Immunothrombosis and the Role of Platelets in Venous Thromboembolic Diseases
Abstract
1. Introduction
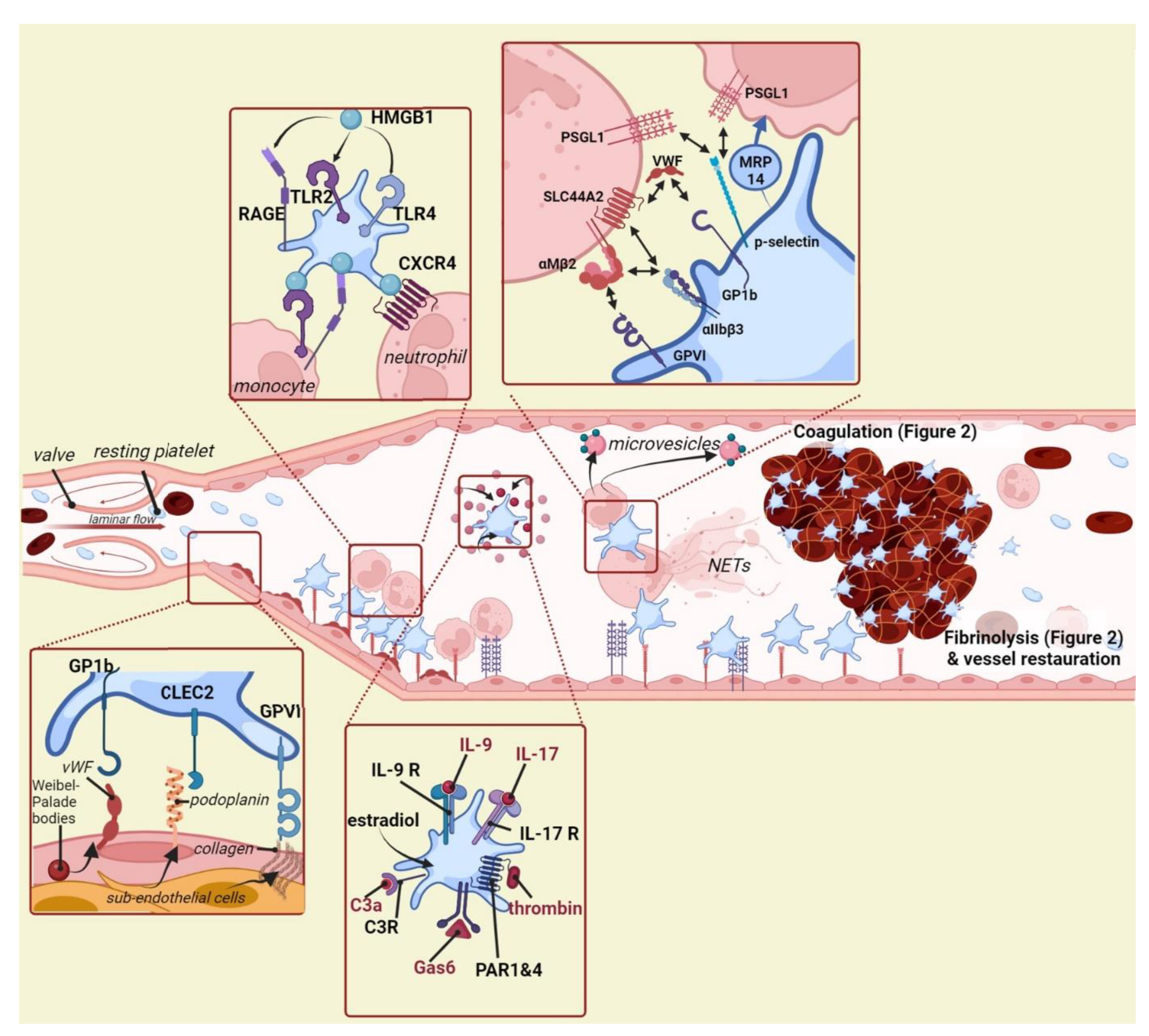
2. Platelets Interact with the Venous Vessel Wall
3. Platelets Interact with and/or Recruit Other Immune Cells
4. Platelet Activators Promoting Venous Thrombosis
5. Platelets and the Coagulation Cascade
6. Platelets during Thrombus Resolution and Vessel Restoration
7. Platelet Inhibition to Prevent Venous Thrombosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, J.N. Platelets. Lancet 2000, 355, 1531–1539. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Platelets in inflammation and infection. Platelets 2015, 26, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2016, 38, 785–791. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New Fundamentals in Hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Di Nisio, M.; van Es, N.; Büller, H.R. Deep vein thrombosis and pulmonary embolism. Lancet 2016, 388, 3060–3073. [Google Scholar] [CrossRef]
- Kahn, S.R.; de Wit, K. Pulmonary Embolism. N. Engl. J. Med. 2022, 387, 45–57. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Association between venous and arterial thrombosis: Clinical implications. Eur. J. Intern. Med. 2012, 23, 333–337. [Google Scholar] [CrossRef]
- Ageno, W. Arterial and Venous Thrombosis: Clinical Evidence for Mechanistic Overlap. Blood 2014, 124, SCI-3. [Google Scholar] [CrossRef]
- Delluc, A.; Lacut, K.; Rodger, M.A. Arterial and venous thrombosis: What’s the link? A narrative review. Thromb. Res. 2020, 191, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P. Venous and arterial thrombosis: Two aspects of the same disease? Eur. J. Intern. Med. 2009, 20, 660–661. [Google Scholar] [CrossRef]
- Carminita, E.; Crescence, L.; Brouilly, N.; Altié, A.; Panicot-Dubois, L.; Dubois, C. DNAse-dependent, NET-independent pathway of thrombus formation in vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2100561118. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Deppermann, C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets 2021, 32, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Hamzeh-Cognasse, H.; Mismetti, P.; Tomas, T.; Eglin, D.; Marotte, H. The Non-Haemostatic Response of Platelets to Stress: An Actor of the Inflammatory Environment on Regenerative Medicine? Front. Immunol. 2021, 12, 741988. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Xiang, S.C.; Rondina, M.T. Platelet secretion in inflammatory and infectious diseases. Platelets 2017, 28, 155–164. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Cognasse, F.; Garraud, O.; Pozzetto, B.; Laradi, S.; Hamzeh-Cognasse, H. How can non-nucleated platelets be so smart? J. Thromb. Haemost. 2016, 14, 794–796. [Google Scholar] [CrossRef]
- Cognasse, F.; Laradi, S.; Berthelot, P.; Bourlet, T.; Marotte, H.; Mismetti, P.; Garraud, O.; Hamzeh-Cognasse, H. Platelet Inflammatory Response to Stress. Front. Immunol. 2019, 10, 1478. [Google Scholar] [CrossRef]
- Shi, G.; Morrell, C.N. Platelets as initiators and mediators of inflammation at the vessel wall. Thromb. Res. 2011, 127, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Jezovnik, M.K. Endothelial Dysfunction and Venous Thrombosis. Angiology 2018, 69, 564–567. [Google Scholar] [CrossRef]
- Nightingale, T.; Cutler, D. The secretion of von W illebrand factor from endothelial cells; an increasingly complicated story. J. Thromb. Haemost. 2013, 11, 192–201. [Google Scholar] [CrossRef]
- Sadler, J.E. BIOCHEMISTRY AND GENETICS OF VON WILLEBRAND FACTOR. Annu. Rev. Biochem. 1998, 67, 395–424. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P. Past, present and future of hemophilia: A narrative review. Orphanet J. Rare Dis. 2012, 7, 24. [Google Scholar] [CrossRef]
- Denorme, F.; Vanhoorelbeke, K.; De Meyer, S.F. von Willebrand Factor and Platelet Glycoprotein Ib: A Thromboinflammatory Axis in Stroke. Front. Immunol. 2019, 10, 2884. [Google Scholar] [CrossRef]
- Brill, A.; Fuchs, T.A.; Chauhan, A.; Yang, J.J.; De Meyer, S.; Koellnberger, M.; Wakefield, T.W.; Lämmle, B.; Massberg, S.; Wagner, D.D. von Willebrand factor–mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 2011, 117, 1400–1407. [Google Scholar] [CrossRef]
- Lankhof, H.L.; van Hoeij, M.; E Schiphorst, M.; Bracke, M.; Wu, Y.-P.; Ijsseldijk, M.J.W.; Vink, T.; de Groot, P.G.; Sixma, J.J. A3 domain is essential for interaction of von Willebrand factor with collagen type III. Thromb. Haemost. 1996, 75, 950–958. [Google Scholar] [CrossRef]
- Flood, V.H.; Lederman, C.A.; Wren, J.S.; Christopherson, P.A.; Friedman, K.D.; Hoffmann, R.G.; Montgomery, R.R. Absent collagen binding in a VWF A3 domain mutant: Utility of the VWF:CB in diagnosis of VWD: Letters to the Editor. J. Thromb. Haemost. 2010, 8, 1431–1433. [Google Scholar] [CrossRef]
- Meng, D.; Luo, M.; Liu, B. The Role of CLEC-2 and Its Ligands in Thromboinflammation. Front. Immunol. 2021, 12, 688643. [Google Scholar] [CrossRef] [PubMed]
- Payne, H.; Ponomaryov, T.; Watson, S.P.; Brill, A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood 2017, 129, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Chandrakanthan, M.; Nguyen, T.Q.; Hasan, Z.; Muralidharan, S.; Vu, T.M.; Li, A.W.L.; Le, U.T.N.; Ha, H.T.T.; Baik, S.-H.; Tan, S.H.; et al. Deletion of Mfsd2b impairs thrombotic functions of platelets. Nat. Commun. 2021, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef]
- Krishnan, H.; Rayes, J.; Miyashita, T.; Ishii, G.; Retzbach, E.P.; Sheehan, S.A.; Takemoto, A.; Chang, Y.; Yoneda, K.; Asai, J.; et al. Podoplanin: An emerging cancer biomarker and therapeutic target. Cancer Sci. 2018, 109, 1292–1299. [Google Scholar] [CrossRef]
- Tsukiji, N.; Osada, M.; Sasaki, T.; Shirai, T.; Satoh, K.; Inoue, O.; Umetani, N.; Mochizuki, C.; Saito, T.; Kojima, S.; et al. Cobalt hematoporphyrin inhibits CLEC-2–podoplanin interaction, tumor metastasis, and arterial/venous thrombosis in mice. Blood Adv. 2018, 2, 2214–2225. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Xu, M.; Jiang, Y.; Zhou, J.; Yang, J.; Gu, H.; Ruan, C.; Wu, J.; Zhao, Y. Blocking podoplanin inhibits platelet activation and decreases cancer-associated venous thrombosis. Thromb. Res. 2021, 200, 72–80. [Google Scholar] [CrossRef]
- Sasano, T.; Gonzalez-Delgado, R.; Muñoz, N.M.; Carlos-Alcade, W.; Cho, M.S.; Sheth, R.A.; Sood, A.K.; Afshar-Kharghan, V. Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer. J. Thromb. Haemost. 2022, 20, 104–114. [Google Scholar] [CrossRef]
- Nieswandt, B. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001, 20, 2120–2130. [Google Scholar] [CrossRef]
- Lockyer, S.; Okuyama, K.; Begum, S.; Le, S.; Sun, B.; Watanabe, T.; Matsumoto, Y.; Yoshitake, M.; Kambayashi, J.; Tandon, N.N. GPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolism. Thromb. Res. 2006, 118, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mammadova-Bach, E.; Ollivier, V.; Loyau, S.; Schaff, M.; Dumont, B.; Favier, R.; Freyburger, G.; Latger-Cannard, V.; Nieswandt, B.; Gachet, C.; et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood 2015, 126, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Kaneva, V.; Loyau, S.; Nechipurenko, D.; Receveur, N.; Le Bris, M.; Janus-Bell, E.; Didelot, M.; Rauch, A.; Susen, S.; et al. Pharmacological Blockade of Glycoprotein VI Promotes Thrombus Disaggregation in the Absence of Thrombin. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2127–2142. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Receveur, N.; Janus-Bell, E.; Mouriaux, C.; Gachet, C.; Jandrot-Perrus, M.; Hechler, B.; Gardiner, E.E.; Mangin, P.H. Respective roles of Glycoprotein VI and FcγRIIA in the regulation of αIIbβ3-mediated platelet activation to fibrinogen, thrombus buildup, and stability. Res. Pract. Thromb. Haemost. 2021, 5, e12551. [Google Scholar] [CrossRef]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating with the Enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, X.; Gu, L.; Zhu, H.; Zhong, Y.; Ye, Y.; Xiong, X.; Jian, Z. New Insight Into Neutrophils: A Potential Therapeutic Target for Cerebral Ischemia. Front. Immunol. 2021, 12, 692061. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- von Brühl, M.-L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762. [Google Scholar] [CrossRef]
- Kapoor, S.; Opneja, A.; Nayak, L. The role of neutrophils in thrombosis. Thromb. Res. 2018, 170, 87–96. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Dyer, M.R.; Chen, Q.; Haldeman, S.; Yazdani, H.; Hoffman, R.; Loughran, P.; Tsung, A.; Zuckerbraun, B.S.; Simmons, R.L.; Neal, M.D. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci. Rep. 2018, 8, 2068. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sonkar, V.K.; Swamy, J.; Ahmed, A.; Sharathkumar, A.A.; Pierce, G.L.; Dayal, S. DNase 1 Protects from Increased Thrombin Generation and Venous Thrombosis during Aging: Cross-Sectional Study in Mice and Humans. J. Am. Heart Assoc. 2022, 11, e021188. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Shi, C.; Erhardt, P.W.; Pavlovsky, A.; Soloviev, D.A.; Bledzka, K.; Ustinov, V.; Zhu, L.; Qin, J.; et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nat. Commun. 2017, 8, 15559. [Google Scholar] [CrossRef]
- Germain, M.; Chasman, D.I.; de Haan, H.; Tang, W.; Lindström, S.; Weng, L.-C.; de Andrade, M.; de Visser, M.C.; Wiggins, K.L.; Suchon, P.; et al. Meta-analysis of 65,734 Individuals Identifies TSPAN15 and SLC44A2 as Two Susceptibility Loci for Venous Thromboembolism. Am. J. Hum. Genet. 2015, 96, 532–542. [Google Scholar] [CrossRef]
- Hinds, D.A.; Buil, A.; Ziemek, D.; Martinez-Perez, A.; Malik, R.; Folkersen, L.; Germain, M.; Mälarstig, A.; Brown, A.; Soria, J.M.; et al. Genome-wide association analysis of self-reported events in 6135 individuals and 252 827 controls identifies 8 loci associated with thrombosis. Hum. Mol. Genet. 2016, 25, 1867–1874. [Google Scholar] [CrossRef]
- Bennett, J.A.; Mastrangelo, M.A.; Ture, S.K.; Smith, C.O.; Loelius, S.G.; Berg, R.A.; Shi, X.; Burke, R.M.; Spinelli, S.L.; Cameron, S.J.; et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat. Commun. 2020, 11, 3479. [Google Scholar] [CrossRef]
- Tilburg, J.; Coenen, D.M.; Zirka, G.; Dólleman, S.; Van Oeveren-Rietdijk, A.M.; Karel, M.F.A.; De Boer, H.C.; Cosemans, J.M.E.M.; Versteeg, H.H.; Morange, P.E.; et al. SLC44A2 deficient mice have a reduced response in stenosis but not in hypercoagulability driven venous thrombosis. J. Thromb. Haemost. 2020, 18, 1714–1727. [Google Scholar] [CrossRef]
- Zirka, G.; Robert, P.; Tilburg, J.; Tishkova, V.; Maracle, C.X.; Legendre, P.; van Vlijmen, B.; Alessi, M.C.; Lenting, P.J.; Morange, P.E.; et al. Impaired adhesion of neutrophils expressing Slc44a2/HNA-3b to VWF protects against NETosis under venous shear rates. Blood 2021, 137, 2256–2266. [Google Scholar] [CrossRef]
- Constantinescu-Bercu, A.; Grassi, L.; Frontini, M.; Salles-Crawley, I.I.; Woollard, K.; Crawley, J.T.B. Activated αIIbβ3 on platelets mediates flow-dependent NETosis via SLC44A2. eLife 2020, 9, e53353. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Evangelista, V.; de Gaetano, G. P-selectin-beta 2-integrin cross-talk: A molecular mechanism for polymorphonuclear leukocyte recruitment at the site of vascular damage. Thromb. Haemost. 1999, 82, 787–793. [Google Scholar]
- Kasthuri, R.S.; Glover, S.L.; Jonas, W.; McEachron, T.; Pawlinski, R.; Arepally, G.M.; Key, N.S.; Mackman, N. PF4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcγRI. Blood 2012, 119, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Apta, B.H.R.; Bonna, A.M.; Harper, M.T. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci. Rep. 2019, 9, 13397. [Google Scholar] [CrossRef]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Wong, D.J.; Park, D.D.; Park, S.S.; Haller, C.A.; Chen, J.; Dai, E.; Liu, L.; Mandhapati, A.R.; Eradi, P.; Dhakal, B.; et al. A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis. Blood 2021, 138, 1182–1193. [Google Scholar] [CrossRef]
- Momi, S.; Canino, J.; Vismara, M.; Galgano, L.; Falcinelli, E.; Guglielmini, G.; Taranta, G.C.; Guidetti, G.F.; Gresele, P.; Torti, M.; et al. Proline-rich tyrosine kinase Pyk2 regulates deep vein thrombosis. Haematologica 2022, 107, 1374–1383. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Jin, R.; Yu, S.; Song, Z.; Zhu, X.; Wang, C.; Yan, J.; Wu, F.; Nanda, A.; Granger, D.N.; Li, G. Soluble CD40 Ligand Stimulates CD40-Dependent Activation of the β2 Integrin Mac-1 and Protein Kinase C Zeda (PKCζ) in Neutrophils: Implications for Neutrophil-Platelet Interactions and Neutrophil Oxidative Burst. PLoS ONE 2013, 8, e64631. [Google Scholar] [CrossRef]
- Canobbio, I.; Visconte, C.; Momi, S.; Guidetti, G.F.; Zarà, M.; Canino, J.; Falcinelli, E.; Gresele, P.; Torti, M. Platelet amyloid precursor protein is a modulator of venous thromboembolism in mice. Blood 2017, 130, 527–536. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Williams, C.M.; Li, Y.; Brown, E.; Poole, A.W. Platelet-specific deletion of SNAP23 ablates granule secretion, substantially inhibiting arterial and venous thrombosis in mice. Blood Adv. 2018, 2, 3627–3636. [Google Scholar] [CrossRef] [PubMed]
- Furie, B.; Furie, B.C.; Flaumenhaft, R. A journey with platelet P-selectin: The molecular basis of granule secretion, signalling and cell adhesion. Thromb. Haemost. 2001, 86, 214–221. [Google Scholar] [CrossRef]
- Ay, C.; Jungbauer, L.V.; Sailer, T.; Tengler, T.; Koder, S.; Kaider, A.; Panzer, S.; Quehenberger, P.; Pabinger, I.; Mannhalter, C. High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clin. Chem. 2007, 53, 1235–1243. [Google Scholar] [CrossRef]
- Ramacciotti, E.; Blackburn, S.; Hawley, A.E.; Vandy, F.; Ballard-Lipka, N.; Stabler, C.; Baker, N.; Guire, K.E.; Rectenwald, J.E.; Henke, P.K.; et al. Evaluation of soluble P-selectin as a marker for the diagnosis of deep venous thrombosis. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2011, 17, 425–431. [Google Scholar] [CrossRef]
- Antonopoulos, C.N.; Sfyroeras, G.S.; Kakisis, J.D.; Moulakakis, K.G.; Liapis, C.D. The role of soluble P selectin in the diagnosis of venous thromboembolism. Thromb. Res. 2014, 133, 17–24. [Google Scholar] [CrossRef]
- Furio, E.; García-Fuster, M.J.; Redon, J.; Marques, P.; Ortega, R.; Sanz, M.J.; Piqueras, L. CX3CR1/CX3CL1 Axis Mediates Platelet–Leukocyte Adhesion to Arterial Endothelium in Younger Patients with a History of Idiopathic Deep Vein Thrombosis. Thromb. Haemost. 2018, 118, 562–571. [Google Scholar] [CrossRef]
- Poruk, K.E.; Firpo, M.A.; Huerter, L.M.; Scaife, C.L.; Emerson, L.L.; Boucher, K.M.; Jones, K.A.; Mulvihill, S.J. Serum Platelet Factor 4 Is an Independent Predictor of Survival and Venous Thromboembolism in Patients with Pancreatic Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2605–2610. [Google Scholar] [CrossRef]
- Riedl, J.; Hell, L.; Kaider, A.; Koder, S.; Marosi, C.; Zielinski, C.; Pamzer, S.; Pabinger, I.; Ay, C. Association of platelet activation markers with cancer-associated venous thromboembolism. Platelets 2016, 27, 80–85. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Kessinger, C.W.; Schmaier, A.; Jaffer, F.A.; Simon, D.I. Myeloid-related protein-14 regulates deep vein thrombosis. JCI Insight 2017, 2, e91356. [Google Scholar] [CrossRef]
- Yun, S.-H.; Sim, E.-H.; Goh, R.-Y.; Park, J.-I.; Han, J.-Y. Platelet Activation: The Mechanisms and Potential Biomarkers. BioMed Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Beutler, B.A. TLRs and innate immunity. Blood 2009, 113, 1399–1407. [Google Scholar] [CrossRef]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like Receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Ebermeyer, T.; Cognasse, F.; Berthelot, P.; Mismetti, P.; Garraud, O.; Hamzeh-Cognasse, H. Platelet Innate Immune Receptors and TLRs: A Double-Edged Sword. Int. J. Mol. Sci. 2021, 22, 7894. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Philippi, V.; Stockhausen, S.; Busse, J.; Antonelli, A.; Miller, M.; Schubert, I.; Hoseinpour, P.; Chandraratne, S.; Von Brühl, M.-L.; et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016, 128, 2435–2449. [Google Scholar] [CrossRef]
- Moser, M.; Nieswandt, B.; Ussar, S.; Pozgajova, M.; Fässler, R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 2008, 14, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, H.; Hu, X.; Zhang, Z.; Ge, S.; Xu, Z.; Gao, J.; Liu, J.; White, G.C.; Ma, Y.-Q. Kindlin-3 in platelets and myeloid cells differentially regulates deep vein thrombosis in mice. Aging 2019, 11, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Xu, Z.; Shi, X.; Liu, S.; Schulte, M.L.; White, G.C.; Ma, Y.-Q. Paxillin binding to the PH domain of kindlin-3 in platelets is required to support integrin αIIbβ3 outside-in signaling. J. Thromb. Haemost. 2021, 19, 3126–3138. [Google Scholar] [CrossRef]
- Larsen, J.B.; Hvas, A.-M. Thrombin: A Pivotal Player in Hemostasis and Beyond. Semin. Thromb. Hemost. 2021, 47, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Greenberg, D.L.; Fujikawa, K.; Xu, W.; Chung, D.W.; Davie, E.W. Protease-activated receptor 1 is the primary mediator of thrombin-stimulated platelet procoagulant activity. Proc. Natl. Acad. Sci. USA 1999, 96, 11189–11193. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Kawano, T.; Grover, S.P.; Bharathi, V.; Martinez, D.; Cowley, D.O.; Mackman, N.; Bergmeier, W.; Antoniak, S. Genetic deletion of platelet PAR4 results in reduced thrombosis and impaired hemostatic plug stability. J. Thromb. Haemost. 2022, 20, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, F.C.; Han, H.; Li, J.; Rumbaut, R.E.; Afshar-Kharghan, V. Abnormal platelet function in C3-deficient mice. J. Thromb. Haemost. 2009, 7, 865–870. [Google Scholar] [CrossRef]
- Subramaniam, S.; Jurk, K.; Hobohm, L.; Jäckel, S.; Saffarzadeh, M.; Schwierczek, K.; Wenzel, P.; Langer, F.; Reinhardt, C.; Ruf, W. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood 2017, 129, 2291–2302. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, M.; Zhu, F.; Zhang, S.; Ding, P.; Wang, M. IL-9 Promotes the Development of Deep Venous Thrombosis by Facilitating Platelet Function. Thromb. Haemost. 2018, 118, 1885–1894. [Google Scholar] [CrossRef]
- Ding, P.; Zhang, S.; Yu, M.; Feng, Y.; Long, Q.; Yang, H.; Li, J.; Wang, M. IL-17A promotes the formation of deep vein thrombosis in a mouse model. Int. Immunopharmacol. 2018, 57, 132–138. [Google Scholar] [CrossRef]
- Valéra, M.-C.; Gratacap, M.-P.; Gourdy, P.; Lenfant, F.; Cabou, C.; Toutain, C.E.; Marcellin, M.; Laurent, N.S.; Sié, P.; Sixou, M.; et al. Chronic estradiol treatment reduces platelet responses and protects mice from thromboembolism through the hematopoietic estrogen receptor α. Blood 2012, 120, 1703–1712. [Google Scholar] [CrossRef]
- Valera, M.-C.; Noirrit-Esclassan, E.; Dupuis, M.; Buscato, M.; Vinel, A.; Guillaume, M.; Briaux, A.; Garcia, C.; Benoit, T.; Lairez, O.; et al. Effect of chronic estradiol plus progesterone treatment on experimental arterial and venous thrombosis in mouse. PLoS ONE 2017, 12, e0177043. [Google Scholar] [CrossRef] [PubMed]
- Law, L.A.; Graham, D.K.; Di Paola, J.; Branchford, B.R. GAS6/TAM Pathway Signaling in Hemostasis and Thrombosis. Front. Med. 2018, 5, 137. [Google Scholar] [CrossRef]
- Angelillo-Scherrer, A.; De Frutos, P.G.; Aparicio, C.; Melis, E.; Savi, P.; Lupu, F.; Arnout, J.; Dewerchin, M.; Hoylaerts, M.F.; Herbert, J.-M.; et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat. Med. 2001, 7, 215–221. [Google Scholar] [CrossRef]
- Angelillo-Scherrer, A.; Burnier, L.; Flores, N.; Savi, P.; DeMol, M.; Schaeffer, P.; Herbert, J.-M.; Lemke, G.; Goff, S.P.; Matsushima, G.K.; et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J. Clin. Investig. 2005, 115, 237–246. [Google Scholar] [CrossRef]
- Gutmann, C.; Siow, R.; Gwozdz, A.M.; Saha, P.; Smith, A. Reactive Oxygen Species in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 1918. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.W.M.; Mattheij, N.J.A.; Cosemans, J.M.E.M. Platelet-based coagulation: Different populations, different functions: Platelet-based coagulation. J. Thromb. Haemost. 2013, 11, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; van Rijn, J.L.; Hemker, H.C.; Zwaal, R.F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur. J. Biochem. 1982, 122, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Lentz, B.R. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003, 42, 423–438. [Google Scholar] [CrossRef]
- Reddy, E.C.; Rand, M.L. Procoagulant Phosphatidylserine-Exposing Platelets in vitro and in vivo. Front. Cardiovasc. Med. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Heestermans, M.; Salloum-Asfar, S.; Streef, T.; Laghmani, E.H.; Salvatori, D.; Luken, B.M.; Zeerleder, S.S.; Spronk, H.M.H.; Korporaal, S.J.; Kirchhofer, D.; et al. Mouse venous thrombosis upon silencing of anticoagulants depends on tissue factor and platelets, not FXII or neutrophils. Blood 2019, 133, 2090–2099. [Google Scholar] [CrossRef]
- Sunnerhagen, M.; Drakenberg, T.; Forsén, S.; Stenflo, J. Effect of Ca2+ on the Structure of Vitamin K-Dependent Coagulation Factors. Pathophysiol. Haemost. Thromb. 1996, 26, 45–53. [Google Scholar] [CrossRef]
- Hur, W.S.; Paul, D.S.; Bouck, E.G.; Negrón, O.A.; Mwiza, J.M.N.; Poole, L.G.; Cline-Fedewa, H.M.; Clark, E.G.; Juang, L.J.; Leung, J.; et al. Hypofibrinogenemia with preserved hemostasis and protection from thrombosis in mice with an Fga truncation mutation. Blood 2022, 139, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Chesney, C.M.; Pifer, D.; Colman, R.W. Subcellular localization and secretion of factor V from human platelets. Proc. Natl. Acad. Sci. USA 1981, 78, 5180–5184. [Google Scholar] [CrossRef] [PubMed]
- Monković, D.D.; Tracy, P.B. Functional characterization of human platelet-released factor V and its activation by factor Xa and thrombin. J. Biol. Chem. 1990, 265, 17132–17140. [Google Scholar] [CrossRef]
- Bouma, B.N.; de Graaf, S.; Slot, J.W.; Zimmerman, T.S. Human blood platelet factor VIII-related antigen: Demonstration of release by α-chymotrypsin. Thromb. Res. 1979, 14, 687–696. [Google Scholar] [CrossRef]
- Yarovoi, H.V.; Kufrin, D.; Eslin, D.E.; Thornton, M.A.; Haberichter, S.L.; Shi, Q.; Zhu, H.; Camire, R.; Fakharzadeh, S.S.; Kowalska, M.A.; et al. Factor VIII ectopically expressed in platelets: Efficacy in hemophilia A treatment. Blood 2003, 102, 4006–4013. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, Q.; Fahs, S.A.; Kuether, E.L.; Walsh, C.E.; Montgomery, R.R. Factor IX ectopically expressed in platelets can be stored in α-granules and corrects the phenotype of hemophilia B mice. Blood 2010, 116, 1235–1243. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Lionikiene, A.S.; Fraser, S.R.; Whyte, C.S.; Booth, N.A.; Mutch, N.J. Functional factor XIII-A is exposed on the stimulated platelet surface. Blood 2014, 124, 3982–3990. [Google Scholar] [CrossRef]
- Somodi, L.; Debreceni, I.B.; Kis, G.; Cozzolino, M.; Kappelmayer, J.; Antal, M.; Panyi, G.; Bárdos, H.; Mutch, N.J.; Muszbek, L. Activation mechanism dependent surface exposure of cellular factor XIII on activated platelets and platelet microparticles. J. Thromb. Haemost. 2022, 20, 1223–1235. [Google Scholar] [CrossRef]
- Gralnick, H.R.; Williams, S.B.; MaKeown, L.P.; Magruder, L.; Hansmann, K.; Vail, M.; Parker, R.I. Platelet von Willebrand Factor. Mayo Clin. Proc. 1991, 66, 634–640. [Google Scholar] [CrossRef]
- Stavenuiter, F.; Davis, N.; Duan, E.; Gale, A.; Heeb, M. Platelet protein S directly inhibits procoagulant activity on platelets and microparticles. Thromb. Haemost. 2013, 109, 229–237. [Google Scholar] [CrossRef]
- Baj-Krzyworzeka, M.; Majka, M.; Pratico, D.; Ratajczak, J.; Vilaire, G.; Kijowski, J.; Reca, R.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp. Hematol. 2002, 30, 450–459. [Google Scholar] [CrossRef]
- Rank, A.; Nieuwland, R.; Delker, R.; Köhler, A.; Toth, B.; Pihusch, V.; Wilkowski, R.; Pihusch, R. Cellular origin of platelet-derived microparticles in vivo. Thromb. Res. 2010, 126, e255–e259. [Google Scholar] [CrossRef]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet microvesicles in health and disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet Extracellular Vesicles: Beyond the Blood. Arterioscler. Thromb. Vasc. Biol. 2020, 41, 87–96. [Google Scholar] [CrossRef]
- Dyer, M.R.; Alexander, W.; Hassoune, A.; Chen, Q.; Brzoska, T.; Alvikas, J.; Liu, Y.; Haldeman, S.; Plautz, W.; Loughran, P.; et al. Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. J. Thromb. Haemost. 2019, 17, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, G.; Afonyushkin, T.; Goederle, L.; Puhm, F.; Schrottmaier, W.C.; Taqi, S.; Schwameis, M.; Ay, C.; Pabinger, I.; Jilma, B.; et al. Natural IgM antibodies inhibit microvesicle-driven coagulation and thrombosis. Blood 2021, 137, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Rectenwald, J.E.; Myers, D.D., Jr.; Hawley, A.E.; Longo, C.; Hemke, P.K.; Guire, K.E.; Schmaier, A.H.; Wakefield, T.W. D-dimer, P-selectin, and microparticles: Novel markers to predict deep venous thrombosis: A pilot study. Thromb. Haemost. 2005, 94, 1312–1317. [Google Scholar] [CrossRef]
- Sahu, A.; Jha, P.K.; Prabhakar, A.; Singh, H.D.; Gupta, N.; Chatterjee, T.; Tyagi, T.; Sharma, S.; Kumari, B.; Singh, S.; et al. MicroRNA-145 Impedes Thrombus Formation via Targeting Tissue Factor in Venous Thrombosis. EBioMedicine 2017, 26, 175–186. [Google Scholar] [CrossRef]
- Boilard, E.; Duchez, A.-C.; Brisson, A. The diversity of platelet microparticles. Curr. Opin. Hematol. 2015, 22, 437–444. [Google Scholar] [CrossRef]
- Müller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renné, T. Platelet Polyphosphates Are Proinflammatory and Procoagulant Mediators In Vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef]
- Gajsiewicz, J.M.; Smith, S.A.; Morrissey, J.H. Polyphosphate and RNA Differentially Modulate the Contact Pathway of Blood Clotting. J. Biol. Chem. 2017, 292, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.J.F.; Barendrecht, A.D.; Nickel, K.F.; Dijkxhoorn, K.; Kenne, E.; Labberton, L.; Mccarty, O.J.T.; Schiffelers, R.; Heijnen, H.F.; Hendrickx, A.P.; et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood 2017, 129, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Labberton, L.; Kenne, E.; Long, A.T.; Nickel, K.F.; Di Gennaro, A.; Rigg, R.A.; Hernandez, J.S.; Butler, L.; Maas, C.; Stavrou, E.; et al. Neutralizing blood-borne polyphosphate in vivo provides safe thromboprotection. Nat. Commun. 2016, 7, 12616. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Smith, S.A.; Morrissey, J.H. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood 2011, 118, 6963–6970. [Google Scholar] [CrossRef]
- Choi, S.; Smith, S.; Morrissey, J. Polyphosphate accelerates factor V activation by factor XIa. Thromb. Haemost. 2015, 113, 599–604. [Google Scholar] [CrossRef]
- Whyte, C.S.; Chernysh, I.N.; Domingues, M.M.; Connell, S.; Weisel, J.W.; Ariens, R.A.S.; Mutch, N.J. Polyphosphate delays fibrin polymerisation and alters the mechanical properties of the fibrin network. Thromb. Haemost. 2016, 116, 897–903. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef]
- Brogren, H.; Karlsson, L.; Andersson, M.; Wang, L.; Erlinge, D.; Jern, S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood 2004, 104, 3943–3948. [Google Scholar] [CrossRef]
- Whyte, C.; Mitchell, J.; Mutch, N. Platelet-Mediated Modulation of Fibrinolysis. Semin. Thromb. Hemost. 2017, 43, 115–128. [Google Scholar] [CrossRef]
- Brogren, H.; Wallmark, K.; Deinum, J.; Karlsson, L.; Jern, S. Platelets Retain High Levels of Active Plasminogen Activator Inhibitor 1. PLoS ONE 2011, 6, e26762. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Buijtenhuijs, P.; Marx, P.F.; Meijers, J.C.M.; Bouma, B.N. Identification of thrombin activatable fibrinolysis inhibitor (TAFI) in human platelets. Blood 2003, 101, 4844–4846. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, E.; Martinod, K.; Cherpokova, D.; Fuchs, T.; Cifuni, S.; Chu, L.; Staudinger, C.; Wagner, D.D. The role of platelets in thrombus fibrosis and vessel wall remodeling after venous thrombosis. J. Thromb. Haemost. 2021, 19, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Jakowitsch, J.; Redwan, B.; Bergmeister, H.; Renner, M.-K.; Panzenböck, H.; Adlbrecht, C.; Georgopoulos, A.; Klepetko, W.; Kneussl, M.; et al. Role for Staphylococci in Misguided Thrombus Resolution of Chronic Thromboembolic Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Kessinger, C.W.; Kim, J.W.; Henke, P.K.; Thompson, B.; McCarthy, J.R.; Hara, T.; Sillesen, M.; Margey, R.J.P.; Libby, P.; Weissleder, R.; et al. Statins Improve the Resolution of Established Murine Venous Thrombosis: Reductions in Thrombus Burden and Vein Wall Scarring. PLoS ONE 2015, 10, e0116621. [Google Scholar] [CrossRef]
- Violi, F.; Calvieri, C.; Ferro, D.; Pignatelli, P. Statins as Antithrombotic Drugs. Circulation 2013, 127, 251–257. [Google Scholar] [CrossRef]
- Xie, Y.; Muller, W.A. Molecular cloning and adhesive properties of murine platelet/endothelial cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1993, 90, 5569–5573. [Google Scholar] [CrossRef]
- Kellermair, J.; Redwan, B.; Alias, S.; Jabkowski, J.; Panzenboeck, A.; Kellermair, L.; Winter, M.P.; Weltermann, A.; Lang, I.M. Platelet endothelial cell adhesion molecule 1 deficiency misguides venous thrombus resolution. Blood 2013, 122, 3376–3384. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Schaff, M.; Peter, K. Current and future antiplatelet therapies: Emphasis on preserving haemostasis. Nat. Rev. Cardiol. 2018, 15, 181–191. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Kunapuli, S.P. Central role of the P2Y12 receptor in platelet activation. J. Clin. Investig. 2004, 113, 340–345. [Google Scholar] [CrossRef]
- Marciniak, S.; Furman, M.I.; Michelson, A.D.; Jakubowski, J.A.; Jordan, R.E.; Marchese, P.J.; Frelinger, A.L.; Mascelli, M.A. An additional mechanism of action of abciximab: Dispersal of newly formed platelet aggregates. Thromb. Haemost. 2002, 87, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Ibbotson, T.; McGavin, J.K.; Goa, K.L. Abciximab: An Updated Review of its Therapeutic Use in Patients with Ischaemic Heart Disease Undergoing Percutaneous Coronary Revascularisation. Drugs 2003, 63, 1121–1163. [Google Scholar] [CrossRef] [PubMed]
- Imbault, P.; Doutremepuich, F.; Aguejouf, O.; Doutremepuich, C. Antithrombotic effects of aspirin and LMWH in a laser-induced model of arterials and venous thrombosis. Thromb. Res. 1996, 82, 469–478. [Google Scholar] [CrossRef]
- Tarantino, E.; Amadio, P.; Squellerio, I.; Porro, B.; Sandrini, L.; Turnu, L.; Cavalca, V.; Tremoli, E.; Barbieri, S.S. Role of thromboxane-dependent platelet activation in venous thrombosis: Aspirin effects in mouse model. Pharmacol. Res. 2016, 107, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Bernat, A.; Maffrand, J. Importance of platelets in experimental venous thrombosis in the rat. Blood 1992, 80, 2281–2286. [Google Scholar] [CrossRef]
- Herbert, J.-M.; Bernat, A.; Maffrand, J.-P. Aprotinin reduces clopidogrel-induced prolongation of the bleeding time in the rat. Thromb. Res. 1993, 71, 433–441. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Vincelette, J.; da Cunha, V.; Martin-McNulty, B.; Mallari, C.; Fitch, R.M.; Alexander, S.; Islam, I.; Buckman, B.O.; Yuan, S.; et al. A novel P2Y(12) adenosine diphosphate receptor antagonist that inhibits platelet aggregation and thrombus formation in rat and dog models. Thromb. Haemost. 2007, 97, 847–855. [Google Scholar]
- Hérault, J.P.; Dol, F.; Gaich, C.; Bernat, A.; Herbert, J.M. Effect of clopidogrel on thrombin generation in platelet-rich plasma in the rat. Thromb. Haemost. 1999, 81, 957–960. [Google Scholar] [CrossRef]
- Guenther, F.; Herr, N.; Mauler, M.; Witsch, T.; Roming, F.; Hein, L.; Boeynaems, J.-M.; Robaye, B.; Idzko, M.; Bode, C.; et al. Contrast ultrasound for the quantification of deep vein thrombosis in living mice: Effects of enoxaparin and P2Y 12 receptor inhibition. J. Thromb. Haemost. 2013, 11, 1154–1162. [Google Scholar] [CrossRef]
- Bird, J.E.; Wang, X.; Smith, P.L.; Barbera, F.; Huang, C.; Schumacher, W.A. A platelet target for venous thrombosis? P2Y1 deletion or antagonism protects mice from vena cava thrombosis. J. Thromb. Thrombolysis 2012, 34, 199–207. [Google Scholar] [CrossRef]
- Cooley, B.C.; Herrera, A.J. Cross-modulatory effects of clopidogrel and heparin on platelet and fibrin incorporation in thrombosis. Blood Coagul. Fibrinolysis 2013, 24, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Mwiza, J.M.N.; Lee, R.H.; Paul, D.S.; Holle, L.A.; Cooley, B.C.; Nieswandt, B.; Schug, W.J.; Kawano, T.; Mackman, N.; Wolberg, A.S.; et al. Both G protein–coupled and immunoreceptor tyrosine-based activation motif receptors mediate venous thrombosis in mice. Blood 2022, 139, 3194–3203. [Google Scholar] [CrossRef] [PubMed]
- Branchford, B.R.; Stalker, T.J.; Law, L.; Acevedo, G.; Sather, S.; Brzezinski, C.; Wilson, K.M.; Minson, K.; Lee-Sherick, A.B.; Davizon-Castillo, P.; et al. The small-molecule MERTK inhibitor UNC2025 decreases platelet activation and prevents thrombosis. J. Thromb. Haemost. 2018, 16, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Dunbar, M.; Murnaghan, J.; Kahn, S.R.; Gross, P.; Forsythe, M.; Pelet, S.; Fisher, W.; Belzile, E.; Dolan, S.; et al. Aspirin or Rivaroxaban for VTE Prophylaxis after Hip or Knee Arthroplasty. N. Engl. J. Med. 2018, 378, 699–707. [Google Scholar] [CrossRef]
- CRISTAL Study Group. Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial. JAMA 2022, 328, 719. [Google Scholar] [CrossRef]
- Becattini, C.; Agnelli, G.; Schenone, A.; Eichinger, S.; Bucherini, E.; Silingardi, M.; Bianchi, M.; Moia, M.; Ageno, W.; Vandelli, M.R.; et al. Aspirin for Preventing the Recurrence of Venous Thromboembolism. N. Engl. J. Med. 2012, 366, 1959–1967. [Google Scholar] [CrossRef]
- Brighton, T.A.; Eikelboom, J.W.; Mann, K.; Mister, R.; Gallus, A.; Ockelford, P.; Gibbs, H.; Hague, W.; Xavier, D.; Diaz, R.; et al. Low-Dose Aspirin for Preventing Recurrent Venous Thromboembolism. N. Engl. J. Med. 2012, 367, 1979–1987. [Google Scholar] [CrossRef]
- Weitz, J.I.; Lensing, A.W.; Prins, M.H.; Bauersachs, R.; Beyer-Westendorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N. Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef]
- Mai, V.; Bertoletti, L.; Cucherat, M.; Jardel, S.; Grange, C.; Provencher, S.; Lega, J.-C. Extended anticoagulation for the secondary prevention of venous thromboembolic events: An updated network meta-analysis. PLoS ONE 2019, 14, e0214134. [Google Scholar] [CrossRef]
- Jourdi, G.; Bachelot-Loza, C.; Mazoyer, E.; Poirault-Chassac, S.; Duchemin, J.; Fontenay, M.; Gaussem, P. Effect of rivaroxaban and dabigatran on platelet functions: In vitro study. Thromb. Res. 2019, 183, 159–162. [Google Scholar] [CrossRef]
- Hernandez-Juarez, J.; Espejo-Godinez, H.G.; Mancilla-Padilla, R.; Hernandez-Lopez, J.R.; Moreno, J.A.A.; Majluf-Cruz, K.; Moreno-Hernández, M.; Isordia-Salas, I.; Majluf-Cruz, A. Effects of Rivaroxaban on Platelet Aggregation. J. Cardiovasc. Pharmacol. 2020, 75, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Trabold, K.; Makhoul, S.; Gambaryan, S.; van Ryn, J.; Walter, U.; Jurk, K. The Direct Thrombin Inhibitors Dabigatran and Lepirudin Inhibit GPIbα-Mediated Platelet Aggregation. Thromb. Haemost. 2019, 119, 916–929. [Google Scholar] [PubMed]
- Kubisz, P.; Stanciakova, L.; Dobrotova, M.; Samos, M.; Mokan, M.; Stasko, J. Apixaban—Metabolism, Pharmacologic Properties and Drug Interactions. Curr. Drug Metab. 2017, 18, 609–621. [Google Scholar] [PubMed]
- Honda, Y.; Kamisato, C.; Morishima, Y. Edoxaban, a direct factor Xa inhibitor, suppresses tissue-factor induced human platelet aggregation and clot-bound factor Xa in vitro: Comparison with an antithrombin-dependent factor Xa inhibitor, fondaparinux. Thromb. Res. 2016, 141, 17–21. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heestermans, M.; Poenou, G.; Duchez, A.-C.; Hamzeh-Cognasse, H.; Bertoletti, L.; Cognasse, F. Immunothrombosis and the Role of Platelets in Venous Thromboembolic Diseases. Int. J. Mol. Sci. 2022, 23, 13176. https://doi.org/10.3390/ijms232113176
Heestermans M, Poenou G, Duchez A-C, Hamzeh-Cognasse H, Bertoletti L, Cognasse F. Immunothrombosis and the Role of Platelets in Venous Thromboembolic Diseases. International Journal of Molecular Sciences. 2022; 23(21):13176. https://doi.org/10.3390/ijms232113176
Chicago/Turabian StyleHeestermans, Marco, Géraldine Poenou, Anne-Claire Duchez, Hind Hamzeh-Cognasse, Laurent Bertoletti, and Fabrice Cognasse. 2022. "Immunothrombosis and the Role of Platelets in Venous Thromboembolic Diseases" International Journal of Molecular Sciences 23, no. 21: 13176. https://doi.org/10.3390/ijms232113176
APA StyleHeestermans, M., Poenou, G., Duchez, A.-C., Hamzeh-Cognasse, H., Bertoletti, L., & Cognasse, F. (2022). Immunothrombosis and the Role of Platelets in Venous Thromboembolic Diseases. International Journal of Molecular Sciences, 23(21), 13176. https://doi.org/10.3390/ijms232113176





