflammatory; role in cancer—nd | [118] | |
| rats; TNBS colitis and C; eO 4, 8, or 16 nmol/kg, 2×/day, 4 days | nt | nt | (1) dose-dependent ↓ the area of colonic damage; (ii) ↑ blood flow in the colon; (iii) ↓ MPO activity and IL-1β in mucosa; (iv) ↓ blood leukocytes | Anti-inflammatory; role in cancer—nd | [117] | |
| (Ghsr−/−) mice; DSS colitis; no eG treatment | nt | nt | (i) in GHS-R KO mice: ↑ disease activity scores, ↑ expression of TNF-α, and IL-1β, and ↓ expression of TJ markers (occludin, claudin 2); (ii) ↑ gut permeability and exacerbated colitis | Anti-inflammatory; role in microbiome homeostasis and gut inflammation during aging; role in cancer—nd | [141] | |
| mice; DSS colitis; GOAT(−/−) mice; no eG treatment | nt | nt | KD of GOAT: ↓ colitis-induced inflammation and ↓ apoptosis by ↓ the intestinal permeability; (ii) GOAT overexpression: ↑ colitis | Proinflammatory role of GOAT; role in cancer—nd | [72] | |
| mice; DSS and TNBS colitis; eG 25–250 µg/kg | nt | nt | (i) protection from apoptosis; (ii) ↓ apoptosis in a dose-dependent manner, reversed by D-Lys3-GHRP-6 | Anti-apoptotic; GHS-R1a/GHS-R1b; UPR; role in CRC—nd | [146] | |
| mice; TNBS colitis; no eG treatment; RT-PCR | ↑ mRNA | ↑ mRNA | ↑ G and GHS-R during acute experimental colitis | Proinflammatory probable; role in cancer—nd | [139] | |
| G(+/+) and G(−/−) mice; DSS colitis; eG 100 nmol kg−1 | nt | nt | (i) ↑ in G(+/+) plasma levels; (ii) ↑ clinical disease activity, ↑ infiltration of neutrophils, and ↑ colonic IL-1β levels; (iii) absence of G did not affect colonic contractility; (iv) in G(−/−) mice: ↓ BW loss, ↓ histological damage, ↓ MPO, IL-1β levels | Proinflammatory; role in cancer—nd | [147] | |
| WT-mice and (Ghsr−/−) mice; DSS colitis; no eG treatment | no changed at day 7th | ↑ mRNA at day 7th | (i) ↓ colonic macrophage infiltration and TLRs expression from DSS-treated (Ghsr−/−) mice vs. WT-mice | Proinflammatory; role in pathogenesis of IBD—probable, role in cancer—nd | [140] | |
| In vitro | normal human colon NCM460 cells transfected with a functional GHS-R; eG 10−9–10−7 M | nt | (+) mRNA | (i) ↑ IκBα phosphorylation and degradation; (ii) stimulation of NF-κB-binding activity and NF-κB p65 subunit phosphorylation; (iii) ↑ TNF-α-induced IL-8 promoter activity and IL-8 protein secretion | Proinflammatory; PKC-dependent NF-κB; role in cancer—nd | [139] |
| NCM460 cells transfected with GHS-R; eG 10−8 M | nt | nt | (i) ↑ COX-2 protein/promoter activity; (ii) ↑ PGE2 secretion; (iii) ↑ phosphorylation of CREB via PKCδ activation; (iv) ↑ phosphorylation of PKCδ | Proinflammatory; PKC; role in cancer—nd | [151] | |
| T cell transfer model of chronic colitis; eG 0–100 ng/mL | nt | nt | (i) directly affected Th cells: ↓ proliferation and ↑ apoptosis; (ii) did not influence Th cell polarization | Regulation of Th cells in gut, anti-proliferative and anti-apoptotic role in IBD—probable; role in cancer—nd | [149] | |
| MA from (Ghsr−/−) LPS-stimulated mice; MA from WT mice; DSS colitis; IHC; ELISA | nt | nt | (i) ↓ IL-6, TNF-α, IL-1β, TLR-2, TLR-4 levels in MA from (Ghsr−/−) mice vs. WT-mice; (ii) D-lys(3) -GHRP6: ↓ LPS-induced MA proinflammatory cytokines from WT mice | GHS-Rs: role in acute colitis and MA activation in vitro; role in pathogenesis of IBD—probable, role in cancer—nd | [140] | |
| human colon HCT116 cells; eG 10−8 M | nt | nt | (i) ↓ ROS via ↑ activity of CAT and MnSOD vs. untreated cells; (ii) ↓ MDA (G + leptin) | Anti-inflammatory; role in pathogenesis of IBD—probable, role in cancer—nd | [150] | |
| TNF-α-induced Caco-2 cells; eG 0.01–10 µmol/L; RT-PCR, WB | nt | nt | (i) ↓ apoptosis | Anti-apoptotic; GHS-R1a; UPR; role in cancer—nd | [146] | |
| IBD Colitis | Ghrelin | Refs. | Obestatin | Refs. |
|---|---|---|---|---|
| Human IBD | A | [122,123,124,125,126,127,128] | A | [125] |
| P | [129] | P | [128] | |
| NS | [130] | nd | ||
| DSS | P | [38,115,141,143,146] | P | [115] |
| A | [140,147] | nd | ||
| TNBS | P | [38,39] | P | [117] |
| AA | P | [107,142,145] | P | [116,118] |
| C. rodentium | P | [138] | nd | |
| AOM/DSS | P | [148] | nd |
| Liver Disease | Ghrelin | Refs. | Obestatin | Ref. |
|---|---|---|---|---|
| NAFLD/NASH | ↓ AG vs. C | [153,154,170] | ↓ O in NAFLD vs. C | [170] |
| ↑ UnAG vs. non-NASH and with more advanced fibrosis | [155] | ↑ O with fibrosis stage | [155] | |
| NS trend to ↑ G tissue expression vs. nonalcoholic steatosis and C | [169] | NS O vs. C | [171] | |
| ↑ G mRNA vs. alcoholic hepatitis, HCV-infected livers, and C | [42] | |||
| Chronic hepatitis B | ↓ AG vs. C | [164] | nd | |
| Chronic hepatitis C | ↓ AG vs. C | [164] | nd | |
| ↓ G vs. C | [42] | |||
| Alcoholic hepatitis | ↓ G vs. C | [42] | nd | |
| Cirrhosis (different etiologies) | ↑ G in Child C cirrhosis vs. CLD with no cirrhosis | [158] | nd | |
| ↑ G vs. C | [172] | |||
| ↓ G in advanced vs. mild fibrosis | [42] | |||
| ↑ AG vs. C | [162] | |||
| ↓ AG and ↑ UnAG associated with cirrhosis severity | [160] | |||
| ↓ AG in viral-associated cirrhosis vs. C | [164] | |||
| ↓ G vs. C | [161] | |||
| ↑ G in PBC vs. C | [173] | |||
| HCC (different etiologies) | ↑ G vs. C | [172] | nd | |
| Autoimmune hepatitis | NS G vs. C | [163] | nd | |
| Acute hepatitis | ↑ G vs. liver after recovery | [174] | nd | |
| Model of the Study | Material and Methods | The Main Effects of Ghrelin | Role in Inflammation/ Signaling Pathway | Ref. |
|---|---|---|---|---|
| Animal | rats; Acetaminophen-induced ALI; eG | (i) ↓ ALT and AST; (ii) ↓ TNF-α | P | [176] |
| rats; CCl4-induced ALI and BDL-induced CLI; WT and G-deficient mice; eG | (i) ↓ necroinflammatory score and ↓ AST; (ii) ↓ inflammatory infiltration; (ii) ↓ apoptosis; (iii) ↓ fibrogenic response and ↓ fibrogenic properties of HSCs (iv) ↓ myofibroblasts accumulation; (v) ↓ extent of OS; (vi) altered gene expression profile in CLI; (vii) G-deficient mice: ↑ fibrosis and damage after CLI | P; Akt/ERK | [42] | |
| rats; CCl4-induced ALI; eG | (i) ↓ plasma/liver MDA, and NO level; (ii) ↑ erythrocyte/hepatic SOD, CAT and GPx; (iii) G alone and G+CCl4: ↑ glucose level; (iv) ↓ histopathological changes | P | [177] | |
| rats; TAA-induced CLI; eG | (i) ↓ ALT, AST and TNF-α levels; (ii) ↓ collagen in liver; (iii) ↓ MDA and Bax gene expression; (iv) ↑ Bcl-2 and eNOS gene expression | P; NO | [178] | |
| mice; concanavalin A-induced ALI; eG | (i) ↓ proinflammatory cytokines (IL-1β, IL-6, and TNF-α); (ii) ↑ Bcl-2, ↓ Bax, and ↓ caspases expression | P; PI3K/Akt/Bcl-2; autophagy | [179] | |
| mice; CCl4-and BDL-induced liver fibrosis; eG | (i) ↓ AST and ALT; (ii) ↓ histopathological changes in both models; (iii) ↓ collagen-I and α-SMA; (iv) ↓ protein expression of TGF-β and p-Smad3; (v) ↓ protein of NF-κB and LC3 in both models; (vi) ↓ ECM formation | P; TGF-β1/Smad3 and NF-κB; autophagy | [191] | |
| rats; sodium metabisulfite (Na2S2O5)-induced liver damage; rat G | ↓ n-6 PUFA levels and ↓ COX and PGE2 levels in liver tissue | P; n-6 PUFA | [180] | |
| rats; HFD-induced NAFLD; eG | (i) ↓ ALT and AST and ↑ hepatic lipid metabolism; (ii) ↓ formation of OS; (iii) ↓ proinflammatory cytokines and apoptotic cells in the liver | P; LKB1/AMPK and PI3K/Akt | [34] | |
| rats; HFD-induced NAFLD; eG (AG) | (i) ↓ TG with concomitant ↑ GPx; (ii) normalized redox state and inflammatory markers (NF-κB and TNF-α) | P; NF- κB; Akt/AMPK | [41] | |
| mice; HFD-induced NAFLD; eG (AG) | (i) ↓ TG; (ii) ↓ TNF-α and IL-6 | P; AMPK/mTOR; NF-κB; autophagy | [181] | |
| rats; HFD-induced NAFLD; sleeve gastrectomy; no eG treatment | After gastrectomy: (i) ↓ UnAG, ↑ AG/UnAG ratio; (ii) ↓ hepatic TGs and lipogenic enzymes Mogat2 and Dgat1; (iii) ↑ mDNA | P; gastrectomy: ↑ AMPK-activated mFFA β-oxidation and autophagy | [84] | |
| rats; HF-high-cholesterol diet-induced NAFLD; no eG treatment | (i) ↑ serum levels of TC, TGs, AST, ALT, hepatic TGs in NAFLD vs. C (ii) ↓ serum UnAG, total G, and the UnAG/AG ratio; (iii) ↑ hypothalamic AG and GHS-R1a | A; AG might induce IR and promote lipid accumulation via central mechanism | [182] | |
| mice; choline-deficient defined l-amino-acid diet-fed-induced NAFLD, melanocortin 4 receptor KO mice; partial hepatectomy mice with/without the blockades of autonomic nerves; no eG treatment | (i) ↑ gastric ghrelin expression through the autonomic pathways; (ii) ↑ GH in pituitary gland; (iii) ↑ hepatic IGF-1; (iv) high levels of ghrelin expression in the arcuate nucleus were correlated with NAFLD progression regardless of the circuits | P; GH/IGF-1 | [183] | |
| rats; HFD-induced NAFLD; eG (UnAG) | (i) ↓ glucose level; (ii) ↓ serum/hepatic cholesterol, TGs, and FFA; (iii) ↓ levels of ROS, lipid peroxides; (iv) ↓ TNF-α and IL-6; (iv) ↓ Bax and caspase-3; (v) ↑ GSH, SOD, and Bcl-2 | P | [186] | |
| mice; WT G(+/+); G KO mice; no eG treatment | G−/− mice: (i) lack of activation of C/EBPα resulted in ↓ of C/EBPα-p300 complexes and ↓ levels of DGAT1 and ↓ TGs | P; C/EBPα-/p300/DGAT1 | [187] | |
| mice; HIRI-induced acute-on-chronic liver failure; eG | (i) ↓ histopathological changes; (ii) ↓ ALT, ↓ MPO expression; (iii) anti-apoptotic and antioxidant effects; (iv) ↑ ECM degradation | P; blocked fibrotic Smad and ERK | [37] | |
| Animal/ In vitro | mice; HFD-induced obese mice, and db/db mice; GHS-R1a KO mice; hepatocytes from WT mice and HepG2 cells; eG | (i) GHS-R antagonism and KO of the gene: ↓ hepatic steatosis by ↓ de novo lipogenesis: (ii) eG: ↑ lipogenesis with ↑ TG in liver, ↑ hepatic lipid accumulation in mice; (iii) in vitro: ↑ lipogenesis via ↑ GHS-R1a activation with ↑ S6 protein | A; GHS-R1a mediated lipogenesis: mTOR/PPARγ | [40] |
| rats; HFD-induced NAFLD; sleeve gastrectomy and RYGB; primary rat hepatocytes under palmitate-induced lipotoxic conditions; eG | After gastrectomy and RYGB: (i) ↓ UnAG, ↑ AG/UnAG ratio; (ii) both strategies: ↓ obesity-associated hepatic steatosis; (iii) ↓ CD68+ and apoptotic cells; (iv) ↓ JNK activation, CRP, TNF and IL-6 transcripts; (v) ↑ mDNA, OXPHOS complexes I and II, ER stress markers; (vi) ↓ GRP78, XBP-1, ATF4, CHOP, and phosphorylated eIF2α; (vii) in vitro: AG and UnAG inhibited steatosis and inflammation with ↑ OXPHOS complexes II, III, and V and downregulated ER stress transducers | A—in vivo; P—in vitro; surgery+ ↑ AG—↓ obesity-associated liver inflammation; ↓ mitochondrial dysfunction, and ↓ ER stress | [87] | |
| mice; LPS-induced NASH in HFD-fed mice; Kupffer cells and hepatocytes isolated from WT, GHSR-1a(−/−) or PPARγ(+/−) mice; eG | (i) ↓ TNF-α and iNOS; (ii) ↑ Arg1 in Kupffer cells treated with LPS | P; GHS-R1a-mediated ↓ of M1 cell polarization; PPARγ mediates the effects of LPS and G on hepatic steatosis | [36] | |
| In vitro | primary rat hepatocytes; eG (Ag and UnAG) | (i) both G isoforms: ↑ intracellular TG content and ↑ mRNA expression of Mogat2 and Dgat1; (ii) AG: ↑ the expression of autophagy-related markers | P; ↑ AMPK-activated mFFA β-oxidation and autophagy | [84] |
| normal human liver cells LO2; eG | (i) ↓ lipid accumulation; ↑ autophagosomes in cells; (iii) G-induced lipid clearance associated with ↑ in autophagy; (iv) ↓ mTOR phosphorylation | P; AMPK/mTOR; autophagy | [181] | |
| primary HSCs (unstimulated and AII-stimulated cells); eG | (i) ↓ expression of collagen-I and TGF-β in HSCs | P; TGF-β | [42] | |
| HepG2 cells; eG (AG, UnAG) | both isoforms of G: (i) ↓ TNF-α-induced apoptosis and pyroptosis; (ii) AG: ↓ TNF-α-activated hepatocyte autophagy | P; AMPK/mTOR; autophagy | [86] |
| Organ | Local/Systemic Effect | Ghrelin | GHS-R | Obestatin | GOAT |
|---|---|---|---|---|---|
| COLON | ↓/↑ TNF-α and ↓/↑ IL-1β secretion | X | X | X | |
| ↓/↑ IL-6 secretion | X | ||||
| ↓ IFN-γ secretion | X | ||||
| ↑ IL-8 gene expression | X | ||||
| ↓/↑ infiltration with neutrophils | X | ||||
| ↓/↑ infiltration with macrophages | X | X | |||
| ↑/↓ TLRs | X | ||||
| ↑ colitis-induced inflammation | X | ||||
| ↑ TGF-β and ↑ IL-10 | X | X | |||
| ↓/↑ NF-κB | X | ||||
| ↑ iNOS and ↑ PGE2 secretion | X | ||||
| ↑ COX-2 protein/promoter activity | X | X | |||
| ↑ CAT and ↑ MnSOD activity | X | ||||
| ↑ GHS | X | ||||
| ↓ MDA | X | X | |||
| ↓/↑ MPO activity | X | X | X | ||
| ↓ proliferation and ↑ apoptosis of Th cells | X | ||||
| ↑ GH | X | ||||
| ↑ IGF-1 | X | ||||
| ↓ blood leukocytes | X | ||||
| LIVER | ↓ TNF-α, IL-1β, and IL-6 secretion | X | X | ||
| ↓ NF-κB | X | ||||
| ↓ inflammatory infiltration | X | ||||
| ↓ TGF-β and p-Smad3 | X | ||||
| ↓ MDA plasma/liver | X | ||||
| ↓ MPO expression | X | ||||
| ↓ NO level | X | X | |||
| ↑ SOD, CAT, GSH, and GPx | X | ||||
| ↑ eNOS expression | X | ||||
| ↓ COX and PGE2 | X | ||||
| ↓ iNOS | X | ||||
| ↓ apoptosis | X | ||||
| ↓ fibrosis | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, A.; Adamek, A. Role of the Ghrelin System in Colitis and Hepatitis as Risk Factors for Inflammatory-Related Cancers. Int. J. Mol. Sci. 2022, 23, 11188. https://doi.org/10.3390/ijms231911188
Kasprzak A, Adamek A. Role of the Ghrelin System in Colitis and Hepatitis as Risk Factors for Inflammatory-Related Cancers. International Journal of Molecular Sciences. 2022; 23(19):11188. https://doi.org/10.3390/ijms231911188
Chicago/Turabian StyleKasprzak, Aldona, and Agnieszka Adamek. 2022. "Role of the Ghrelin System in Colitis and Hepatitis as Risk Factors for Inflammatory-Related Cancers" International Journal of Molecular Sciences 23, no. 19: 11188. https://doi.org/10.3390/ijms231911188
APA StyleKasprzak, A., & Adamek, A. (2022). Role of the Ghrelin System in Colitis and Hepatitis as Risk Factors for Inflammatory-Related Cancers. International Journal of Molecular Sciences, 23(19), 11188. https://doi.org/10.3390/ijms231911188





