A Tissue Engineering Acoustophoretic (TEA) Set-up for the Enhanced Osteogenic Differentiation of Murine Mesenchymal Stromal Cells (mMSCs)
Abstract
1. Introduction
2. Results
2.1. Speed of Sound (SOS) Measurement
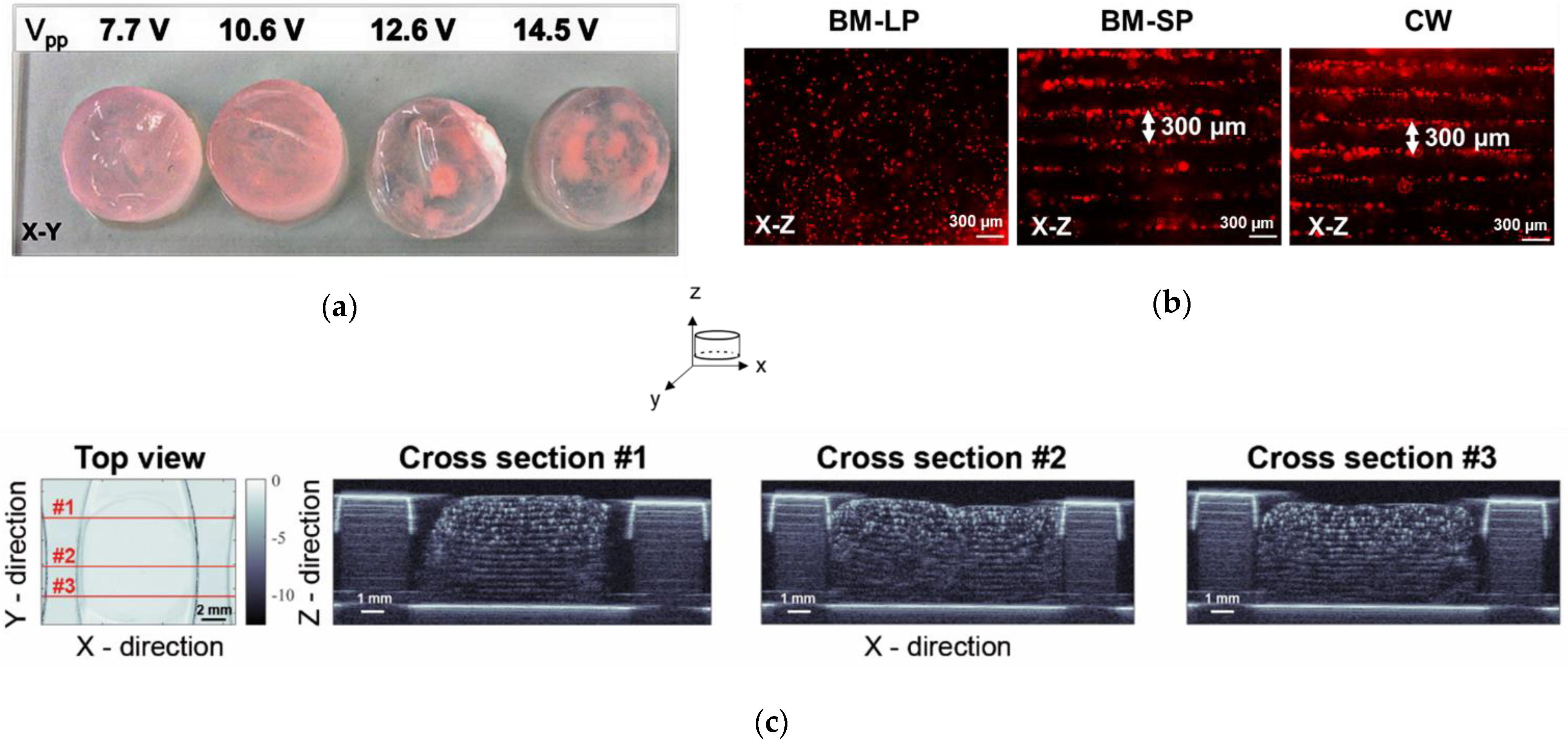
2.2. Ultrasound Wave Mode and Transmission Voltage in the TEA Set-Up
2.3. Cell Patterning in 3-D Fibrin Hydrogels
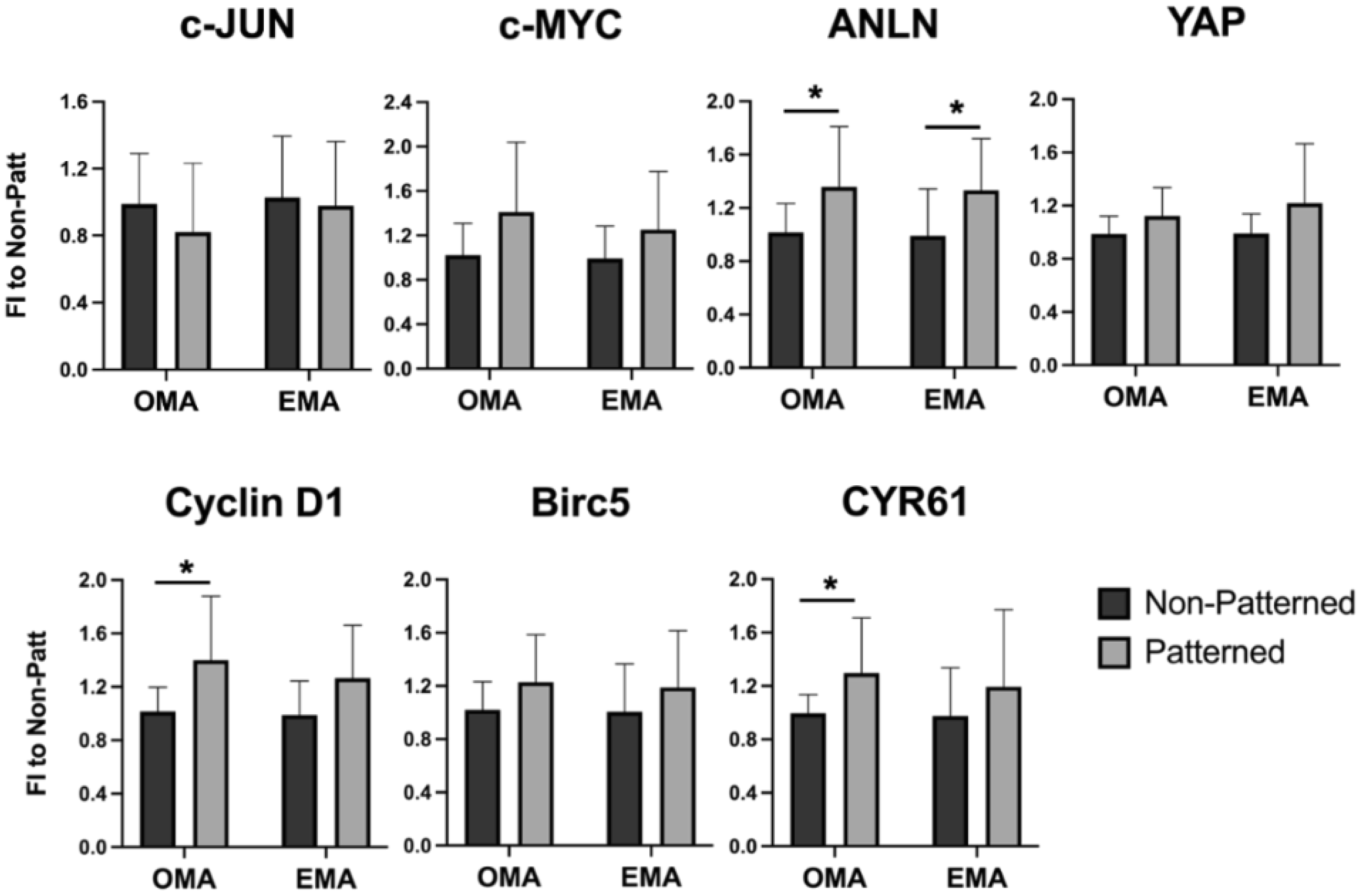
2.4. Impact of Patterning on Cell Behavior
2.5. Gene Expression in the Patterned Fibrin Constructs in Media with and without Osteogenic Supplements
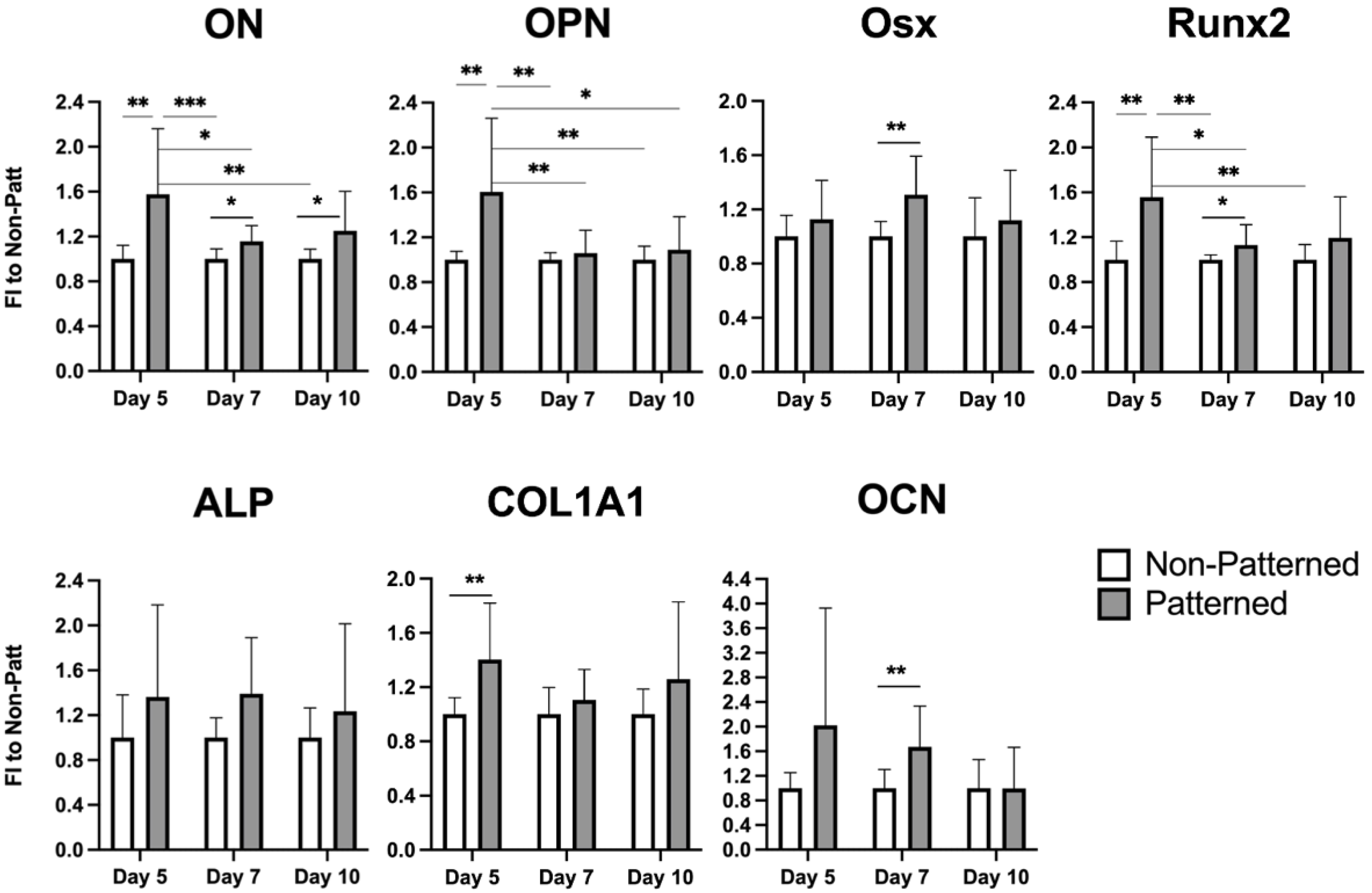
2.6. Expression of Osteogenic Genes in the Patterned Fibrin Constructs Cultured for 10 Days in Osteoinductive Media
3. Discussion
4. Materials and Methods
4.1. Tissue Engineering Acoustophoretic (TEA) Set-Up
4.2. Fibrin Hydrogel Preparation
4.3. Measurement of Transmission Voltage
4.4. Speed of Sound Measurement
4.5. Cell Culture
4.6. Construct Culturing in Expansion and Osteogenic Media
4.7. Cell Viability Assay
4.8. Fluorescent Staining
4.9. Scanning Acoustic Microscopy (SAM)
4.10. Quantitative Real-Time Polymerase Chain Reaction Assay (qRT-PCR)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Avila, H.M.; Hagg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L. Bottom-up tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Guillemot, F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011, 29, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Tiruvannamalai-Annamalai, R.; Armant, D.R.; Matthew, H.W.T. A Glycosaminoglycan Based, Modular Tissue Scaffold System for Rapid Assembly of Perfusable, High Cell Density, Engineered Tissues. PLoS ONE 2014, 9, e84287. [Google Scholar] [CrossRef]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Kollmannsberger, P.; Bidan, C.M.; Dunlop, J.W.C.; Fratzl, P.; Vogel, V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci. Adv. 2018, 4, eaao4881. [Google Scholar] [CrossRef]
- Bidan, C.M.; Kollmannsberger, P.; Gering, V.; Ehrig, S.; Joly, P.; Petersen, A.; Vogel, V.; Fratzl, P.; Dunlop, J.W. Gradual conversion of cellular stress patterns into pre-stressed matrix architecture during in vitro tissue growth. J. R. Soc. Interface 2016, 13, 20160136. [Google Scholar] [CrossRef]
- Bankoff, A.D.P. Biomechanical Characteristics of the Bone; InTech: London, UK, 2012. [Google Scholar]
- Wang, H.; Abhilash, A.S.; Chen, C.S.; Wells, R.G.; Shenoy, V.B. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys. J. 2014, 107, 2592–2603. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kikuchi, A.; Aoyagi, T.; Okano, T. Cell sheet tissue engineering: Cell sheet preparation, harvesting/manipulation, and transplantation. J. Biomed. Mate.r Res. A 2019, 107, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, W.; Wang, J.; Yang, G.; Yin, S.; Tang, T.; Yu, C.; Jiang, X. Recent advances in cell sheet technology for bone and cartilage regeneration: From preparation to application. Int. J. Oral Sci. 2019, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2020, 11 (Suppl. S1), S118–S124. [Google Scholar] [CrossRef] [PubMed]
- Zaszczynska, A.; Moczulska-Heljak, M.; Gradys, A.; Sajkiewicz, P. Advances in 3D Printing for Tissue Engineering. Materials 2021, 14, 3149. [Google Scholar] [CrossRef] [PubMed]
- Goranov, V.; Shelyakova, T.; De Santis, R.; Haranava, Y.; Makhaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Peluso, V.; Rinaldi, L.; Russo, T.; Oliviero, O.; Di Vito, A.; Garbi, C.; Giudice, A.; De Santis, R.; Gloria, A.; D’Anto, V. Impact of Magnetic Stimulation on Periodontal Ligament Stem Cells. Int. J. Mol. Sci. 2021, 23, 188. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.; Bhavsar, M.; Han, Z.; Barker, J.H. Effects of Electrical Stimulation on Stem Cells. Curr. Stem Cell Res. Ther. 2020, 15, 441–448. [Google Scholar] [CrossRef]
- Puts, R.; Vico, R.; Beilfuss, N.; Shaka, M.; Padilla, F.; Raum, K. Pulsed ultrasound for bone regeneration-outcomes and hurdles in the clinical application: A systematic review. Eur. Cells Mater. 2021, 42, 281–311. [Google Scholar] [CrossRef]
- Dalecki, D.; Hocking, D.C. Ultrasound technologies for biomaterials fabrication and imaging. Ann. Biomed. Eng. 2015, 43, 747–761. [Google Scholar] [CrossRef]
- Crasto, G.J.; Kartner, N.; Reznik, N.; Spatafora, M.V.; Chen, H.; Williams, R.; Burns, P.N.; Clokie, C.; Manolson, M.F.; Peel, S.A. Controlled bone formation using ultrasound-triggered release of BMP-2 from liposomes. J. Control. Release 2016, 243, 99–108. [Google Scholar] [CrossRef]
- Zamuner, A.; Cavo, M.; Scaglione, S.; Messina, G.M.L.; Russo, T.; Gloria, A.; Marletta, G.; Dettin, M. Design of Decorated Self-Assembling Peptide Hydrogels as Architecture for Mesenchymal Stem Cells. Materials 2016, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Petersson, F.; Aberg, L.; Sward-Nilsson, A.M.; Laurell, T. Free flow acoustophoresis: Microfluidic-based mode of particle and cell separation. Anal. Chem. 2007, 79, 5117–5123. [Google Scholar] [CrossRef] [PubMed]
- Garvin, K.A.; Hocking, D.C.; Dalecki, D. Controlling the spatial organization of cells and extracellular matrix proteins in engineered tissues using ultrasound standing wave fields. Ultrasound Med. Biol. 2010, 36, 1919–1932. [Google Scholar] [CrossRef]
- Garvin, K.A.; Dalecki, D.; Yousefhussien, M.; Helguera, M.; Hocking, D.C. Spatial patterning of endothelial cells and vascular network formation using ultrasound standing wave fields. J. Acoust. Soc. Am. 2013, 134, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Garvin, K.A.; Dalecki, D.; Hocking, D.C. Vascularization of three-dimensional collagen hydrogels using ultrasound standing wave fields. Ultrasound Med. Biol. 2011, 37, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, C.; Chen, P.; Guven, S.; Demirtas, T.T.; Nieland, T.J.; Padilla, F.; Demirci, U. A Bio-Acoustic Levitational (BAL) Assembly Method for Engineering of Multilayered, 3D Brain-Like Constructs, Using Human Embryonic Stem Cell Derived Neuro-Progenitors. Adv. Mater. 2016, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tait, A.; Glynne-Jones, P.; Hill, A.R.; Smart, D.E.; Blume, C.; Hammarstrom, B.; Fisher, A.L.; Grossel, M.C.; Swindle, E.J.; Hill, M.; et al. Engineering multi-layered tissue constructs using acoustic levitation. Sci. Rep. 2019, 9, 9789. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef]
- Bilaniuk, N.; Wong, G.S.K. Speed of sound in pure water as a function of temperature. J. Acoust. Soc. Am. 1993, 93, 1609–1612. [Google Scholar] [CrossRef]
- Kulterer, B.; Friedl, G.; Jandrositz, A.; Sanchez-Cabo, F.; Prokesch, A.; Paar, C.; Scheideler, M.; Windhager, R.; Preisegger, K.H.; Trajanoski, Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genom. 2007, 8, 70. [Google Scholar] [CrossRef]
- Infante, A.; Rodriguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Karsenty, G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52–54, 78–87. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Khanna, A.; Nelmes, R.T.; Gougoulias, N.; Maffulli, N.; Gray, J. The effects of LIPUS on soft-tissue healing: A review of literature. Br. Med. Bull. 2009, 89, 169–182. [Google Scholar] [CrossRef]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef]
- Shi, M.; Liu, B.; Liu, G.; Wang, P.; Yang, M.; Li, Y.; Zhou, J. Low intensity-pulsed ultrasound induced apoptosis of human hepatocellular carcinoma cells in vitro. Ultrasonics 2016, 64, 43–53. [Google Scholar] [CrossRef]
- Sheehan, K.; Sheehan, D.; Sulaiman, M.; Padilla, F.; Moore, D.; Sheehan, J.; Xu, Z. Investigation of the tumoricidal effects of sonodynamic therapy in malignant glioblastoma brain tumors. J. Neuro-Oncol. 2020, 148, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Chen, C.S. Cell-cell signaling by direct contact increases cell proliferation via a PI3K-dependent signal. FEBS Lett. 2002, 514, 238–242. [Google Scholar] [CrossRef]
- Gumbiner, B.M.; Kim, N.G. The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 2014, 127 Pt 4, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. A revisited concept: Contact inhibition of growth. From cell biology to malignancy. Exp. Cell Res. 2017, 359, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, Y.; Chen, D. Biological functions and role of CCN1/Cyr61 in embryogenesis and tumorigenesis in the female reproductive system (Review). Mol. Med. Rep. 2018, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Johnson, J.M.; Lera, R.F.; Brahma, S.; Burkard, M.E. Anillin Phosphorylation Controls Timely Membrane Association and Successful Cytokinesis. PLoS Genet. 2017, 13, e1006511. [Google Scholar] [CrossRef]
- Chen, G.; Xu, R.; Zhang, C.; Lv, Y. Responses of MSCs to 3D Scaffold Matrix Mechanical Properties under Oscillatory Perfusion Culture. ACS Appl. Mater. Interfaces 2017, 9, 1207–1218. [Google Scholar] [CrossRef]
- Provin, C.; Takano, K.; Sakai, Y.; Fujii, T.; Shirakashi, R. A method for the design of 3D scaffolds for high-density cell attachment and determination of optimum perfusion culture conditions. J. Biomech. 2008, 41, 1436–1449. [Google Scholar] [CrossRef]
- Yamada, S.; Yassin, M.A.; Schwarz, T.; Mustafa, K.; Hansmann, J. Optimization and Validation of a Custom-Designed Perfusion Bioreactor for Bone Tissue Engineering: Flow Assessment and Optimal Culture Environmental Conditions. Front. Bioeng. Biotechnol. 2022, 10, 811942. [Google Scholar] [CrossRef]
- Padilla, F.; Puts, R.; Vico, L.; Guignandon, A.; Raum, K. Stimulation of Bone Repair with Ultrasound. Adv. Exp. Med. Biol. 2016, 880, 385–427. [Google Scholar] [PubMed]
- Puts, R.; Rikeit, P.; Ruschke, K.; Knaus, P.; Schreivogel, S.; Raum, K. Functional regulation of YAP mechanosensitive transcriptional coactivator by Focused Low-Intensity Pulsed Ultrasound (FLIPUS) enhances proliferation of murine mesenchymal precursors. PLoS ONE 2018, 13, e0206041. [Google Scholar] [CrossRef] [PubMed]
- Puts, R.; Rikeit, P.; Ruschke, K.; Kadow-Romacker, A.; Hwang, S.; Jenderka, K.V.; Knaus, P.; Raum, K. Activation of Mechanosensitive Transcription Factors in Murine C2C12 Mesenchymal Precursors by Focused Low-Intensity Pulsed Ultrasound (FLIPUS). IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Carina, V.; Costa, V.; Raimondi, L.; Pagani, S.; Sartori, M.; Figallo, E.; Setti, S.; Alessandro, R.; Fini, M.; Giavaresi, G. Effect of low-intensity pulsed ultrasound on osteogenic human mesenchymal stem cells commitment in a new bone scaffold. J. Appl. Biomater. Funct. Mater. 2017, 15, e215–e222. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, X.; Cao, H.; Qin, L.; Hou, W.; Wu, L. A Comparison of 1- and 3.2-MHz Low-Intensity Pulsed Ultrasound on Osteogenesis on Porous Titanium Alloy Scaffolds: An In Vitro and In Vivo Study. J. Ultrasound Med. 2019, 38, 191–202. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, Y.; Wang, H.; Cao, Q.; Yang, Y.; Zhou, Y.; Tan, T.; Huang, X.; Zhou, Q. Low-intensity pulsed ultrasound promotes cell viability and inhibits apoptosis of H9C2 cardiomyocytes in 3D bioprinting scaffolds via PI3K-Akt and ERK1/2 pathways. J. Biomater. Appl. 2022, 37, 8853282221102669. [Google Scholar] [CrossRef]
- Zhou, X.; Castro, N.J.; Zhu, W.; Cui, H.; Aliabouzar, M.; Sarkar, K.; Zhang, L.G. Improved Human Bone Marrow Mesenchymal Stem Cell Osteogenesis in 3D Bioprinted Tissue Scaffolds with Low Intensity Pulsed Ultrasound Stimulation. Sci. Rep. 2016, 6, 32876. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- McBride, S.H.; Falls, T.; Tate, M.L.K. Modulation of stem cell shape and fate B: Mechanical modulation of cell shape and gene expression. Tissue Eng. Part A 2008, 14, 1573–1580. [Google Scholar] [CrossRef]
- McBride, S.H.; Tate, M.L.K. Modulation of stem cell shape and fate A: The role of density and seeding protocol on nucleus shape and gene expression. Tissue Eng. Part A 2008, 14, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.; Nino, J.L.G.; Graney, P.; Razal, J.M.; Minett, A.I.; Ribas, J.; Ovalle-Robles, R.; Biro, M.; Zreiqat, H. Relationship between nanotopographical alignment and stem cell fate with live imaging and shape analysis. Sci. Rep. 2016, 6, 37909. [Google Scholar] [CrossRef] [PubMed]
- Namgung, S.; Baik, K.Y.; Park, J.; Hong, S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano 2011, 5, 7383–7390. [Google Scholar] [CrossRef] [PubMed]
- Tapscott, S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 2005, 132, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; d’Aquino, R.; Cusella-De Angelis, M.G.; Laino, G.; Piattelli, A.; Pacifici, M.; De Rosa, A.; Papaccio, G. Concave pit-containing scaffold surfaces improve stem cell-derived osteoblast performance and lead to significant bone tissue formation. PLoS ONE 2007, 2, e496. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Liu, Y.S.; Liu, Y.A.; Wu, Y.C.; Del Alamo, J.C.; Chiou, A.; Lee, O.K. Bio-chemical and physical characterizations of mesenchymal stromal cells along the time course of directed differentiation. Sci. Rep. 2016, 6, 31547. [Google Scholar] [CrossRef]
- Chansoria, P.; Narayanan, L.K.; Schuchard, K.; Shirwaiker, R. Ultrasound-assisted biofabrication and bioprinting of preferentially aligned three-dimensional cellular constructs. Biofabrication 2019, 11, 035015. [Google Scholar] [CrossRef]
- Chansoria, P.; Shirwaiker, R. Characterizing the Process Physics of Ultrasound-Assisted Bioprinting. Sci. Rep. 2019, 9, 13889. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Nowicki, M.; Zhou, X.; Khademhosseini, A.; Zhang, L.G. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs via Dual 3D Bioprinting: Integrating Regional Bioactive Factors into Architectural Design. Adv. Healthc. Mater. 2016, 5, 2174–2181. [Google Scholar] [CrossRef]
- Flegeau, K.; Toquet, C.; Rethore, G.; d’Arros, C.; Messager, L.; Halgand, B.; Dupont, D.; Autrusseau, F.; Lesoeur, J.; Veziers, J.; et al. In Situ Forming, Silanized Hyaluronic Acid Hydrogels with Fine Control Over Mechanical Properties and In Vivo Degradation for Tissue Engineering Applications. Adv. Healthc. Mater. 2020, 9, e2000981. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Sannachi, L.; Koch, T.; Brand, S.; Maennicke, N.; Wicke, M.; Moerlein, D.; Raum, K. Prediction of the intramuscular fat content in loin muscle of pig carcasses by quantitative time-resolved ultrasound. Meat Sci. 2012, 90, 216–225. [Google Scholar]
- Maennicke, N.; Schoene, M.; Gottwald, M.; Goebel, F.; Oelze, M.; Raum, K. 3-D high-frequency ultrasound backscatter analysis of human articular cartilage. Ultrasound Med. Biol. 2013, 40, 244–257. [Google Scholar] [CrossRef] [PubMed]





| H2O (dd) | Fibrinogen | Fibrinogen with Cells | Fibrinogen with Beads | |
|---|---|---|---|---|
| Mean, m/s | 1489.9 | 1498.3 *** | 1497.9 *** | 1501.0 *** |
| SD | 0.8 | 0.5 | 0.7 | 1.7 |
| Gene | Forward | Reverse |
|---|---|---|
| Cyclin D1 | 5′–CGTGGCCTCTAAGATGAAGG–3′ | 5′–CCACTTGAGCTTGTTCACCA–3′ |
| BIRC5 | 5′–CATCGCCACCTTCAAGAACT–3′ | 5′–AAAACACTGGGCCAAATCAG–3′ |
| CYR61 | 5′–TGCTGTAAGGTCTGCGCTAA–3′ | 5′–AGGGTCTGCCTTCTGACTGA–3′ |
| YAP | 5′–AAGGAGAGACTGCGGTTGAA–3′ | 5′–CCTGAGACATCCCAGGAGAA–3′ |
| ANLN | 5′–TCAATAGCAGCAGTGTTCAGC–3′ | 5′–GATTTTGTGCCTCACGGTTT–3′ |
| c-MYC | 5′–GCTGTTTGAAGGCTGGATTT–3′ | 5′–CTCTGCTGTTGCTGGTGATAG–3′ |
| c-JUN | 5′–TGTTTGTTTGTTTGGGTGTCC–3′ | 5′–GAGGTTGGGGGCTACTTTTC–3′ |
| OPN | 5′–GCTTGGCTTATGGACTGAGG–3′ | 5′–GGGATGACATCGAGGGACT–3′ |
| OCN | 5′–CGCTCTGTCTCTCTGACCTC–3′ | 5′–GACTGAGGCTCCAAGGTAGC–3′ |
| ON | 5′–ACATTGCACCACACGTTTC–3′ | 5′–GGGACACATCAGAGGGAGAG–3′ |
| Osx | 5′–CCCTTCTCAAGCACCAATGG–3′ | 5′–AGGGTGGGTAGTCATTTGCATAG–3′ |
| RUNX2 | 5′–TAAGAAGAGCCAGGCAGGTG–3′ | 5′–TAGTGCATTCGTGGGTTGG–3′ |
| ALP | 5′–AACCCAGACACAAGCATTCC–3′ | 5′–GAGAGCGAAGGGTCAGTCAG–3′ |
| COL1A1 | 5′–GCCTCCCAGAACATCACCTA–3′ | 5′–GACTGTCTTGCCCCAAGTTC–3′ |
| GAPDH | 5′–TGCACCACCAACTGCTTAG–3′ | 5′–GAGGCAGGGATGATGTTC–3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Beilfuss, N.; Zabarylo, U.; Raum, K.; Puts, R. A Tissue Engineering Acoustophoretic (TEA) Set-up for the Enhanced Osteogenic Differentiation of Murine Mesenchymal Stromal Cells (mMSCs). Int. J. Mol. Sci. 2022, 23, 11473. https://doi.org/10.3390/ijms231911473
Zhang H, Beilfuss N, Zabarylo U, Raum K, Puts R. A Tissue Engineering Acoustophoretic (TEA) Set-up for the Enhanced Osteogenic Differentiation of Murine Mesenchymal Stromal Cells (mMSCs). International Journal of Molecular Sciences. 2022; 23(19):11473. https://doi.org/10.3390/ijms231911473
Chicago/Turabian StyleZhang, Hui, Nirina Beilfuss, Urszula Zabarylo, Kay Raum, and Regina Puts. 2022. "A Tissue Engineering Acoustophoretic (TEA) Set-up for the Enhanced Osteogenic Differentiation of Murine Mesenchymal Stromal Cells (mMSCs)" International Journal of Molecular Sciences 23, no. 19: 11473. https://doi.org/10.3390/ijms231911473
APA StyleZhang, H., Beilfuss, N., Zabarylo, U., Raum, K., & Puts, R. (2022). A Tissue Engineering Acoustophoretic (TEA) Set-up for the Enhanced Osteogenic Differentiation of Murine Mesenchymal Stromal Cells (mMSCs). International Journal of Molecular Sciences, 23(19), 11473. https://doi.org/10.3390/ijms231911473






