Anticoagulation Monitoring with Activated Partial ThromboPlastin Time and Anti-Xa Activity in Intensive Care Unit Patients: Interest of Thrombin Generation Assay
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. aPTT, Anti-Xa Activity, and Their Correlation with Inflammatory Response
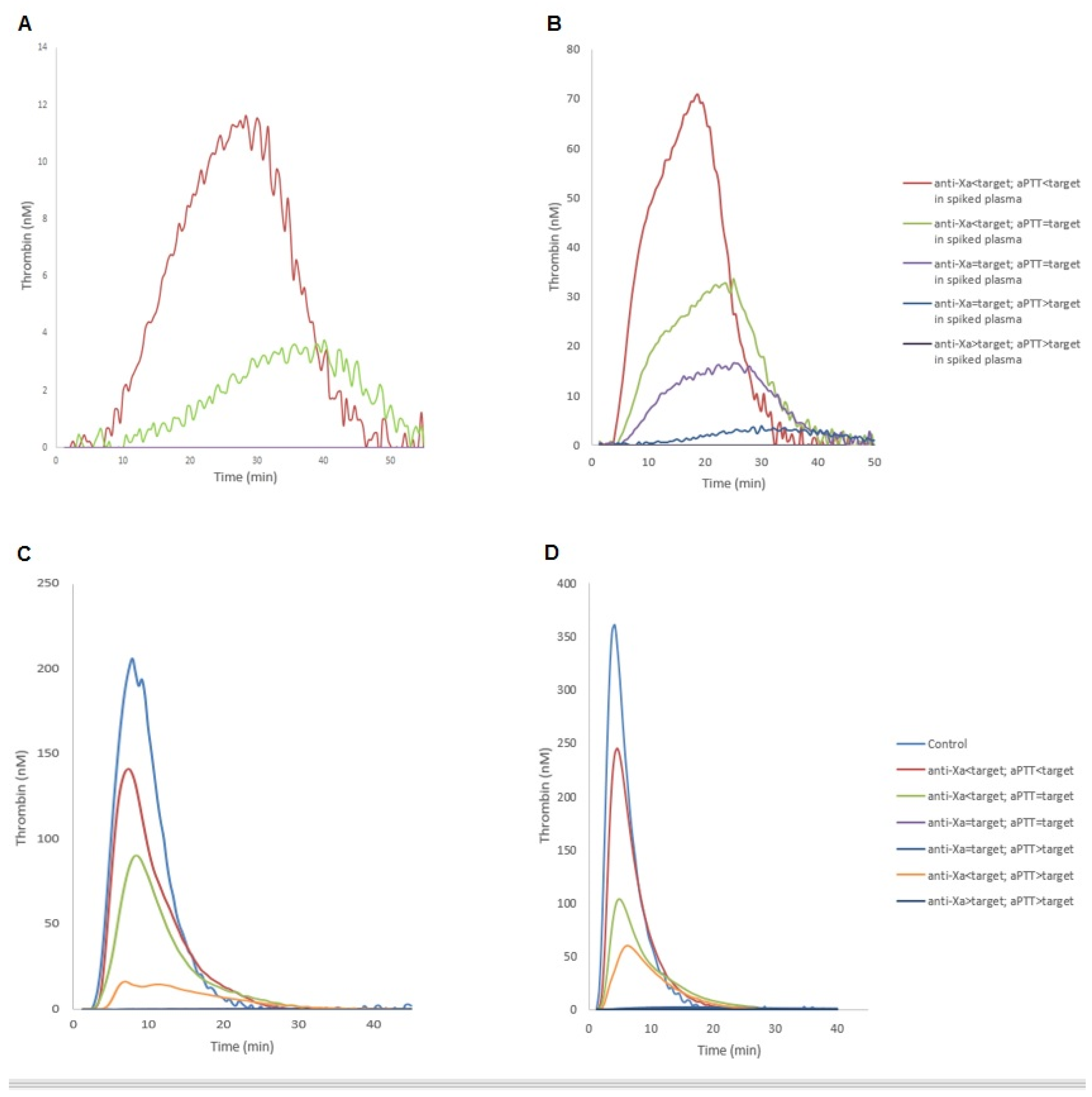
2.3. Thrombin Generation Assay
3. Discussion
4. Materials and Methods
4.1. Population Selection
4.2. Study Procedures
- aPTT < 50.3 s. (aPTT < T): underdosed UFH target;
- 50.3s. ≤ aPTT ≤ 83.8 s. (aPTT = T): under therapeutical target;
- aPTT > 83.8 s. (aPTT > T): overdosed UFH target.
- anti-Xa < 0.3 UI/mL (anti-Xa < T): underdosed UFH target;
- 0.3 UI/mL ≤ anti-Xa ≤ 0.7 UI/mL (anti-Xa = T): under therapeutical target;
- Anti-Xa > 0.7 UI/mL (anti-Xa > T): overdosed UFH target.
4.3. Blood Collection
4.4. Laboratory Assays
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehot, J.; Clec’h, C.; Bonhomme, F.; Brauner, M.; Chemouni, F.; de Mesmay, M.; Gayat, E.; Guidet, B.; Hejblum, G.; Hernu, R.; et al. Pertinence de la prescription des examens biologiques et de la radiographie thoracique en réanimation RFE commune SFAR-SRLF. Méd. Intensive Réanim. 2019, 28, 172–189. [Google Scholar] [CrossRef]
- Takemoto, C.M.; Streiff, M.B.; Shermock, K.M.; Kraus, P.S.; Chen, J.; Jani, J.; Kickler, T. Activated Partial Thromboplastin Time and Anti-Xa Measurements in Heparin Monitoring: Biochemical Basis for Discordance. Am. J. Clin. Pathol. 2013, 139, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Guervil, D.J.; Rosenberg, A.F.; Winterstein, A.G.; Harris, N.S.; Johns, T.E.; Zumberg, M.S. Activated Partial Thromboplastin Time versus Antifactor Xa Heparin Assay in Monitoring Unfractionated Heparin by Continuous Intravenous Infusion. Ann. Pharmacother. 2011, 45, 861–868. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Hirsh, J. Monitoring Unfractionated Heparin with the APTT: Time for a Fresh Look. Thromb. Haemost. 2006, 96, 547–552. [Google Scholar] [PubMed]
- Harr, J.N.; Moore, E.E.; Chin, T.L.; Ghasabyan, A.; Gonzalez, E.; Wohlauer, M.V.; Sauaia, A.; Banerjee, A.; Silliman, C.C. Postinjury Hyperfibrinogenemia Compromises Efficacy of Heparin-Based Venous Thromboembolism Prophylaxis. Shock Augusta Ga 2014, 41, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Billoir, P.; Clavier, T.; Guilbert, A.; Barbay, V.; Chrétien, M.H.; Fresel, M.; Abriou, C.; Girault, C.; Le Cam Duchez, V. Is Citrate Theophylline Adenosine Dipyridamole (CTAD) Better than Citrate to Survey Unfractionated Heparin Treatment? Has Delayed Centrifugation a Real Impact on This Survey? J. Thromb. Thrombolysis 2019, 48, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Connors, J.M. Heparin Resistance—Clinical Perspectives and Management Strategies. N. Engl. J. Med. 2021, 385, 826–832. [Google Scholar] [CrossRef]
- Levine, S.P.; Sorenson, R.R.; Harris, M.A.; Knieriem, L.K. The Effect of Platelet Factor 4 (PF4) on Assays of Plasma Heparin. Br. J. Haematol. 1984, 57, 585–596. [Google Scholar] [CrossRef]
- Billoir, P.; Duflot, T.; Fresel, M.; Chrétien, M.H.; Barbay, V.; Le Cam Duchez, V. Thrombin Generation Profile in Non-Thrombotic Factor V Leiden Carriers. J. Thromb. Thrombolysis 2019, 47, 473–477. [Google Scholar] [CrossRef]
- Billoir, P.; Miranda, S.; Damian, L.; Richard, V.; Benhamou, Y.; Duchez, V.L.C. Development of a Thrombin Generation Test in Cultured Endothelial Cells: Evaluation of the Prothrombotic Effects of Antiphospholipid Antibodies. Thromb. Res. 2018, 169, 87–92. [Google Scholar] [CrossRef]
- Billoir, P.; Alexandre, K.; Duflot, T.; Roger, M.; Miranda, S.; Goria, O.; Joly, L.M.; Demeyere, M.; Feugray, G.; Brunel, V.; et al. Investigation of Coagulation Biomarkers to Assess Clinical Deterioration in SARS-CoV-2 Infection. Front. Med. 2021, 8, 670694. [Google Scholar] [CrossRef] [PubMed]
- Billoir, P.; Le Cam Duchez, V.; Miranda, S.; Richard, V.; Benhamou, Y. Thrombin generation assay in autoimmune disease. Rev. Med. Interne 2021, 42, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Thomas, O.; Lybeck, E.; Strandberg, K.; Tynngård, N.; Schött, U. Monitoring Low Molecular Weight Heparins at Therapeutic Levels: Dose-Responses of, and Correlations and Differences between APTT, Anti-Factor Xa and Thrombin Generation Assays. PLoS ONE 2015, 10, e0116835. [Google Scholar] [CrossRef]
- Hemker, H.C.; Al Dieri, R.; Béguin, S. Heparins: A Shift of Paradigm. Front. Med. 2019, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.P.; Pognani, C.; Meisner, M.; Stuani, A.; Bellomi, D.; Sgarbi, L. Procalcitonin and C-Reactive Protein during Systemic Inflammatory Response Syndrome, Sepsis and Organ Dysfunction. Crit. Care Lond. Engl. 2004, 8, R234. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H. Heparin Resistance and Antithrombin: Should It Still Be Called Heparin Resistance? J. Cardiothorac. Vasc. Anesth. 2004, 18, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Marlar, R.A.; Clement, B.; Gausman, J. Activated Partial Thromboplastin Time Monitoring of Unfractionated Heparin Therapy: Issues and Recommendations. Semin. Thromb. Hemost. 2017, 43, 253–260. [Google Scholar] [CrossRef]
- van Roessel, S.; Middeldorp, S.; Cheung, Y.W.; Zwinderman, A.H.; de Pont, A.C.J.M. Accuracy of APTT Monitoring in Critically Ill Patients Treated with Unfractionated Heparin. Neth. J. Med. 2014, 72, 305–310. [Google Scholar]
- Dargaud, Y.; Sorensen, B.; Shima, M.; Hayward, C.; Srivastava, A.; Negrier, C. Global Haemostasis and Point of Care Testing. Haemoph. Off. J. World Fed. Hemoph. 2012, 18 (Suppl. 4), 81–88. [Google Scholar] [CrossRef]
- Kasonga, F.; Feugray, G.; Chamouni, P.; Barbay, V.; Fresel, M.; Hélène Chretien, M.; Brunel, S.; LE Cam Duchez, V.; Billoir, P. Evaluation of Thrombin Generation Assay in Factor XI Deficiency. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 523, 348–354. [Google Scholar] [CrossRef]
- Feugray, G.; Kasonga, F.; Chamouni, P.; Barbay, V.; Fresel, M.; Hélène Chretien, M.; Brunel, S.; Le Cam Duchez, V.; Billoir, P. Factor XII Deficiency Evaluated by Thrombin Generation Assay. Clin. Biochem. 2021, 100, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.A.; Szlam, F.; Sun, H.Y.; Taketomi, T.; Levy, J.H. Thrombin Generation Assay and Viscoelastic Coagulation Monitors Demonstrate Differences in the Mode of Thrombin Inhibition between Unfractionated Heparin and Bivalirudin. Anesth. Analg. 2007, 105, 933–939. [Google Scholar] [CrossRef]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Ohman, E.M.; Dalen, J.E. Heparin and Low-Molecular-Weight Heparin: Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety. Chest 2001, 119, 64S–94S. [Google Scholar] [CrossRef]
- Levy, J.H.; Tanaka, K.A. Inflammatory Response to Cardiopulmonary Bypass. Ann. Thorac. Surg. 2003, 75, S715–S720. [Google Scholar] [CrossRef]
- Ljungkvist, M.; Strandberg, K.; Berntorp, E.; Chaireti, R.; Holme, P.A.; Larsen, O.H.; Lassila, R.; Jouppila, A.; Szanto, T.; Zetterberg, E. Evaluation of a Standardized Protocol for Thrombin Generation Using the Calibrated Automated Thrombogram: A Nordic Study. Haemoph. Off. J. World Fed. Hemoph. 2019, 25, 334–342. [Google Scholar] [CrossRef]
- Moussa, M.D.; Soquet, J.; Lamer, A.; Labreuche, J.; Gantois, G.; Dupont, A.; Abou-Arab, O.; Rousse, N.; Liu, V.; Brandt, C.; et al. Evaluation of Anti-Activated Factor X Activity and Activated Partial Thromboplastin Time Relations and Their Association with Bleeding and Thrombosis during Veno-Arterial ECMO Support: A Retrospective Study. J. Clin. Med. 2021, 10, 2158. [Google Scholar] [CrossRef] [PubMed]
- Vandiver, J.W.; Vondracek, T.G. Antifactor Xa Levels versus Activated Partial Thromboplastin Time for Monitoring Unfractionated Heparin. Pharmacotherapy 2012, 32, 546–558. [Google Scholar] [CrossRef]
- Uprichard, J.; Manning, R.A.; Laffan, M.A. Monitoring Heparin Anticoagulation in the Acute Phase Response. Br. J. Haematol. 2010, 149, 613–619. [Google Scholar] [CrossRef]
- Szlam, F.; Sreeram, G.; Solomon, C.; Levy, J.H.; Molinaro, R.J.; Tanaka, K.A. Elevated Factor VIII Enhances Thrombin Generation in the Presence of Factor VIII-Deficiency, Factor XI-Deficiency or Fondaparinux. Thromb. Res. 2011, 127, 135–140. [Google Scholar] [CrossRef]
- Gausman, J.N.; Marlar, R.A. Inaccuracy of a “Spiked Curve” for Monitoring Unfractionated Heparin Therapy. Am. J. Clin. Pathol. 2011, 135, 870–876. [Google Scholar] [CrossRef]
- Onasoga-Jarvis, A.A.; Leiderman, K.; Fogelson, A.L.; Wang, M.; Manco-Johnson, M.J.; Di Paola, J.A.; Neeves, K.B. The Effect of Factor VIII Deficiencies and Replacement and Bypass Therapies on Thrombus Formation under Venous Flow Conditions in Microfluidic and Computational Models. PLoS ONE 2013, 8, e78732. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Gallus, A.; Hirsh, J.; Cade, J. A Prospective Study of the Value of Monitoring Heparin Treatment with the Activated Partial Thromboplastin Time. N. Engl. J. Med. 1972, 287, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Rosborough, T.K. Monitoring Unfractionated Heparin Therapy with Antifactor Xa Activity Results in Fewer Monitoring Tests and Dosage Changes than Monitoring with the Activated Partial Thromboplastin Time. Pharmacotherapy 1999, 19, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, H.J.M. Global Assays and the Management of Oral Anticoagulation. Thromb. J. 2015, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Number of Patients | 30 |
|---|---|
| Age (years) | 62.7 ± 15.8 |
| Male | 24 (80%) |
| Body mass index (kg/m2) | 27.4 ± 6.7 |
| SAPS II score | 34.3 ± 14.5 |
| Reason for ICU admission | |
| Post-operative ICU | 13 (43.4%) |
| Unprogrammed ICU admission | 17 (56.6%) |
| Indication of anticoagulation | |
| Vascular surgery requiring anticoagulation | 17 (56.6%) |
| Transitory atrial fibrillation | 5 (16.6%) |
| DVT/PE development | 4 (13.3%) |
| Arterial thrombosis | 1 (3.3%) |
| Others | 3 (10%) |
| Duration of stay (days) | |
| In ICU | 12.8 ± 19.6 |
| In hospital | 28.1 ± 30.0 |
| Survival 28 days after ICU admission | 29 (96%) |
| aPTT (s.) (N: 28.5–38.5) | 57.0 [48.6–72.0] |
| Anti-Xa (UI/mL) (N: 0.35–0.7UI/mL) | 0.13 [0.1–1.6] |
| Fibrinogen (g/L) (N: 2–4 g/l) | 6.0 [5.0–7.3] |
| FVIII (%) (N: 50–150%) | 235 [183–368] |
| CRP (mg/L) (N < 5 mg/L) | 127.5 [57–188.3] |
| aPTT < 50.3 s. | 50.3 s.< aPTT < 83.8 s. | aPTT > 83.8 s. | |
|---|---|---|---|
| Anti-Xa activity <0.3 UI/mL | n = 32 2 bleeding complications 1 thromboembolic complication | n = 29 2 thromboembolic complications | n = 14 1 bleeding complication |
| 0.3 UI/Ml <Anti-Xa activity <0.7 UI/mL | n = 2 No complication | n = 8 No complication | n = 6 No complication |
| Anti-Xa activity >0.7 UI/mL | n = 0 | n = 1 No complication | n = 9 1 thromboembolic complication |
| Group | UFH Target | TGA Condition | Lagtime (min) | TTP (min) | ETP (nM.min) | Peak (nM) | Velocity (nM/min) |
|---|---|---|---|---|---|---|---|
| Control | NA | 5 pM | 3.13 [2.92–3.33] | 6.67 [5.94–7.82] | 1344 [1190–1575] | 199 [157–253] | 52.6 [37.3–86.0] |
| NA | 20 pM | 1.67 [1.67–2.08] | 4.17 [4.07–4.7] | 1662 [1446–1782] | 332 [283–398] | 138.7 [108.9–174.7] | |
| ICU | NA | 5 pM | 5.84 [4.58–9.07] a | 9.58 [7.29–15.0] a | 1199 [553–1520] | 200 [41.5–290] | 64.25 [8.18–124.2] |
| (N = 99) | NA | 20 pM | 2.92 [2.14–3.75] a | 5.83 [4.58–8.75] a | 1264 [465–1557] a | 231 [52–304] a | 84.2 [11.5–140] a |
| ICU | aPTT < T | 5 pM | 5.42 [4.17–8.96] a | 8.12 [6.67–13.12] a | 1360 [826–1489] | 251 [78–297] | 98.4 [124.6] |
| (N = 27) | Anti-Xa < T | 20 pM | 2.09 [2.08–2.92] a | 4.79 [4.17–5.62] | 1442 [1227–1553] | 291 [235–338] | 128 [89.2–161] |
| ICU | aPTT = T | 5 pM | 5.94 [5.0–8.7] a | 9.69 [8.07–16.3] a | 1170 [451–1649] | 144 [25–298] | 45.2 [6.0–119.2] |
| (N = 47) | Anti-Xa < T | 20 pM | 2.72 [2.5–3.75] a | 6.25 [4.59–8.75] a,b | 1154 [450–1617] a | 210 [51–304] a | 53.4 [7.5–122.4] a,b |
| ICU | aPTT = T | 5 pM | IC | IC | IC | IC | IC |
| (N = 9) | Anti-Xa = T | 20 pM | IC | IC | IC | IC | IC |
| ICU | aPTT > T | 5 pM | 9.58 [8.12–12.08] a | 19.37 [11.87–44.37] a | 446 [316–577] | 25 [1–72] | 11.3 [3.4–19.2] |
| (N=4) | Anti-Xa < T | 20 pM | 3.44 [2.97–3.91] a | 8.65 [6.77–14.74] a,b | 595 [171–1182] a | 71 [9–139] a,b | 14.1 [1.26–49.7] a,b |
| ICU | aPTT > T | 5 pM | IC | IC | IC | IC | IC |
| (N = 12) | Anti-Xa = T | 20 pM | IC | IC | IC | IC | IC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billoir, P.; Elie, T.; Levy, J.H.; Besnier, E.; Dureuil, B.; Veber, B.; Le Cam-Duchez, V.; Clavier, T. Anticoagulation Monitoring with Activated Partial ThromboPlastin Time and Anti-Xa Activity in Intensive Care Unit Patients: Interest of Thrombin Generation Assay. Int. J. Mol. Sci. 2022, 23, 11219. https://doi.org/10.3390/ijms231911219
Billoir P, Elie T, Levy JH, Besnier E, Dureuil B, Veber B, Le Cam-Duchez V, Clavier T. Anticoagulation Monitoring with Activated Partial ThromboPlastin Time and Anti-Xa Activity in Intensive Care Unit Patients: Interest of Thrombin Generation Assay. International Journal of Molecular Sciences. 2022; 23(19):11219. https://doi.org/10.3390/ijms231911219
Chicago/Turabian StyleBilloir, Paul, Thomas Elie, Jerrold H. Levy, Emmanuel Besnier, Bertrand Dureuil, Benoit Veber, Véronique Le Cam-Duchez, and Thomas Clavier. 2022. "Anticoagulation Monitoring with Activated Partial ThromboPlastin Time and Anti-Xa Activity in Intensive Care Unit Patients: Interest of Thrombin Generation Assay" International Journal of Molecular Sciences 23, no. 19: 11219. https://doi.org/10.3390/ijms231911219
APA StyleBilloir, P., Elie, T., Levy, J. H., Besnier, E., Dureuil, B., Veber, B., Le Cam-Duchez, V., & Clavier, T. (2022). Anticoagulation Monitoring with Activated Partial ThromboPlastin Time and Anti-Xa Activity in Intensive Care Unit Patients: Interest of Thrombin Generation Assay. International Journal of Molecular Sciences, 23(19), 11219. https://doi.org/10.3390/ijms231911219







