RB49-like Bacteriophages Recognize O Antigens as One of the Alternative Primary Receptors
Abstract
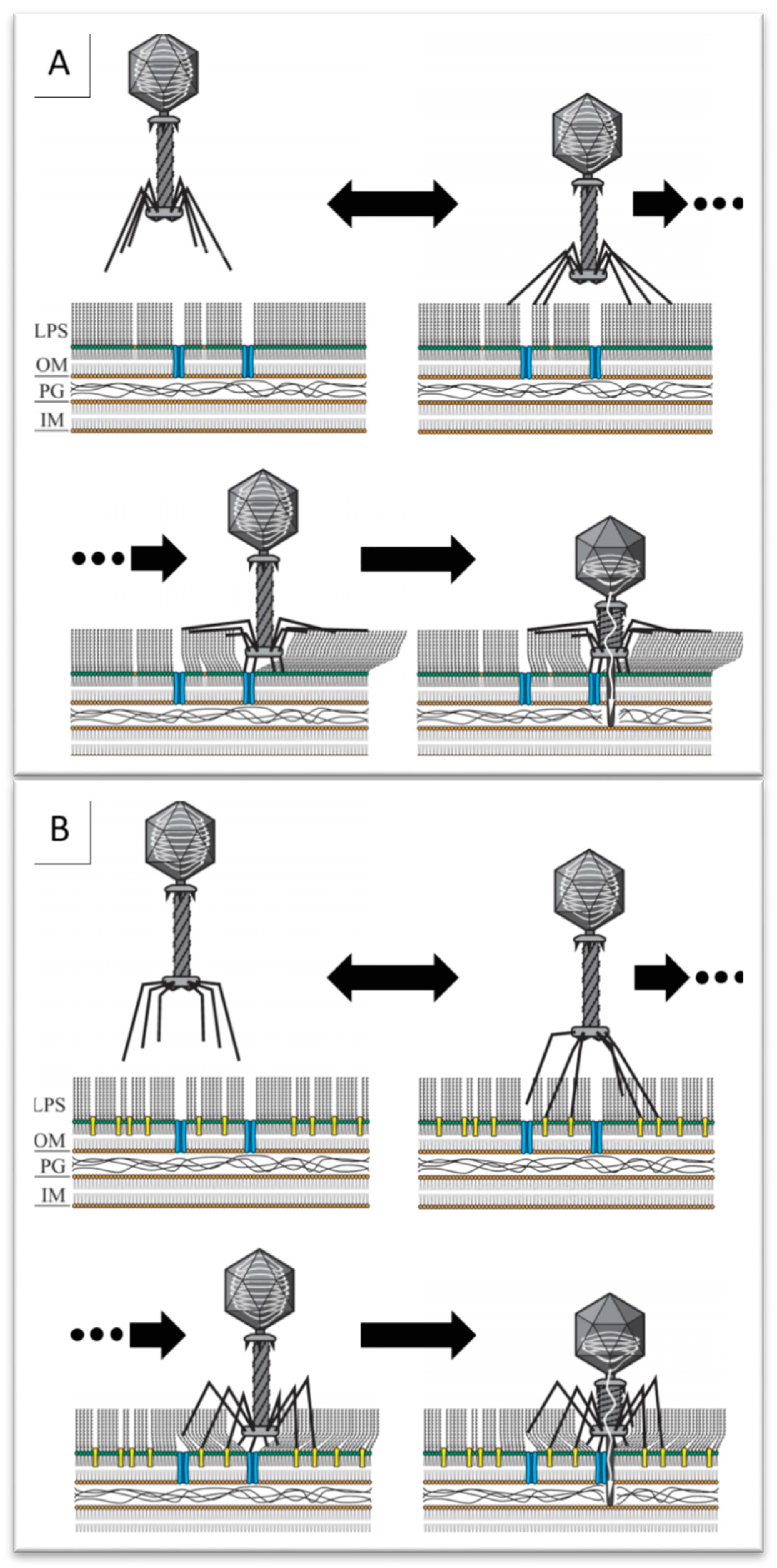
1. Introduction
2. Results
2.1. RB49-like Bacteriophages Have Different Adsorption Specificity towards E. Coli Strains with Different O Antigen Types
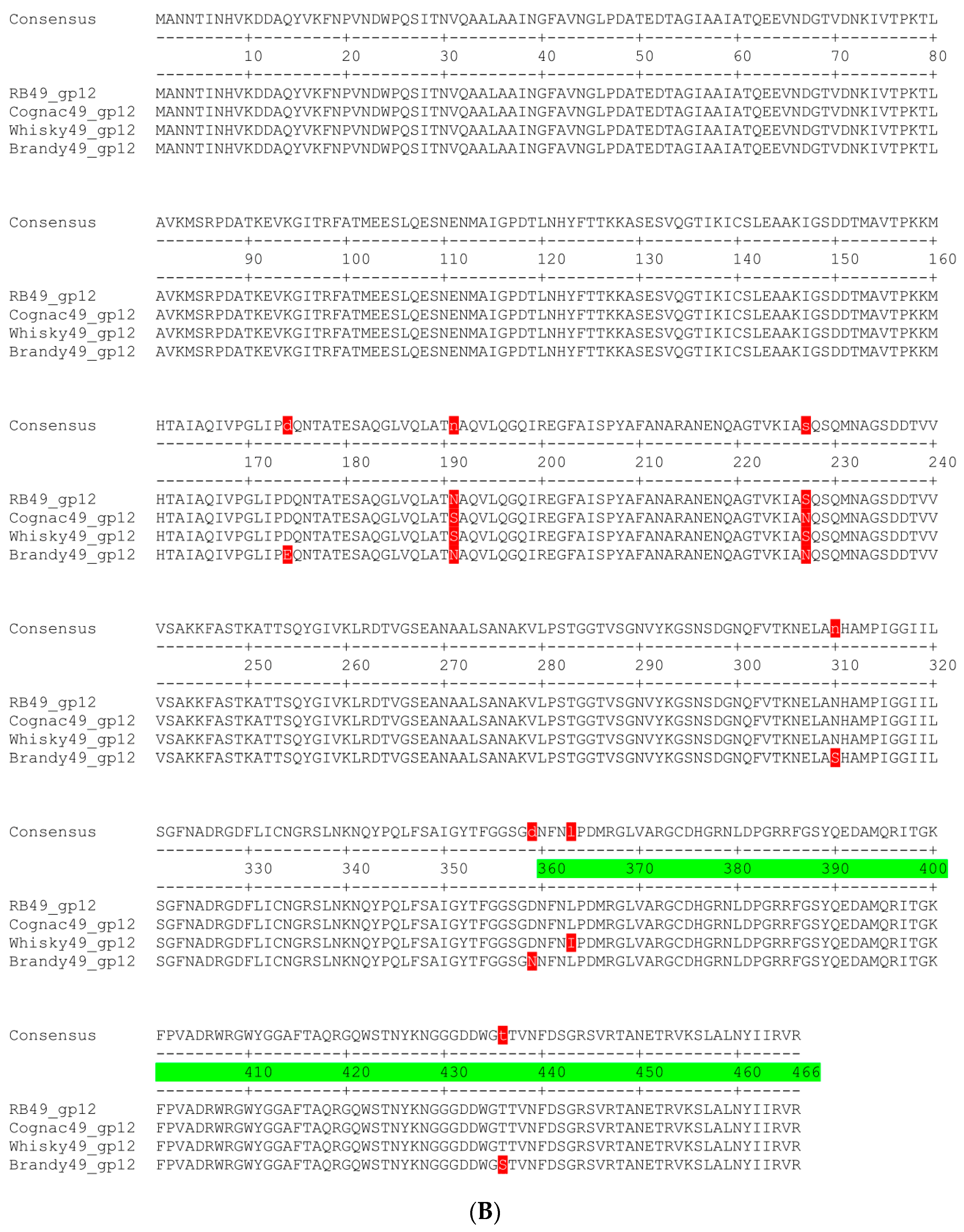
2.2. Genetic Determination of the Host Range
2.3. Sensitivity of the Host Strains Rough Mutants to RB49-like Phages
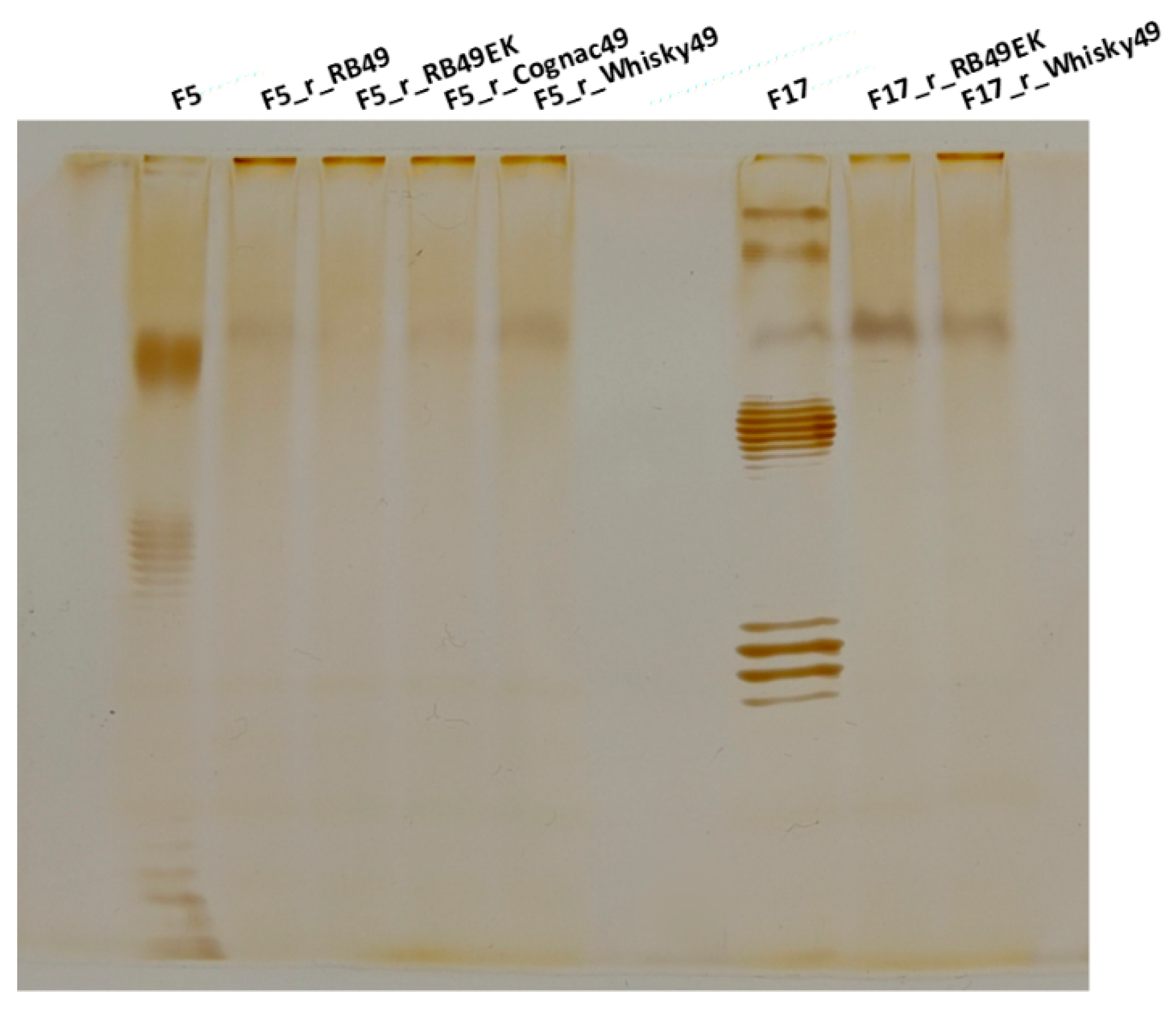
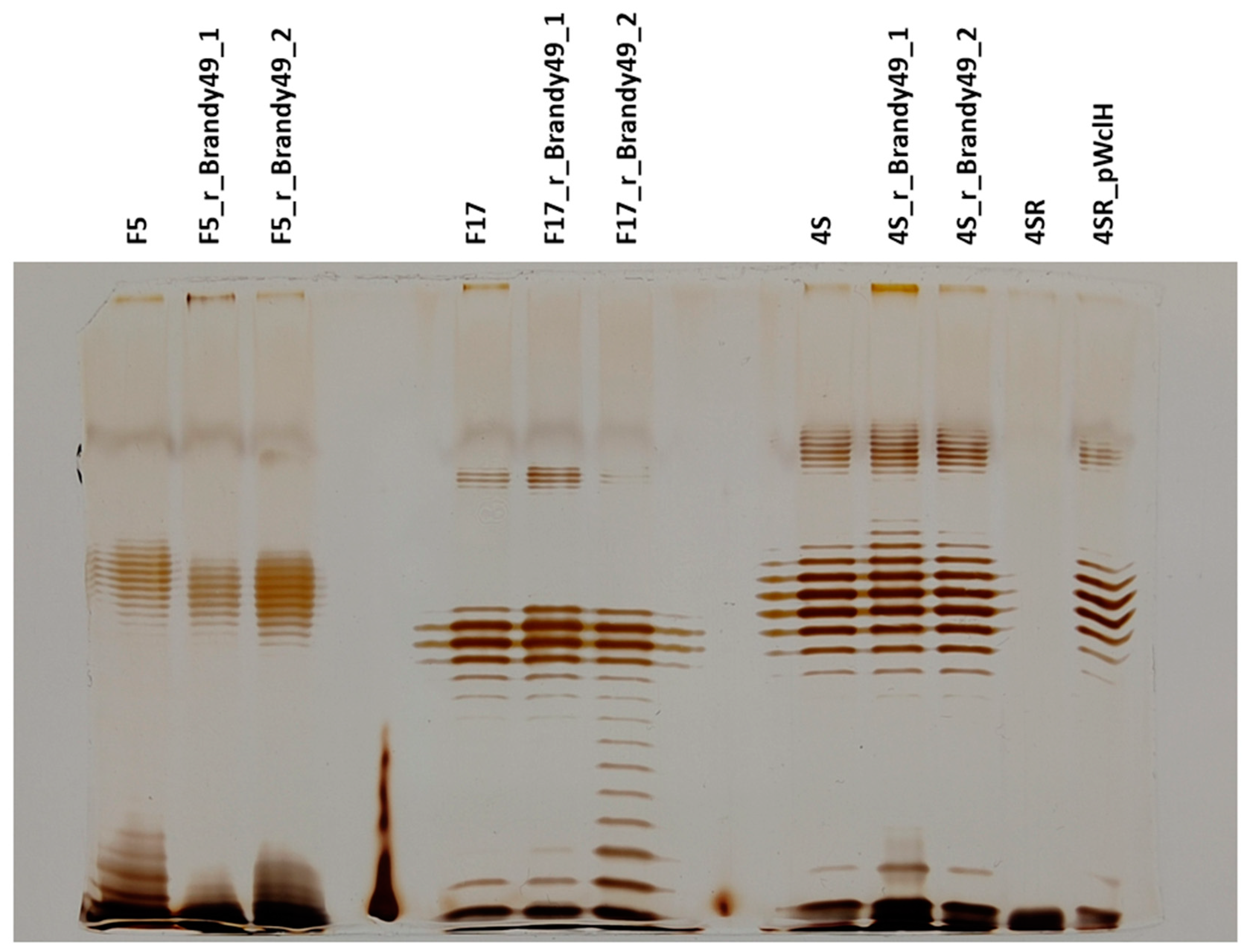
2.4. O Antigen Alterations in Phage-Resistant Mutants
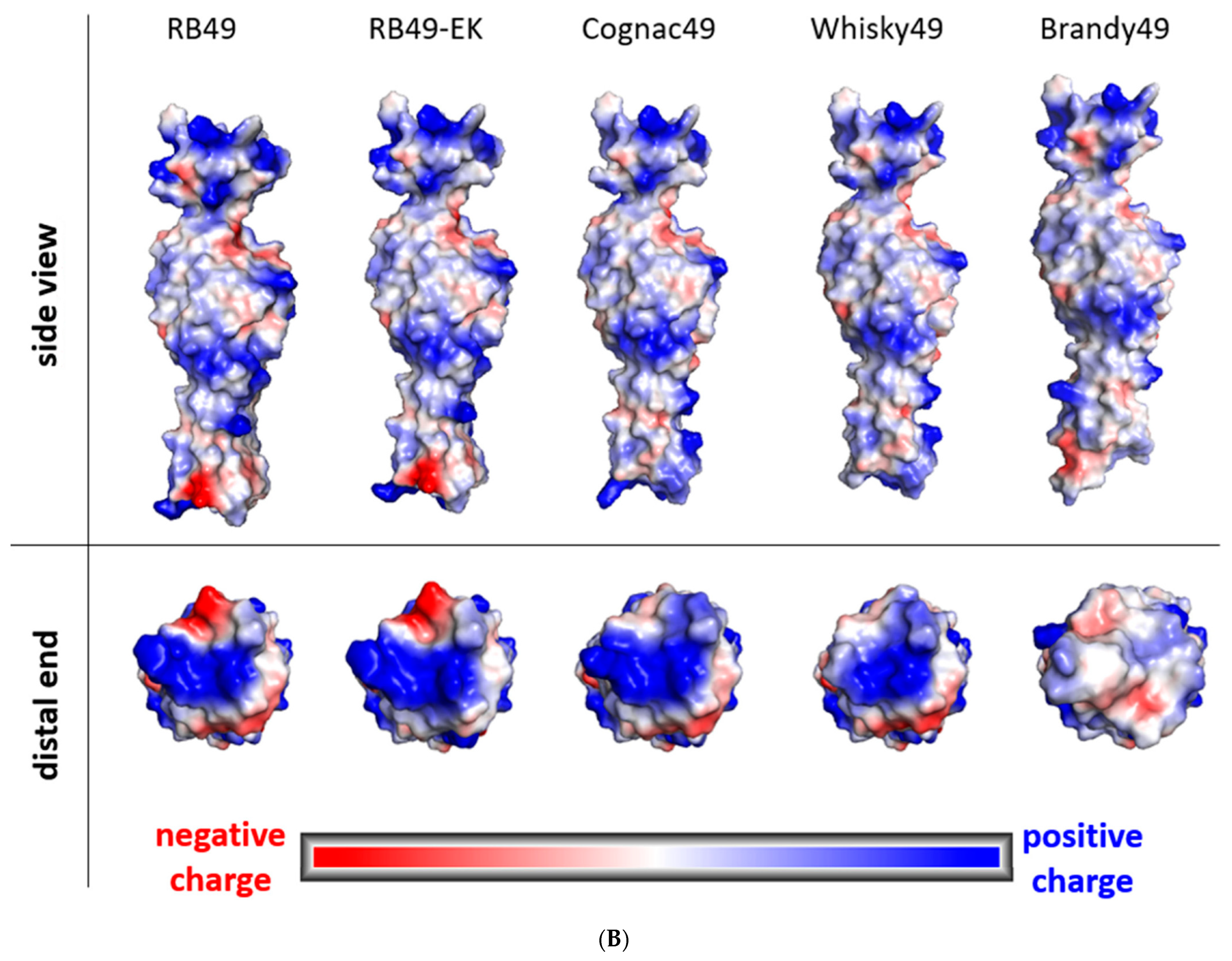
2.5. Phage Brandy49 Has a Distinct Mechanism of O Antigen Penetration
3. Discussion
4. Materials and Methods
4.1. Bacterial and Bacteriophage Strains
4.2. Bacteriophage Adsorption Assay
4.3. Selection of Bacteriophage-Resistant Mutants
4.4. Genomic Sequencing
4.5. Lipopolysaccharide (LPS) Profiling
4.6. Construction of pOmpA Plasmid and Complementation
4.7. Bioinformatic Analysis and Structural Modelling of Phage Proteins
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thanki, A.M.; Taylor-Joyce, G.; Dowah, A.; Nale, J.Y.; Malik, D.; Clokie, M.R.J. Unravelling the Links between Phage Adsorption and Successful Infection in Clostridium difficile. Viruses 2018, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.; Hao, H.; Shabbir, M.Z.; Wu, Q.; Sattar, A.; Yuan, Z. Bacteria vs. Bacteriophages: Parallel Evolution of Immune Arsenals. Front. Microbiol. 2016, 7, 1292. [Google Scholar] [CrossRef] [PubMed]
- Letarov, A.; Kulikov, E. Adsorption of bacteriophages on bacterial cells. Biochemistry 2017, 82, 1632–1658. [Google Scholar] [CrossRef] [PubMed]
- Huss, P.; Meger, A.; Leander, M.; Nishikawa, K.; Raman, S. Mapping the functional landscape of the receptor binding domain of T7 bacteriophage by deep mutational scanning. Elife 2021, 10, e63775. [Google Scholar] [CrossRef] [PubMed]
- Parent, K.N.; Erb, M.L.; Cardone, G.; Nguyen, K.; Gilcrease, E.B.; Porcek, N.B.; Pogliano, J.; Baker, T.S.; Casjens, S.R. OmpA and OmpC are critical host factors for bacteriophage Sf6 entry in Shigella. Mol. Microbiol. 2014, 92, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Prokhorov, N.S.; Riccio, C.; Zdorovenko, E.L.; Shneider, M.M.; Browning, C.; Knirel, Y.A.; Leiman, P.G.; Letarov, A.V. Function of bacteriophage G7C esterase tailspike in host cell adsorption. Mol. Microbiol. 2017, 105, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, E.E.; Golomidova, A.K.; Prokhorov, N.S.; Ivanov, P.A.; Letarov, A.V. High-throughput LPS profiling as a tool for revealing of bacteriophage infection strategies. Sci. Rep. 2019, 9, 2958. [Google Scholar] [CrossRef]
- Heller, K.; Braun, V. Polymannose O-antigens of Escherichia coli, the binding sites for the reversible adsorption of bacteriophage T5+ via the L-shaped tail fibers. J. Virol. 1982, 41, 222–227. [Google Scholar] [CrossRef]
- Golomidova, A.K.; Naumenko, O.I.; Senchenkova, S.N.; Knirel, Y.A.; Letarov, A.V. The O-polysaccharide of Escherichia coli F5, which is structurally related to that of E. coli O28ab, provides cells only weak protection against bacteriophage attack. Arch. Virol. 2019, 164, 2783–2787. [Google Scholar] [CrossRef]
- Golomidova, A.K.; Kulikov, E.E.; Prokhorov, N.S.; Guerrero-Ferreira, R.C.; Knirel, Y.A.; Kostryukova, E.S.; Tarasyan, K.K.; Letarov, A.V. Branched lateral tail fiber organization in T5-like bacteriophages DT57C and DT571/2 is revealed by genetic and functional analysis. Viruses 2016, 8, 26. [Google Scholar] [CrossRef]
- Golomidova, A.K.; Efimov, A.D.; Kulikov, E.E.; Kuznetsov, A.S.; Belalov, I.S.; Letarov, A.V. O antigen restricts lysogenization of non-O157 Escherichia coli strains by Stx-converting bacteriophage phi24B. Sci. Rep. 2021, 11, 3035. [Google Scholar] [CrossRef]
- Golomidova, A.; Efimov, A.; Kulikov, E.; Kuznetsov, A.; Letarov, A. The lysogenization of the non-O157 Escherichia coli strains by stx-converting bacteriophage phi24B is associated with the O antigen loss and reduced fitness. Biorxiv 2019, 2019, 860106. [Google Scholar]
- Kulikov, E.E.; Golomidova, A.K.; Efimov, A.D.; Belalov, I.S.; Letarova, M.A.; Zdorovenko, E.L.; Knirel, Y.A.; Dmitrenok, A.S.; Letarov, A.V. Equine Intestinal O-Seroconverting Temperate Coliphage Hf4s: Genomic and Biological Characterization. Appl. Environ. Microbiol. 2021, 87, e0112421. [Google Scholar] [CrossRef]
- Maffei, E.; Shaidullina, A.; Burkolter, M.; Heyer, Y.; Estermann, F.; Druelle, V.; Sauer, P.; Willi, L.; Michaelis, S.; Hilbi, H.; et al. Systematic exploration of Escherichia coli phage-host interactions with the BASEL phage collection. PLoS Biol. 2021, 19, e3001424. [Google Scholar] [CrossRef]
- Kaczorowska, J.; Casey, E.; Neve, H.; Franz, C.; Noben, J.P.; Lugli, G.A.; Ventura, M.; Sinderen, D.V.; Mahony, J. A Quest of Great Importance-Developing a Broad Spectrum Escherichia coli Phage Collection. Viruses 2019, 11, 899. [Google Scholar] [CrossRef]
- Mohan Raj, J.R.; Vittal, R.; Huilgol, P.; Bhat, U.; Karunasagar, I. T4-like Escherichia coli phages from the environment carry blaCTX-M. Lett. Appl. Microbiol. 2018, 67, 9–14. [Google Scholar] [CrossRef]
- Wei, X.; Ge, T.; Wu, C.; Wang, S.; Mason-Jones, K.; Li, Y.; Zhu, Z.; Hu, Y.; Liang, C.; Shen, J.; et al. T4-like Phages Reveal the Potential Role of Viruses in Soil Organic Matter Mineralization. Environ. Sci. Technol. 2021, 55, 6440–6448. [Google Scholar] [CrossRef]
- Mathieu, A.; Dion, M.; Deng, L.; Tremblay, D.; Moncaut, E.; Shah, S.A.; Stokholm, J.; Krogfelt, K.A.; Schjorring, S.; Bisgaard, H.; et al. Virulent coliphages in 1-year-old children fecal samples are fewer, but more infectious than temperate coliphages. Nat. Commun. 2020, 11, 378. [Google Scholar] [CrossRef]
- Sorensen, P.E.; Van Den Broeck, W.; Kiil, K.; Jasinskyte, D.; Moodley, A.; Garmyn, A.; Ingmer, H.; Butaye, P. New insights into the biodiversity of coliphages in the intestine of poultry. Sci. Rep. 2020, 10, 15220. [Google Scholar] [CrossRef]
- Hu, B.; Margolin, W.; Molineux, I.J.; Liu, J. Structural remodeling of bacteriophage T4 and host membranes during infection initiation. Proc. Natl. Acad. Sci. USA 2015, 112, E4919–E4928. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.Y.; Shiomi, D.; Niki, H.; Margolin, W. Visualization of bacteriophage P1 infection by cryo-electron tomography of tiny Escherichia coli. Virology 2011, 417, 304–311. [Google Scholar] [CrossRef]
- Esteves, N.C.; Scharf, B.E. Flagellotropic Bacteriophages: Opportunities and Challenges for Antimicrobial Applications. Int. J. Mol. Sci. 2022, 23, 7084. [Google Scholar] [CrossRef]
- Guerrero-Ferreira, R.C.; Hupfeld, M.; Nazarov, S.; Taylor, N.M.; Shneider, M.M.; Obbineni, J.M.; Loessner, M.J.; Ishikawa, T.; Klumpp, J.; Leiman, P.G. Structure and transformation of bacteriophage A511 baseplate and tail upon infection of Listeria cells. EMBO J. 2019, 38, e99455. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; van Raaij, M.J.; Leiman, P.G. Contractile injection systems of bacteriophages and related systems. Mol. Microbiol. 2018, 108, 6–15. [Google Scholar] [CrossRef]
- Taylor, N.M.; Prokhorov, N.S.; Guerrero-Ferreira, R.C.; Shneider, M.M.; Browning, C.; Goldie, K.N.; Stahlberg, H.; Leiman, P.G. Structure of the T4 baseplate and its function in triggering sheath contraction. Nature 2016, 533, 346–352. [Google Scholar] [CrossRef]
- Desplats, C.; Krisch, H.M. The diversity and evolution of the T4-type bacteriophages. Res. Microbiol. 2003, 154, 259–267. [Google Scholar] [CrossRef]
- Monod, C.; Repoila, F.; Kutateladze, M.; Tetart, F.; Krisch, H.M. The genome of the pseudo T-even bacteriophages, a diverse group that resembles T4. J. Mol. Biol. 1997, 267, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Desplats, C.; Dez, C.; Tetart, F.; Eleaume, H.; Krisch, H.M. Snapshot of the genome of the pseudo-T-even bacteriophage RB49. J. Bacteriol. 2002, 184, 2789–2804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trojet, S.N.; Caumont-Sarcos, A.; Perrody, E.; Comeau, A.M.; Krisch, H.M. The gp38 adhesins of the T4 superfamily: A complex modular determinant of the phage’s host specificity. Genome Biol. Evol. 2011, 3, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P.; van Raaij, M. Bacteriophage T4 long tail fiber domains. Biophys Rev. 2018, 10, 463–471. [Google Scholar] [CrossRef]
- Dunne, M.; Denyes, J.M.; Arndt, H.; Loessner, M.J.; Leiman, P.G.; Klumpp, J. Salmonella Phage S16 Tail Fiber Adhesin Features a Rare Polyglycine Rich Domain for Host Recognition. Structure 2018, 26, 1573–1582. [Google Scholar] [CrossRef]
- Swanson, N.A.; Cingolani, G. A Tail of Phage Adhesins. Structure 2018, 26, 1565–1567. [Google Scholar] [CrossRef]
- Efimov, A.; Kulikov, E.; Golomidova, A.; Belalov, I.; Babenko, V.; Letarov, A. Isolation and sequencing of three RB49-like bacteriophages infecting O antigen-producing E. coli strains. F1000 Res. 2021, 10, 1113. [Google Scholar] [CrossRef]
- Liu, D.; Reeves, P.R. Escherichia coli K12 regains its O antigen. Microbiology 1994, 140, 49–57. [Google Scholar] [CrossRef]
- Islam, M.Z.; Fokine, A.; Mahalingam, M.; Zhang, Z.; Garcia-Doval, C.; van Raaij, M.J.; Rossmann, M.G.; Rao, V.B. Molecular anatomy of the receptor binding module of a bacteriophage long tail fiber. PLoS Pathog. 2019, 15, e1008193. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006–2008. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Ivanov, P.A.; Senchenkova, S.N.; Naumenko, O.I.; Ovchinnikova, O.O.; Shashkov, A.S.; Golomidova, A.K.; Babenko, V.V.; Kulikov, E.E.; Letarov, A.V. Structure and gene cluster of the O antigen of Escherichia coli F17, a candidate for a new O-serogroup. Int. J. Biol. Macromol. 2019, 124, 389–395. [Google Scholar] [CrossRef]
- Islam, M.R.; Martinez-Soto, C.E.; Lin, J.T.; Khursigara, C.M.; Barbut, S.; Anany, H. A systematic review from basics to omics on bacteriophage applications in poultry production and processing. Crit. Rev. Food Sci. Nutr. 2021, 1–33. [Google Scholar] [CrossRef]
- Pereira, C.; Costa, P.; Duarte, J.; Balcao, V.M.; Almeida, A. Phage therapy as a potential approach in the biocontrol of pathogenic bacteria associated with shellfish consumption. Int. J. Food Microbiol. 2020, 338, 108995. [Google Scholar] [CrossRef]
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, A.; Almuina-Gonzalez, R.; de Miguel, T.; Sanchez, S.; Sanchez-Perez, A.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef]
- Batinovic, S.; Wassef, F.; Knowler, S.A.; Rice, D.T.F.; Stanton, C.R.; Rose, J.; Tucci, J.; Nittami, T.; Vinh, A.; Drummond, G.R.; et al. Bacteriophages in Natural and Artificial Environments. Pathogens 2019, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Lojewska, E.; Sakowicz, T. An Alternative to Antibiotics: Selected Methods to Combat Zoonotic Foodborne Bacterial Infections. Curr. Microbiol. 2021, 78, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Brives, C.; Pourraz, J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Gutierrez, B.; Domingo-Calap, P. Phage Therapy in Gastrointestinal Diseases. Microorganisms 2020, 8, 1420. [Google Scholar] [CrossRef]
- Vlassov, V.V.; Tikunova, N.V.; Morozova, V.V. Bacteriophages as Therapeutic Preparations: What Restricts Their Application in Medicine. Biochem. Biokhimiia 2020, 85, 1350–1361. [Google Scholar] [CrossRef]
- Kornienko, M.; Fisunov, G.; Bespiatykh, D.; Kuptsov, N.; Gorodnichev, R.; Klimina, K.; Kulikov, E.; Ilina, E.; Letarov, A.; Shitikov, E. Transcriptional Landscape of Staphylococcus aureus Kayvirus Bacteriophage vB_SauM-515A1. Viruses 2020, 12, 1320. [Google Scholar] [CrossRef]
- Isaev, A.B.; Musharova, O.S.; Severinov, K.V. Microbial Arsenal of Antiviral Defenses-Part I. Biochem. Biokhimiia 2021, 86, 319–337. [Google Scholar] [CrossRef]
- Zdorovenko, E.L.; Wang, Y.; Shashkov, A.S.; Chen, T.; Ovchinnikova, O.G.; Liu, B.; Golomidova, A.K.; Babenko, V.V.; Letarov, A.V.; Knirel, Y.A. O-Antigens of Escherichia coli strains O81 and HS3-104 are structurally and genetically related, except O-Antigen glucosylation in E. coli HS3-104. Biochem. Biokhimiia 2018, 83, 534–541. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Prokhorov, N.S.; Shashkov, A.S.; Ovchinnikova, O.G.; Zdorovenko, E.L.; Liu, B.; Kostryukova, E.S.; Larin, A.K.; Golomidova, A.K.; Letarov, A.V. Variations in O-antigen biosynthesis and O-acetylation associated with altered phage sensitivity in Escherichia coli 4s. J. Bacteriol. 2015, 197, 905–912. [Google Scholar] [CrossRef]
- Fernandes, S.; Sao-Jose, C. Enzymes and Mechanisms Employed by Tailed Bacteriophages to Breach the Bacterial Cell Barriers. Viruses 2018, 10, 396. [Google Scholar] [CrossRef]
- Latka, A.; Maciejewska, B.; Majkowska-Skrobek, G.; Briers, Y.; Drulis-Kawa, Z. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl. Microbiol. Biotechnol. 2017, 101, 3103–3119. [Google Scholar] [CrossRef]
- Stenutz, R.; Weintraub, A.; Widmalm, G. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 2006, 30, 382–403. [Google Scholar] [CrossRef]
- Coimbra, R.S.; Grimont, F.; Lenormand, P.; Burguiere, P.; Beutin, L.; Grimont, P.A. Identification of Escherichia coli O-serogroups by restriction of the amplified O-antigen gene cluster (rfb-RFLP). Res. Microbiol. 2000, 151, 639–654. [Google Scholar] [CrossRef]
- Mare, A.; Man, A.; Ciurea, C.N.; Pintea-Simon, I.A.; Ianosi, E.S.; Girbovan, C.E.; Toma, F. Serogroups and genetic diversity of diarrheagenic strains of Escherichia coli: A retrospective study. J. Infect. Dev. Ctries 2022, 16, 827–834. [Google Scholar] [CrossRef]
- Tanabe, R.H.S.; Dias, R.C.B.; Orsi, H.; de Lira, D.R.P.; Vieira, M.A.; Dos Santos, L.F.; Ferreira, A.M.; Rall, V.L.M.; Mondelli, A.L.; Gomes, T.A.T.; et al. Characterization of Uropathogenic Escherichia coli Reveals Hybrid Isolates of Uropathogenic and Diarrheagenic (UPEC/DEC) E. coli. Microorganisms 2022, 10, 645. [Google Scholar] [CrossRef]
- Verma, S.; Venkatesh, V.; Kumar, R.; Kashyap, S.; Kumar, M.; Maurya, A.K.; Dhole, T.N.; Singh, M. Etiological agents of diarrhea in hospitalized pediatric patients with special emphasis on diarrheagenic Escherichia coli in North India. J. Lab. Physicians 2019, 11, 68–74. [Google Scholar] [CrossRef]
- Kato, H.; Yamaguchi, H.; Ito, Y.; Imuta, N.; Nishi, J.; Kasai, M. Escherichia coli O157 Enterocolitis Followed by Non-diarrheagenic Escherichia coli Bacteremia. Indian J. Pediatr. 2019, 86, 750. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, X.; Li, R.; Han, Y.; Hao, G.; Wang, G.; Sun, S. Companion Animals as Potential Reservoirs of Antibiotic Resistant Diarrheagenic Escherichia coli in Shandong, China. Antibiotics 2022, 11, 828. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, N.; Malhotra, S.; Madan, P.; Ahmad, W.; Hans, C. Serotyping and Antimicrobial Susceptibility Pattern of Escherichia coli Isolates from Urinary Tract Infections in Pediatric Population in a Tertiary Care Hospital. J. Pathog. 2016, 2016, 2548517. [Google Scholar] [CrossRef]
- Kornienko, M.; Kuptsov, N.; Gorodnichev, R.; Bespiatykh, D.; Guliaev, A.; Letarova, M.; Kulikov, E.; Veselovsky, V.; Malakhova, M.; Letarov, A.; et al. Contribution of Podoviridae and Myoviridae bacteriophages to the effectiveness of anti-staphylococcal therapeutic cocktails. Sci. Rep. 2020, 10, 18612. [Google Scholar] [CrossRef]
- Morozova, V.V.; Kozlova, Y.N.; Ganichev, D.A.; Tikunova, N.V. Bacteriophage Treatment of Infected Diabetic Foot Ulcers. Methods Mol. Biol. 2018, 1693, 151–158. [Google Scholar] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Fokine, A.; Rossmann, M.G. Molecular architecture of tailed double-stranded DNA phages. Bacteriophage 2014, 4, e28281. [Google Scholar] [CrossRef] [PubMed]
- Effantin, G.; Boulanger, P.; Neumann, E.; Letellier, L.; Conway, J.F. Bacteriophage T5 structure reveals similarities with HK97 and T4 suggesting evolutionary relationships. J. Mol. Biol. 2006, 361, 993–1002. [Google Scholar] [CrossRef]
- Zivanovic, Y.; Confalonieri, F.; Ponchon, L.; Lurz, R.; Chami, M.; Flayhan, A.; Renouard, M.; Huet, A.; Decottignies, P.; Davidson, A.R.; et al. Insights into bacteriophage T5 structure from analysis of its morphogenesis genes and protein components. J. Virol. 2014, 88, 1162–1174. [Google Scholar] [CrossRef]
- Letarov, A.V.; Krisch, H.M. The episodic evolution of fibritin: Traces of ancient global environmental alterations may remain in the genomes of T4-like phages. Ecol. Evol. 2013, 3, 3628–3635. [Google Scholar] [CrossRef]
- Letarov, A.; Manival, X.; Desplats, C.; Krisch, H.M.M. Gpwac of the T4-type bacteriophages: Structure, function and evolution of a segmented coiled-coil protein that controls viral infectivity. J. Bacteriol. 2005, 187, 1055–1066. [Google Scholar] [CrossRef]
- Fokine, A.; Zhang, Z.; Kanamaru, S.; Bowman, V.D.; Aksyuk, A.A.; Arisaka, F.; Rao, V.B.; Rossmann, M.G. The molecular architecture of the bacteriophage T4 neck. J. Mol. Biol. 2013, 425, 1731–1744. [Google Scholar] [CrossRef]
- Golomidova, A.; Kulikov, E.; Isaeva, A.; Manykin, A.; Letarov, A. The diversity of coliphages and coliforms in horse feces reveals a complex pattern of ecological interactions. Appl. Environ. Microbiol. 2007, 73, 5975–5981. [Google Scholar] [CrossRef]
- Kulikov, E.; Golomidova, A.; Babenko, V.; Letarov, A.J.M. A Simple Method for Extraction of the Horse Feces Virome DNA, Suitable for Oxford Nanopore Sequencing. Microbiology 2020, 89, 246–249. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]


| Phages | Host Strain (O Antigen Type) | |||||||
|---|---|---|---|---|---|---|---|---|
| 4s (O22) | 4sR (Rough) | F17 (New) | F17 WbbL (Rough) | F5 (O28) | F5:24B (Rough) | MG1665 K-12 (Rough) | C600 (Rough) | |
| RB49 | − | − | − | − | + | + | + | + |
| RB49-EK | − | − | + | − | + | + | + | + |
| Whisky49 | − | − | + | − | + | +/− | +/− | +/− |
| Cognac49 | − | − | − | − | + | + | + | + |
| Brandy49 | + | + | + | + | + | + | + | + |
| Host Strains | Bacteriophages | |||||
|---|---|---|---|---|---|---|
| Cognac49 | Whisky49 | RB49 | RB49-EK | Brandy49 | FimX | |
| F5 wt | + | + | + | + | + | − (EOP~10−6) |
| F5-rCognac49 | − | − | − | − | + | + |
| F5-rWhisky49 | − | − | − | − | + | + |
| F5-rRB49 | − | − | − | − | + | + |
| F5-rRB49-EK | − | − | − | − | + | + |
| F5-rRB49:pOmpA | − | − | − | − | + | + |
| F17 wt | − | + | − | + | + | − |
| F17-rWhisky49 | − | − | − | − | + | + |
| F17-rRB49-EK | − | − | − | − | + | + |
| F17-wbbL:pOmpA | − | − | − | − | + | + |
| K-12 MG1665 | + | +/− | + | + | + | + |
| K-12 ΔompA | − | − | − | − | + | + |
| K-12 ΔompA:pOmpA | + | +/− | + | + | + | + |
| K-12-rRB49 | − | − | − | − | + | + |
| K-12-rRB49:pOmpA | − | − | − | − | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimov, A.D.; Golomidova, A.K.; Kulikov, E.E.; Belalov, I.S.; Ivanov, P.A.; Letarov, A.V. RB49-like Bacteriophages Recognize O Antigens as One of the Alternative Primary Receptors. Int. J. Mol. Sci. 2022, 23, 11329. https://doi.org/10.3390/ijms231911329
Efimov AD, Golomidova AK, Kulikov EE, Belalov IS, Ivanov PA, Letarov AV. RB49-like Bacteriophages Recognize O Antigens as One of the Alternative Primary Receptors. International Journal of Molecular Sciences. 2022; 23(19):11329. https://doi.org/10.3390/ijms231911329
Chicago/Turabian StyleEfimov, Alexandr D., Alla K. Golomidova, Eugene E. Kulikov, Ilya S. Belalov, Pavel A. Ivanov, and Andrey V. Letarov. 2022. "RB49-like Bacteriophages Recognize O Antigens as One of the Alternative Primary Receptors" International Journal of Molecular Sciences 23, no. 19: 11329. https://doi.org/10.3390/ijms231911329
APA StyleEfimov, A. D., Golomidova, A. K., Kulikov, E. E., Belalov, I. S., Ivanov, P. A., & Letarov, A. V. (2022). RB49-like Bacteriophages Recognize O Antigens as One of the Alternative Primary Receptors. International Journal of Molecular Sciences, 23(19), 11329. https://doi.org/10.3390/ijms231911329






