Melatonin Type 2 Receptor Activation Regulates Blue Light Exposure-Induced Mouse Corneal Epithelial Damage by Modulating Impaired Autophagy and Apoptosis
Abstract
:1. Introduction
2. Results
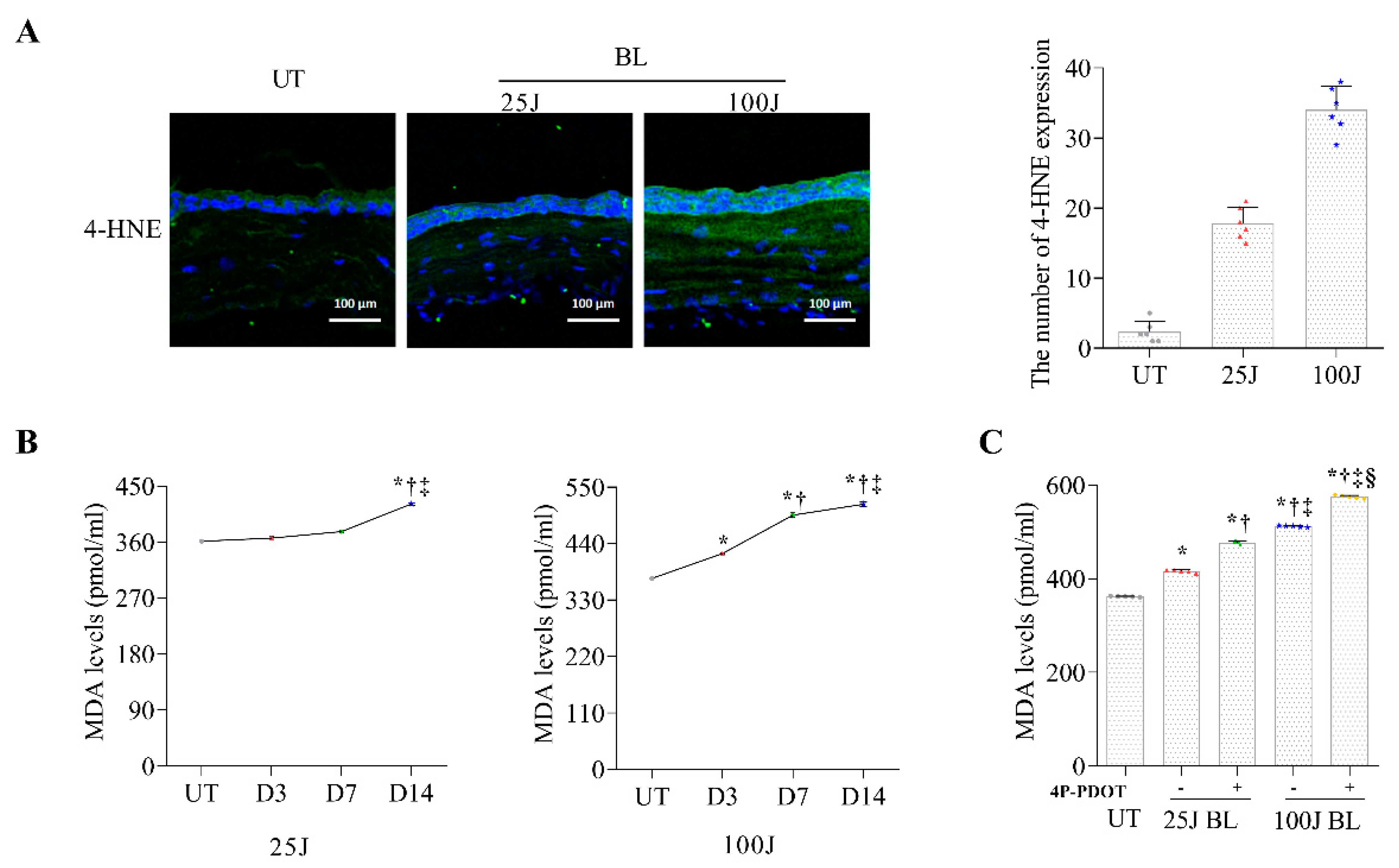
2.1. Oxidative Stress Levels in the Cornea Induced by BL Exposuree
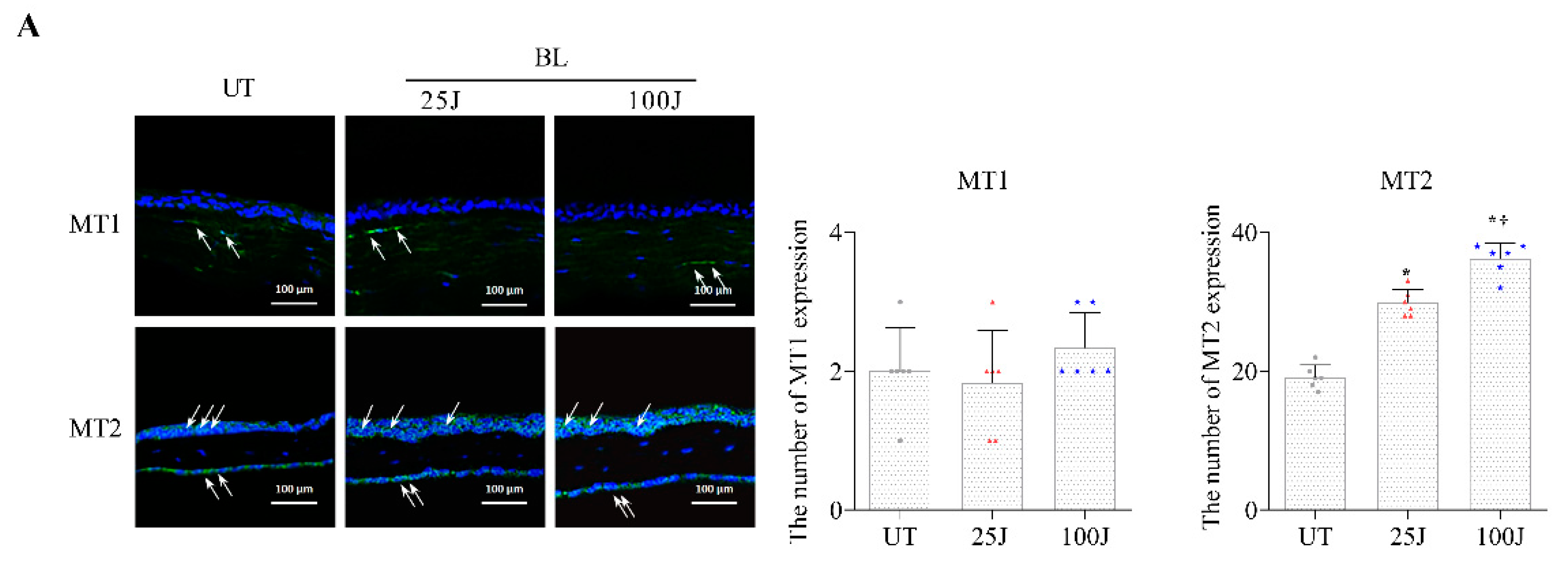
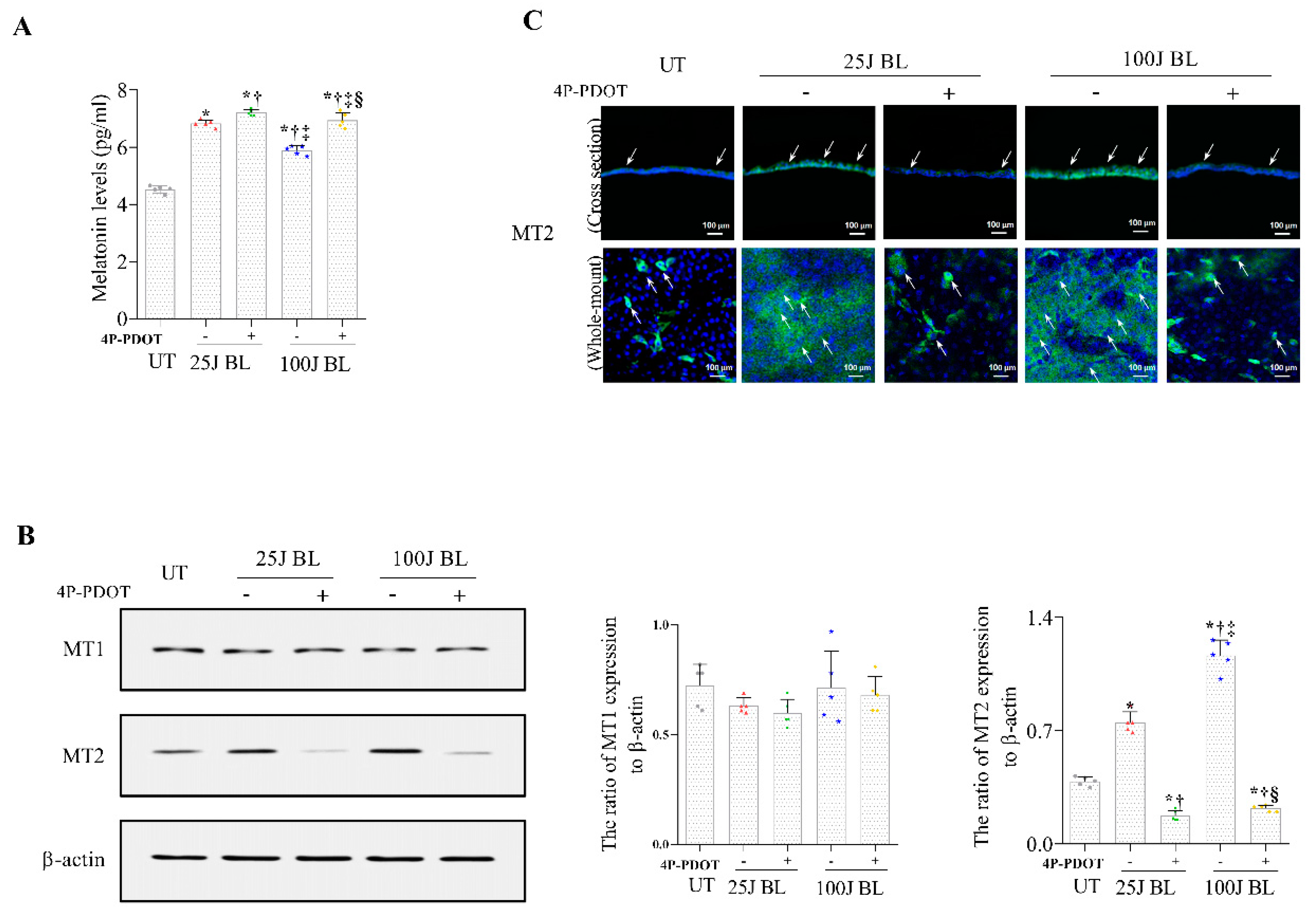
2.2. Melatonin Secretion and MT1/2 Activation in Corneal Tissues Due to BL Exposure
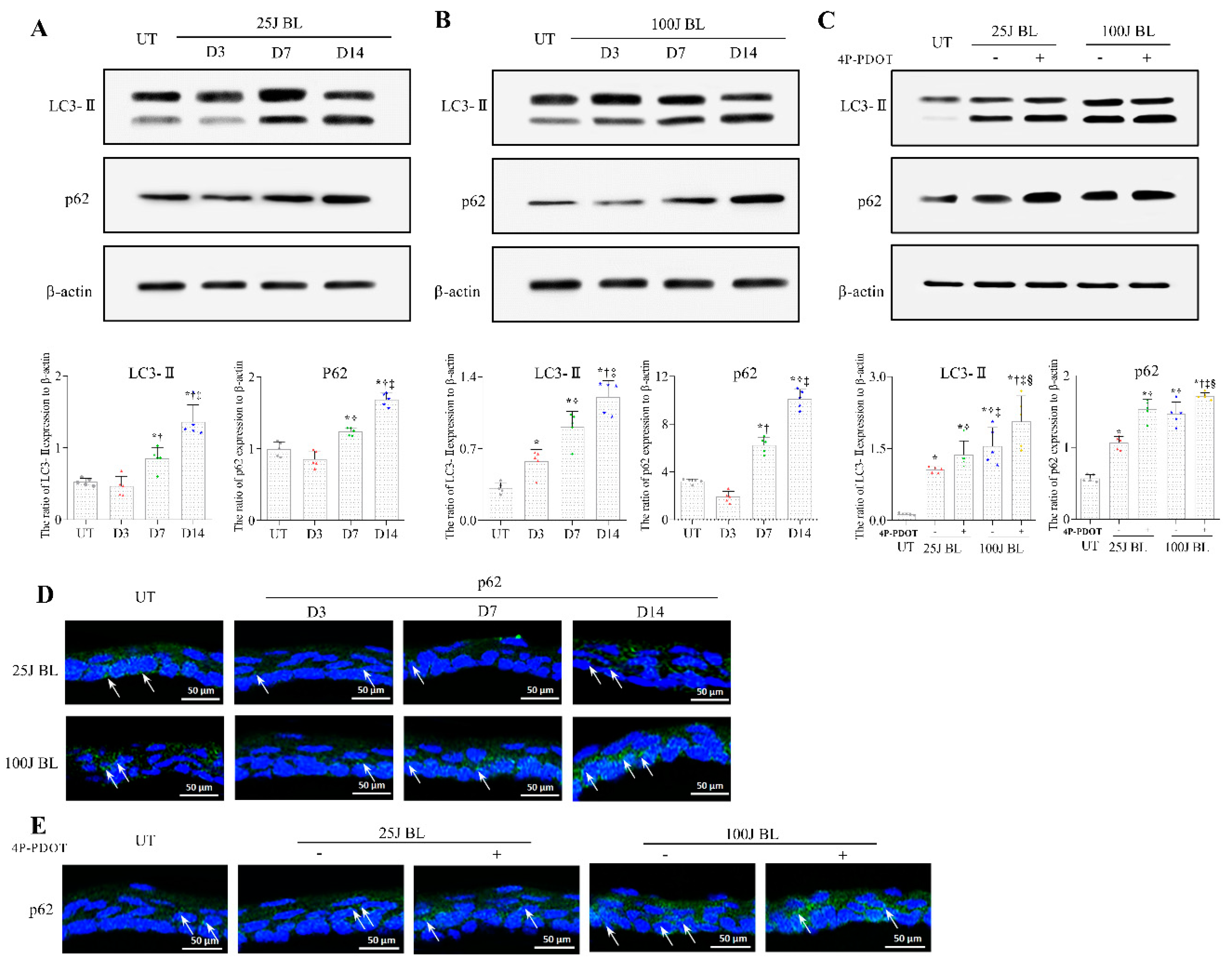
2.3. Autophagy Flux Defects in the Mouse Cornea Due to BL Exposure
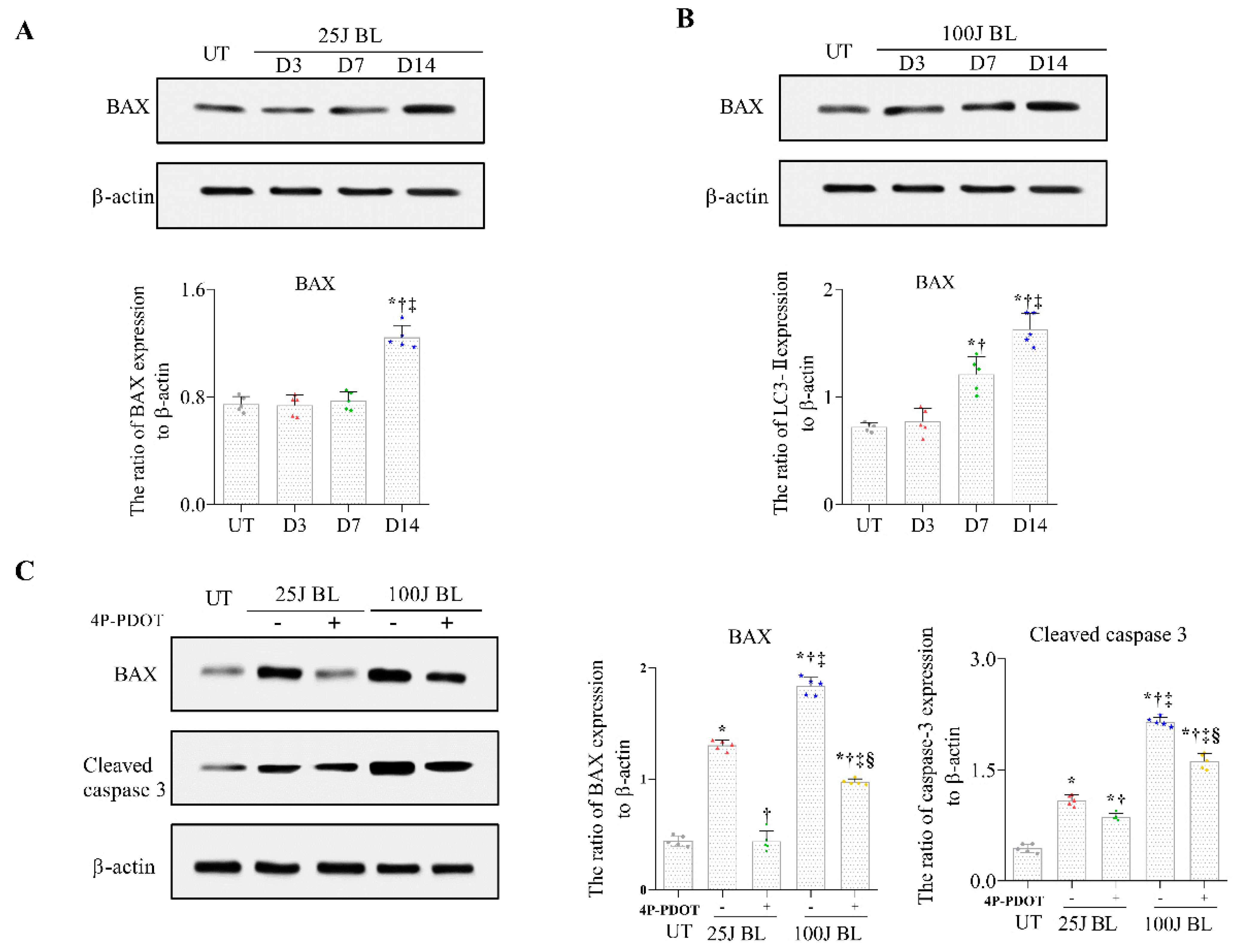
2.4. BAX and Cleaved Caspase 3 Expression in Corneal Tissue induced by BL Exposure
2.5. Corneal Epithelial Damage
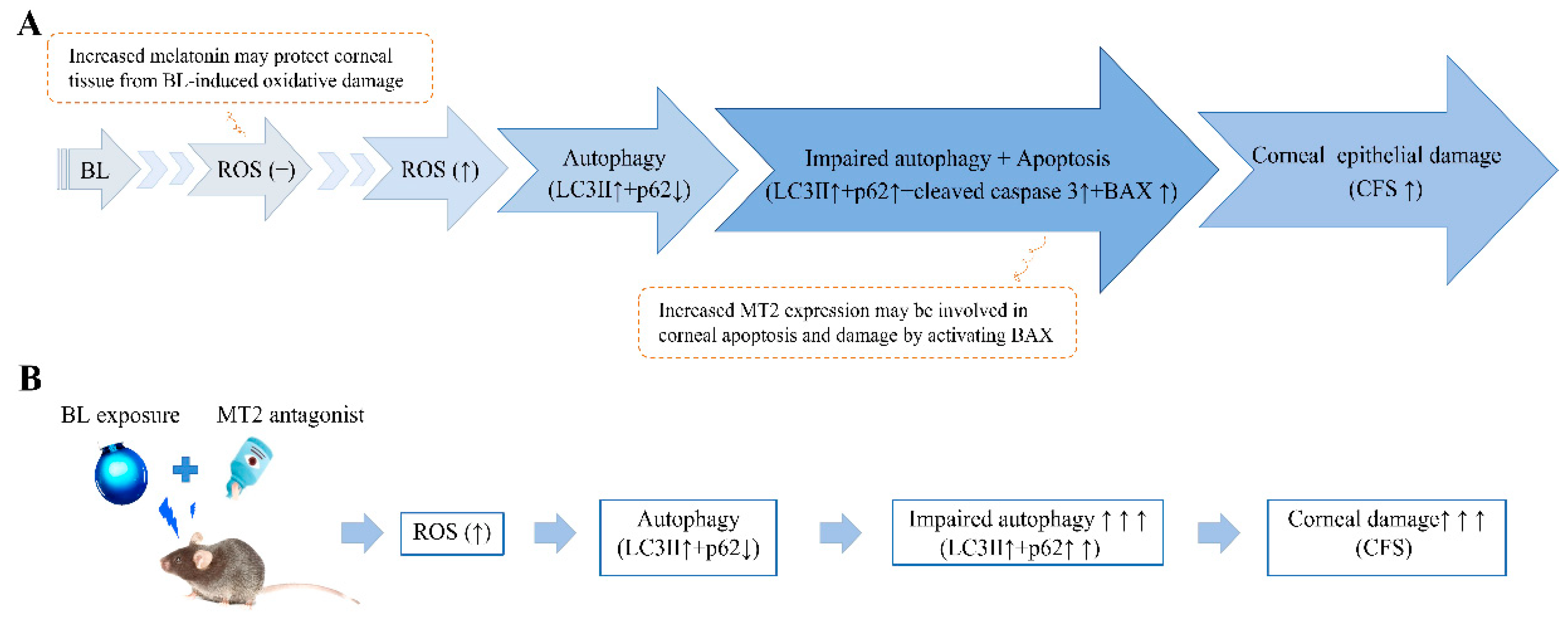
3. Discussion
4. Materials and Methods
4.1. Designs of Mouse Model and Experiments
4.2. ELISA
4.3. Immunohistochemistry
4.4. Western Blotting
4.5. Ocular Surface Parameters
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lavker, R.M.; Kaplan, N.; Wang, J.; Peng, H. Corneal epithelial biology: Lessons stemming from old to new. Exp. Eye Res. 2020, 198, 108094. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial stem cells-physiology, pathophysiology and therapeutic options. Cells 2021, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Cui, L.; Li, Y.; Choi, J.S.; Choi, J.H.; Li, Z.; Kim, G.E.; Choi, W.; Yoon, K.C. Influence of light emitting diode-derived blue light overexposure on mouse ocular surface. PLoS ONE 2016, 11, e0161041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, R.; Li, L.; Choi, J.S.; Kim, J.; Yoon, H.J.; Park, J.H.; Yoon, K.C. Blue light induces impaired autophagy through nucleotide-binding oligomerization domain 2 activation on the mouse ocular surface. Int. J. Mol. Sci. 2021, 22, 2015. [Google Scholar] [CrossRef]
- Choi, J.H.; Li, Y.; Kim, S.H.; Jin, R.; Kim, Y.H.; Choi, W.; You, I.C.; Yoon, K.C. The influences of smartphone use on the status of the tear film and ocular surface. PLoS ONE 2018, 13, e0206541. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar] [PubMed]
- Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-dose oral N-acetylcysteine in parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef]
- Dreaden Kasson, T.M.; Rexroth, S.; Barry, B.A. Light-induced oxidative stress, N-formylkynurenine, and oxygenic photosynthesis. PLoS ONE 2012, 7, e42220. [Google Scholar] [CrossRef]
- Ding, Y.; Aredo, B.; Zhong, X.; Zhao, C.X.; Ufret-Vincenty, R.L. Increased susceptibility to fundus camera-delivered light-induced retinal degeneration in mice deficient in oxidative stress response proteins. Exp. Eye Res. 2017, 159, 58–68. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, Y.; Zhu, X.; Wang, J.; Gao, F.; Yang, M.; Mao, L.; Zhang, Z.; Sun, B. Edaravone dexborneol treatment attenuates neuronal apoptosis and improves neurological function by suppressing 4-HNE-associated oxidative stress after subarachnoid hemorrhage. Front. Pharamacol. 2022, 13, 848529. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Suprachiasmatic nucleus and melatonin: Reciprocal interactions and clinical correlations. Neurology 2008, 71, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Cahill, G.M. A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J. Neurochem. 1999, 72, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Bahna, S.G.; Niles, L.P. Epigenetic regulation of melatonin receptors in neuropsychiatric disorders. Br. J. Pharmacol. 2018, 175, 3209–3219. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Baba, K.; Benleulmi-Chaachoua, A.; Journé, A.S.; Kamal, M.; Guillaume, J.L.; Dussaud, S.; Gbahou, F.; Yettou, K.; Liu, C.; Contreras-Alcantara, S.; et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci. Signal. 2013, 6, ra89. [Google Scholar] [CrossRef]
- Berbets, A.M.; Davydenko, I.S.; Barbe, A.M.; Konkov, D.H.; Albota, O.M.; Yuzko, O.M. Melatonin 1A and 1B receptors’ expression decreases in the placenta of women with fetal growth restriction. Reprod. Sci. 2021, 28, 197–206. [Google Scholar] [CrossRef]
- He, Y.M.; Deng, H.H.; Shi, M.H.; Bodinga, B.M.; Chen, H.L.; Han, Z.S.; Jiang, Z.L.; Li, Q.W. Melatonin modulates the functions of porcine granulosa cells via its membrane receptor MT2 in vitro. Anim. Reprod. Sci. 2016, 172, 164–172. [Google Scholar] [CrossRef]
- Espino, J.; Rodriguez, A.B.; Pariente, J.A. Melatonin and oxidative stress in the diabetic state: Clinical implications and potential therapeutic applications. Curr. Med. Chem. 2019, 26, 4178–4190. [Google Scholar] [CrossRef]
- Zhao, W.J.; Zhang, M.; Miao, Y.; Yang, X.L.; Wang, Z. Melatonin potentiates glycine currents through a PLC/PKC signalling pathway in rat retinal ganglion cells. J. Physiol. 2010, 588, 2605–2619. [Google Scholar] [CrossRef]
- Aierken, A.; Li, B.; Liu, P.; Cheng, X.; Kou, Z.; Tan, N.; Zhang, M.; Yu, S.; Shen, Q.; Du, X.; et al. Melatonin treatment improves human umbilical cord mesenchymal stem cell therapy in a mouse model of type II diabetes mellitus via the PI3K/AKT signaling pathway. Stem. Cell Res. Ther. 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin-mediated MT2 attenuates colitis induced by dextran sodium sulfate via PI3K/AKT/Nrf2/SIRT1/RORalpha/NF-kappaB signaling pathways. Int. Immunopharmacol. 2021, 96, 107779. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, H.J.; Jung, Y.H.; Kim, J.S.; Choi, S.H.; Han, H.J. Melatonin inhibits apoptotic cell death induced by Vibrio vulnificus VvhA via melatonin receptor 2 coupling with NCF-1. Cell Death Dis. 2018, 9, 48. [Google Scholar] [CrossRef]
- Wiechmann, A.F.; Wirsig-Wiechmann, C.R. Melatonin receptor mRNA and protein expression in Xenopus laevis nonpigmented ciliary epithelial cells. Exp. Eye Res. 2001, 73, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.Q.; Green, L.; Wang, C.; Alssadi, R.; Godley, B.F. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp. Eye Res. 2004, 78, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Maitra, S.K. Influence of altered photoperiods on serum melatonin and its receptors (MT1 and MT2) in the brain, retina, and ovary in carp Catla catla. Chronobiol. Int. 2012, 29, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Crooke, A.; Guzman-Aranguez, A.; Mediero, A.; Alarma-Estrany, P.; Carracedo, G.; Pelaez, T.; Peral, A.; Pintor, J. Effect of melatonin and analogues on corneal wound healing: Involvement of Mt2 melatonin receptor. Curr. Eye Res. 2015, 40, 56–65. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Kageyama, S.; Gudmundsson, S.R.; Sou, Y.S.; Ichimura, Y.; Tamura, N.; Kazuno, S.; Ueno, T.; Miura, Y.; Noshiro, D.; Abe, M.; et al. p62/SQSTM1-droplet serves as a platform for autophagosome formation and anti-oxidative stress response. Nat. Commun. 2021, 12, 16. [Google Scholar] [CrossRef]
- Katsuragi, Y.; Ichimura, Y.; Komatsu, M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015, 282, 4672–4678. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective autophagy receptor p62/SQSTM1, a pivotal player in stress and aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef] [PubMed]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Yapislar, H.; Haciosmanoglu, E.; Sarioglu, T.; Ekmekcioglu, C. The melatonin MT2 receptor is involved in the anti-apoptotic effects of melatonin in rats with type 2 diabetes mellitus. Tissue Cell 2022, 76, 101763. [Google Scholar] [CrossRef]
- Hutchinson, A.J.; Ma, J.; Liu, J.; Hudson, R.L.; Dubocovich, M.L. Role of MT1 melatonin receptors in methamphetamine-induced locomotor sensitization in C57BL/6 mice. Psychopharmacology 2014, 231, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.M.; Khan, S.; Chaudhury, N.K. Radiomitigation by Melatonin in C57BL/6 Mice: Possible Implications as Adjuvant in Radiotherapy and Chemotherapy. Int. J. Clin. Pediatr. Den. 2022, 36, 1203–1221. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Belagodu, A.; Reiter, R.J.; Ray, S.K.; Banik, N.L. Cytoprotective effects of melatonin on C6 astroglial cells exposed to glutamate excitotoxicity and oxidative stress. J. Pineal Res. 2008, 45, 117–124. [Google Scholar] [CrossRef]
- Bantseev, V.; McCanna, D.J.; Driot, J.Y.; Sivak, J.G. The effects of toxicological agents on the optics and mitochondria of the lens and the mitochondria of the corneal epithelium. Semin. Cell Dev. Biol. 2008, 19, 150–159. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Rogov, V.; Dötsch, V.; Johansen, T.; Kirkin, V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell. 2014, 53, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011, 7, 279–296. [Google Scholar] [CrossRef]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Murrow, L.; Debnath, J. Autophagy as a stress-response and quality-control mechanism: Implications for cell injury and human disease. Annu. Rev. Pathol. 2013, 8, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef]
- Robertson, D.M.; Ladage, P.M.; Yamamoto, N.; Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Bcl-2 and Bax regulation of corneal homeostasis in genetically altered mice. Eye Contact Lens. 2006, 32, 3–7. [Google Scholar] [CrossRef]
- Radogna, F.; Albertini, M.C.; De Nicola, M.; Diederich, M.; Bejarano, I.; Ghibelli, L. Melatonin promotes Bax sequestration to mitochondria reducing cell susceptibility to apoptosis via the lipoxygenase metabolite 5-hydroxyeicosatetraenoic acid. Mitochondrion 2015, 21, 113–121. [Google Scholar] [CrossRef]
- Radogna, F.; Cristofanon, S.; Paternoster, L.; D’Alessio, M.; De Nicola, M.; Cerella, C.; Dicato, M.; Diederich, M. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J. Pineal Res. 2008, 44, 316–325. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.; Wen, A.; Yang, B.; Pang, X. Luzindole and 4P-PDOT block the effect of melatonin on bovine granulosa cell apoptosis and cell cycle depending on its concentration. PeerJ 2021, 9, e10627. [Google Scholar] [CrossRef]
- Oshima, Y.; Fujio, Y.; Nakanishi, T.; Itoh, N.; Yamamoto, Y.; Negoro, S.; Tanaka, K.; Kishimoto, T.; Kawase, I.; Azuma, J. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc. Res. 2005, 65, 428–435. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Li, Y.; Jin, H.; Yoon, H.S.; Choi, J.S.; Kim, J.; Yoon, H.J.; Yoon, K.C. Melatonin Type 2 Receptor Activation Regulates Blue Light Exposure-Induced Mouse Corneal Epithelial Damage by Modulating Impaired Autophagy and Apoptosis. Int. J. Mol. Sci. 2022, 23, 11341. https://doi.org/10.3390/ijms231911341
Jin R, Li Y, Jin H, Yoon HS, Choi JS, Kim J, Yoon HJ, Yoon KC. Melatonin Type 2 Receptor Activation Regulates Blue Light Exposure-Induced Mouse Corneal Epithelial Damage by Modulating Impaired Autophagy and Apoptosis. International Journal of Molecular Sciences. 2022; 23(19):11341. https://doi.org/10.3390/ijms231911341
Chicago/Turabian StyleJin, Rujun, Ying Li, Hui Jin, Hee Su Yoon, Ji Suk Choi, Jonghwa Kim, Hyeon Jeong Yoon, and Kyung Chul Yoon. 2022. "Melatonin Type 2 Receptor Activation Regulates Blue Light Exposure-Induced Mouse Corneal Epithelial Damage by Modulating Impaired Autophagy and Apoptosis" International Journal of Molecular Sciences 23, no. 19: 11341. https://doi.org/10.3390/ijms231911341




