α-Gal Nanoparticles Mediated Homing of Endogenous Stem Cells for Repair and Regeneration of External and Internal Injuries by Localized Complement Activation and Macrophage Recruitment
Abstract
1. Introduction
2. Anti-Gal and the α-Gal Nanoparticles
3. In Situ Effects of α-Gal Nanoparticles
3.1. Hypothesis
3.2. Experimental Animal Models
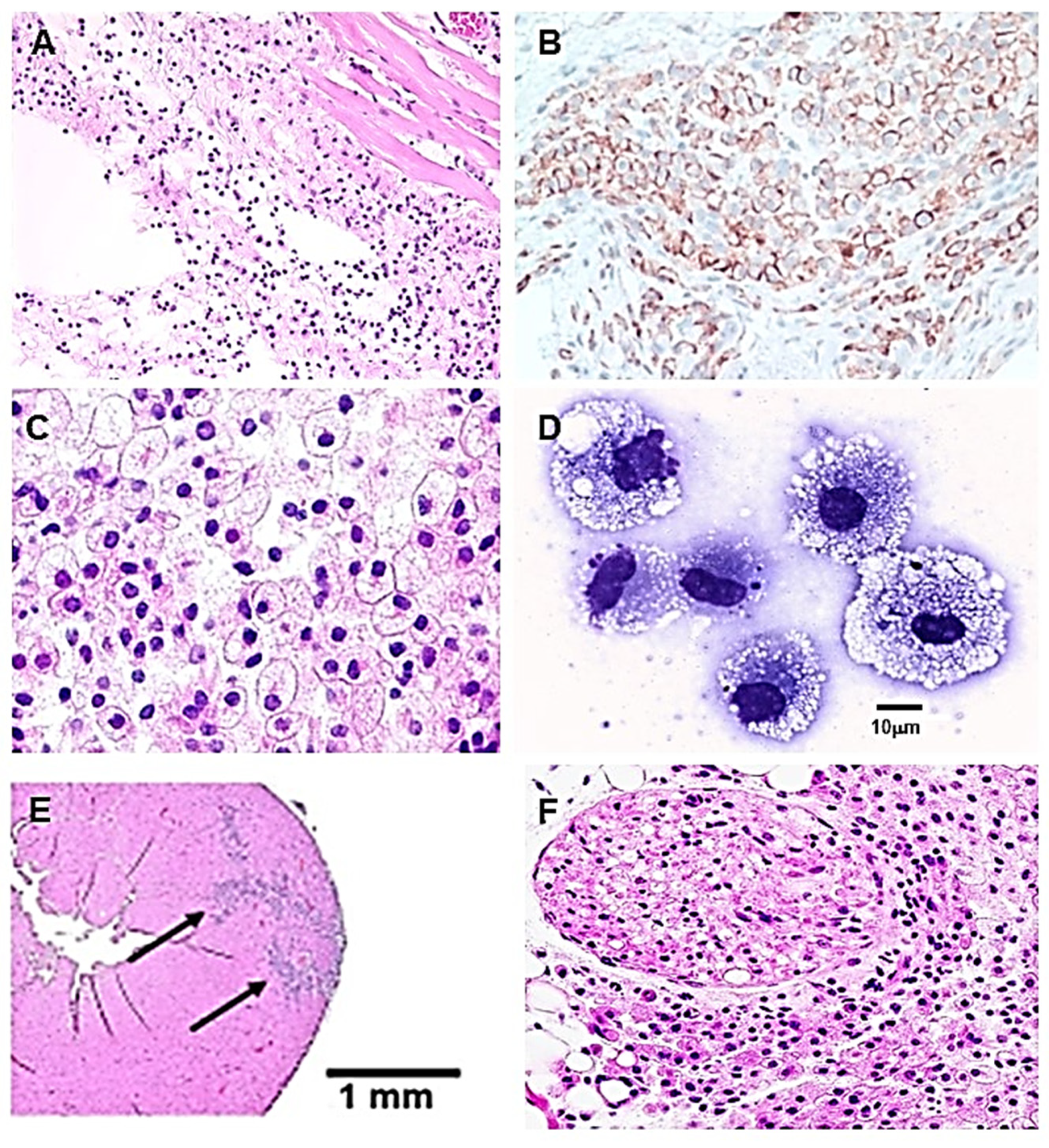
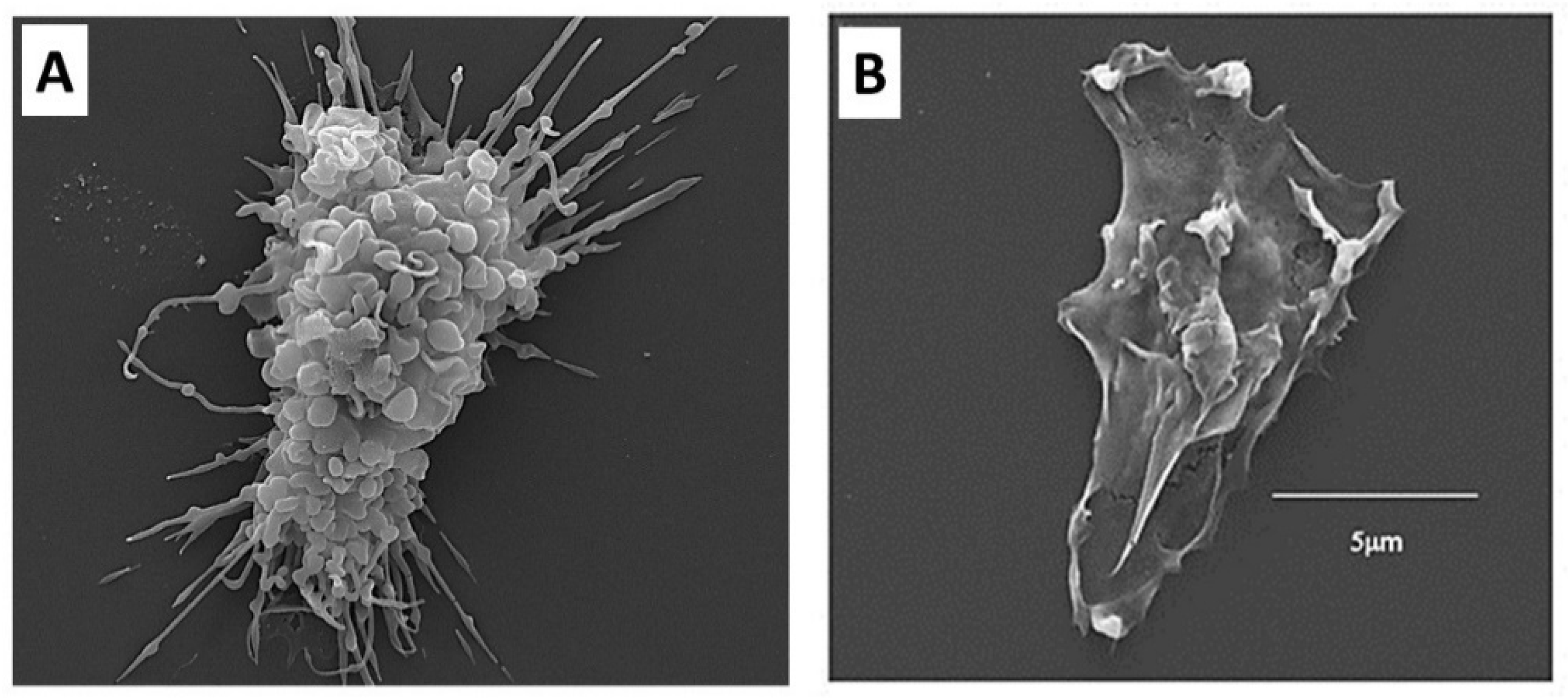
3.3. Recruitment of Macrophages by α-Gal Nanoparticles
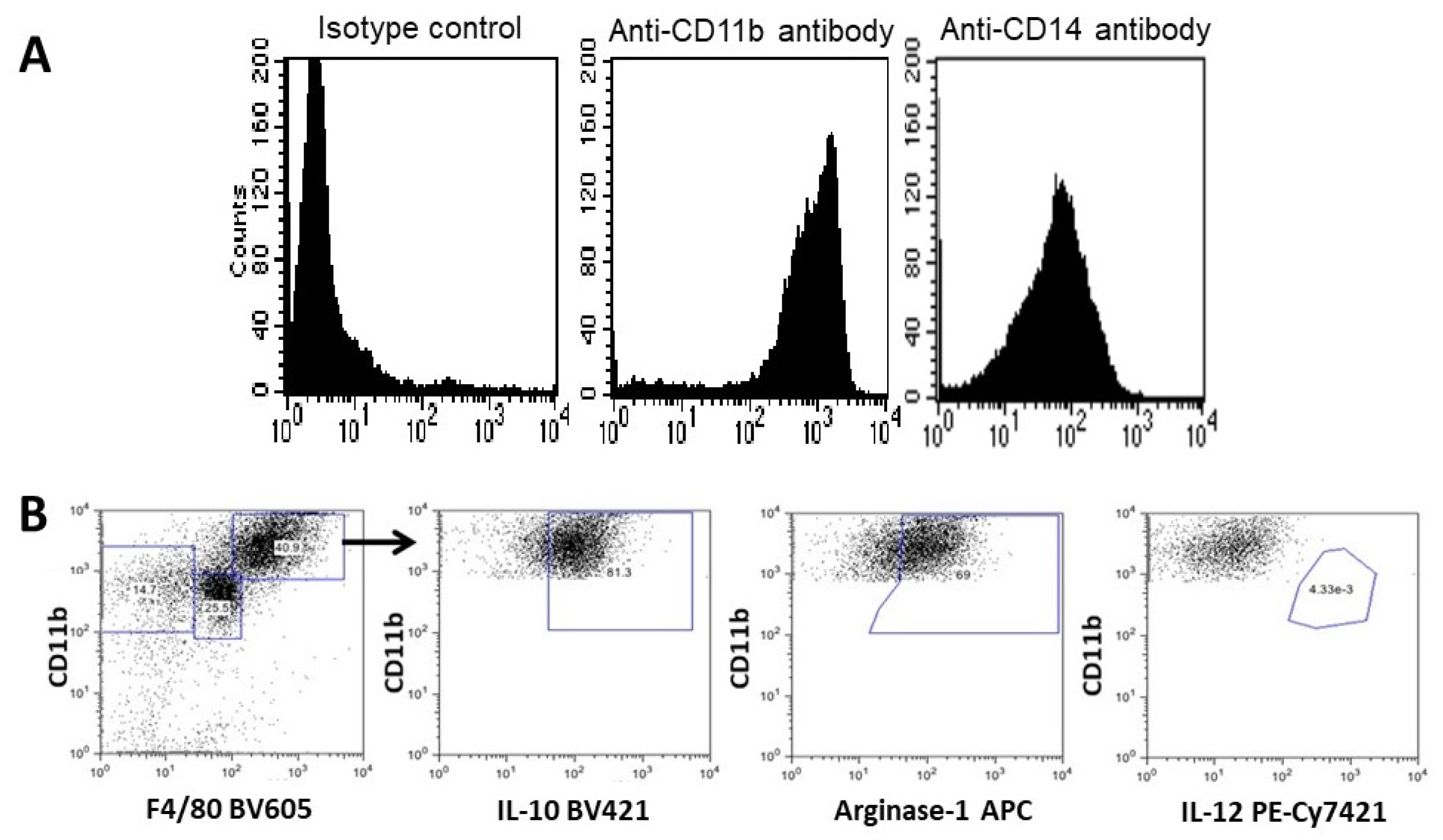
3.4. Characterization of the Recruited Macrophages as M2 Polarized Macrophages
3.5. Homing of Stem Cells
3.6. Cartilage Regeneration in PVA Sponge Discs
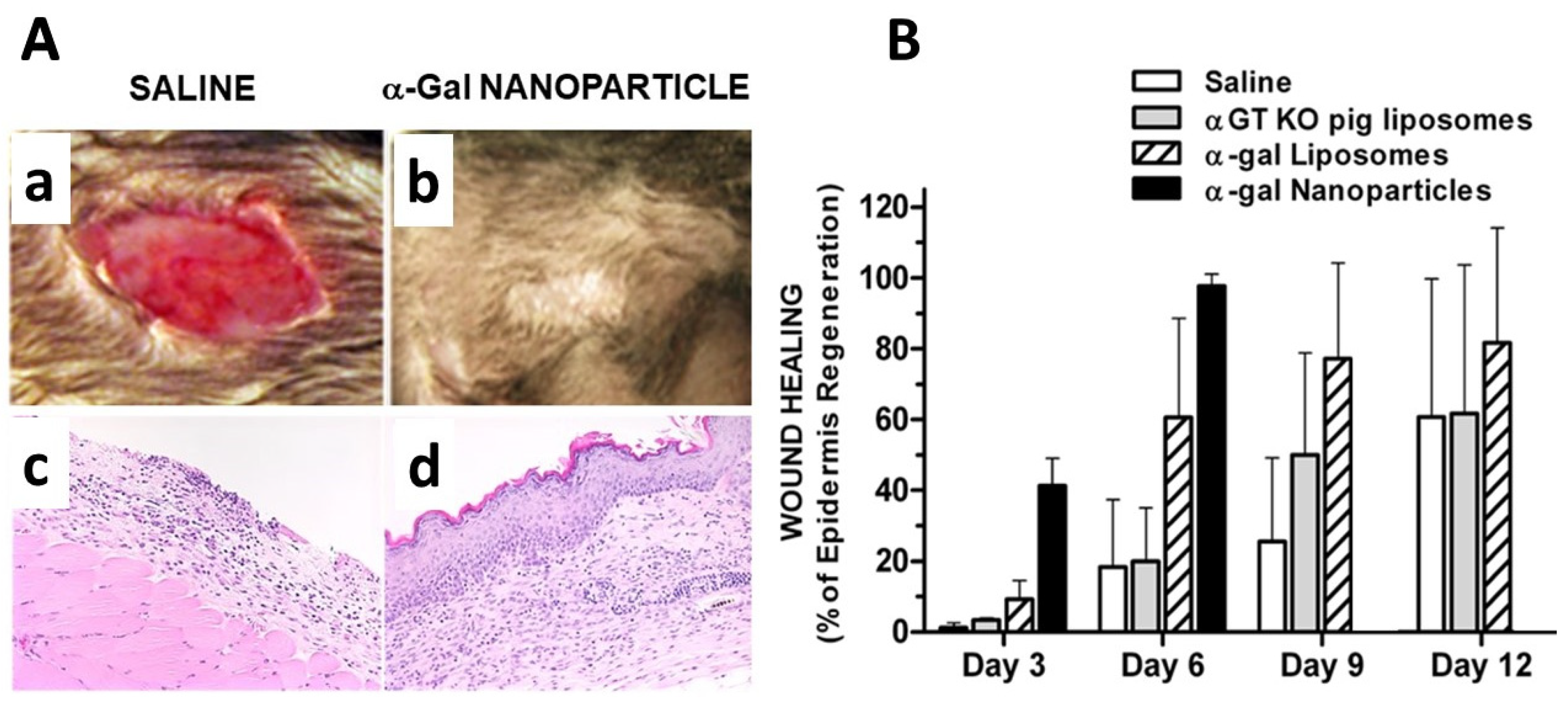
4. Regeneration of Skin Wounds by α-Gal Nanoparticles Prevents Scar Formation
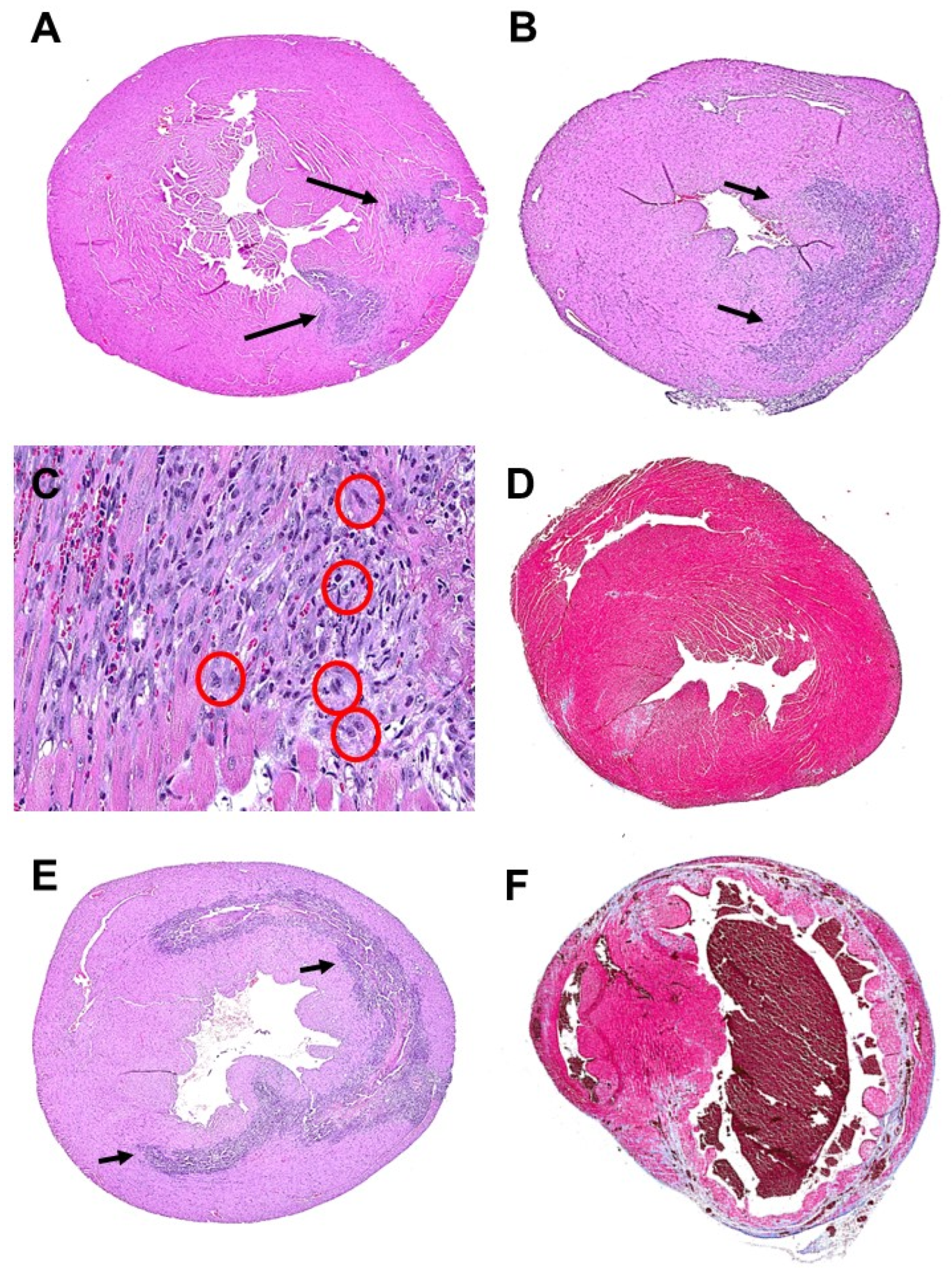
5. Regeneration of Myocardium by α-Gal Nanoparticles Post-Myocardial Infarction
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godwin, J. The promise of perfect adult tissue repair and regeneration in mammals: Learning from regenerative amphibians and fish. Bioessays 2014, 36, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Pinto, A.R.; Epelman, S.; Kopecky, B.J.; Clemente-Casares, X.; Godwin, J.; Jason, N.; Kovacic, C. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4). J. Am. Coll. Cardiol. 2018, 72, 2213–2230. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, M.J.; Sughrue, M.E.; Kane, A.J.; Ahn, B.J.; Fang, S.; Parsa, A.T. The complement cascade as a mediator of tissue growth and regeneration. Inflamm. Res. 2010, 59, 897–905. [Google Scholar] [CrossRef][Green Version]
- Flink, I.L. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: Confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat. Embryol. 2002, 205, 235–244. [Google Scholar]
- Nguyev-Chi, M.; Laplace-Builhé, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 2017, 8, e2979. [Google Scholar] [CrossRef]
- Godwin, J.W.; Debuque, R.; Salimova, E.; Rosenthal, N.A. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen. Med. 2017, 2, 22. [Google Scholar] [CrossRef]
- Laurson, J.; Selden, C.; Hodgson, H.J. Hepatocyte progenitors in man and in rodents - multiple pathways, multiple candidates. Int. J. Exp. Pathol. 2005, 86, 1–18. [Google Scholar]
- Simkin, J.; Sammarco, M.C.; Marrero, L.; Dawson, L.A.; Yan, M.; Tucker, C.; Cammack, A.; Muneoka, K. Macrophages are required to coordinate mouse digit tip regeneration. Development 2017, 144, 3907–3916. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.L.; Wurdinger, T.; Figueiredo, J.S.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Haubner, B.J.; Adamowicz-Brice, M.; Khadayate, S.; Tiefenthaler, V.; Metzler, B.; Aitman, T.; Penninger, J.M. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 2012, 4, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef]
- Ye, L.; D’Agostino, G.; Loo, S.J.; Wang, C.X.; Su, L.P.; Tan, S.H.; Tee, G.Z.; Pua, C.J.; Pena, E.M.; Cheng, R.B.; et al. Early regenerative capacity in the porcine heart. Circulation 2018, 138, 2798–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative potential of neonatal porcine hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.I.; Porrello, E.R. Turning back the cardiac regenerative clock: Lessons from the neonate. Trends Cardiovasc. Med. 2012, 22, 128–133. [Google Scholar] [CrossRef]
- Galili, U.; Zhu, Z.; Chen, J.; Goldufsky, J.W.; Schaer, G.L. Near complete repair after myocardial infarction in adult mice by altering the inflammatory response with intramyocardial injection of α-gal nanoparticles. Front. Cardiovasc. Med. 2021, 8, 719160. [Google Scholar] [CrossRef]
- Del Rio-Tsonis, K.; Tsonis, P.A.; Zarkadis, I.K.; Tsagas, A.G.; Lambris, J.D. Expression of the third component of complement, C3, in regenerating limb blastema cells of urodeles. J. Immunol. 1998, 161, 6819–6824. [Google Scholar]
- Mastellos, D.; Papadimitriou, J.C.; Franchini, S.; Tsonis, P.A.; Lambris, J.D. A novel role of complement: Mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 2001, 166, 2479–2486. [Google Scholar] [CrossRef]
- Kimura, Y.; Madhavan, M.; Call, M.K.; Santiago, W.; Tsonis, P.A.; Lambris, J.D.; Del Rio-Tsonis, K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 2003, 170, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Mastellos, D.C.; Deangelis, R.A.; Lambris, J.D. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 2013, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Abbas, Y.; Bryant, D.M.; Gonzalez-Rosa, J.M.; Sharpe, M.; Uygur, A.; Cocco-Delgado, L.H.; Ho, N.N.; Gerard, N.P.; Gerard, C.J.; et al. Complement Receptor C5aR1 Plays an Evolutionarily Conserved Role in Successful Cardiac Regeneration. Circulation 2018, 137, 2152–2165. [Google Scholar] [CrossRef]
- Bolaños-Castro, L.A.; Walters, H.E.; García Vázquez, R.O.; Yun, M.H. Immunity in salamander regeneration: Where are we standing and where are we headed? Dev. Dyn. 2021, 250, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Rachmilewitz, E.A.; Peleg, A.; Flechner, I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J. Exp. Med. 1984, 160, 1519–1531. [Google Scholar] [CrossRef]
- Avila, J.L.; Rojas, M.; Galili, U. Immunogenic Gal alpha 1----3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J. Immunol. 1989, 142, 2828–2834. [Google Scholar] [PubMed]
- McMorrow, I.M.; Comrack, C.A.; Sachs, D.H.; DerSimonian, H. Heterogeneity of human anti-pig natural antibodies cross-reactive with the Gal(α1,3)Galactose epitope. Transplantation 1997, 64, 501–510. [Google Scholar] [CrossRef]
- Galili, U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. [Google Scholar] [CrossRef]
- Zappe, A.; Rosenlöcher, J.; Kohla, G.; Hinderlich, S.; Parr, M.K. Purification and Characterization of Antibodies Directed against the α-Gal Epitope. BioChem 2021, 1, 81–97. [Google Scholar] [CrossRef]
- Wang, L.; Anaraki, F.; Henion, T.R.; Galili, U. Variations in activity of the human natural anti-Gal antibody in young and elderly populations. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M227–M233. [Google Scholar] [CrossRef]
- Galili, U.; Mandrell, R.E.; Hamadeh, R.M.; Shohet, S.B.; Griffiss, J.M. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 1988, 56, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Bernth Jensen, J.M.; Petersen, M.S.; Ellerman-Eriksen, S.; Møller, B.K.; Jensenius, J.C.; Skov Sørensen, U.B.; Thiel, S. Abundant human anti-Galα3Gal antibodies display broad pathogen reactivity. Sci. Rep. 2020, 10, 4611. [Google Scholar] [CrossRef]
- Posekany, K.J.; Pittman, H.K.; Bradfield, J.F.; Haisch, C.E.; Verbanac, K.M. Induction of cytolytic anti-Gal antibodies in α-1,3-galactosyltransferase gene knockout mice by oral inoculation with Escherichia coli O86:B7 bacteria. Infect. Immun. 2002, 70, 6215–6222. [Google Scholar] [CrossRef] [PubMed]
- Mañez, R.; Blanco, F.J.; Díaz, I.; Centeno, A.; Lopez-Pelaez, E.; Hermida, M.; Davies, H.F.; Katopodis, A. Removal of bowel aerobic gram-negative bacteria is more effective than immunosuppression with cyclophosphamide and steroids to decrease natural α-galactosyl IgG antibodies. Xenotransplantation 2001, 8, 15–23. [Google Scholar] [CrossRef]
- Han, W.; Cai, L.; Wu, B.; Li, L.; Xiao, Z.; Cheng, J.; Wang, P.G. The wciN gene encodes an α-1,3-galactosyltransferase involved in the biosynthesis of the capsule repeating unit of Streptococcus pneumoniae serotype 6B. Biochemistry 2012, 51, 5804–5810. [Google Scholar] [CrossRef]
- Boussamet, L.; Montassier, E.; Soulillou, J.P.; Berthelot, L. Anti α1-3Gal antibodies and Gal content in gut microbiota in immune disorders and multiple sclerosis. Clin. Immunol. 2022, 235, 108693. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Macher, B.A.; Buehler, J.; Shohet, S.B. Human natural anti-α-galactosyl IgG. II. The specific recognition of α[1,3]-linked galactose residues. J. Exp. Med. 1985, 162, 573–582. [Google Scholar] [CrossRef]
- Towbin, H.; Rosenfelder, G.; Wieslander, J.; Avila, J.L.; Rojas, M.; Szarfman, A.; Esser, K.; Nowack, H.; Timpl, R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl [α1-3]-galactose epitopes. J. Exp. Med. 1987, 166, 419–432. [Google Scholar] [CrossRef]
- Teneberg, S.; Lönnroth, I.; Torres Lopez, J.F.; Galili, U.; Olwegard Halvarsson, M.; Angstrom, J.; Angstrom, J.; Karlsson, K.A. Molecular mimicry in the recognition of glycosphingolipids by Galα3Galβ4GlcNAcβ-binding Clostridium difficile toxin A, human natural anti-α-galactosyl IgG and the monoclonal antibody Gal-13: Characterization of a binding-active human glycosphingolipid, non-identical with the animal receptor. Glycobiology 1996, 6, 599–609. [Google Scholar]
- Yu, P.B.; Parker, W.; Everett, M.L.; Fox, I.J.; Platt, J.L. Immunochemical properties of anti-Galα1-3Gal antibodies after sensitization with xenogeneic tissues. J. Clin. Immunol. 1999, 19, 116–126. [Google Scholar] [CrossRef]
- Galili, U.; Clark, M.R.; Shohet, S.B.; Buehler, J.; Macher, B.A. Evolutionary relationship between the anti-Gal antibody and the Galα1-3Gal epitope in primates. Proc. Natl. Acad. Sci. USA 1987, 84, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Shohet, S.B.; Kobrin, E.; Stults, C.L.M.; Macher, B.A. Man, apes, and Old-World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988, 263, 17755–17762. [Google Scholar] [CrossRef]
- Oriol, R.; Candelier, J.J.; Taniguchi, S.; Balanzino, L.; Peters, L.; Niekrasz, M.; Hammer, C.; Cooper, D.K. Major carbohydrate epitopes in tissues of domestic and African wild animals of potential interest for xenotransplantation research. Xenotransplantation 1999, 6, 79–89. [Google Scholar] [CrossRef]
- Galili, U. Evolution in primates by “Catastrophic-selection” interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. Am. J. Phys. Anthropol. 2019, 168, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Good, A.H.; Koren, E.; Oriol, R.; Malcolm, A.J.; Ippolito, R.M.; Neethling, F.A.; Ye, Y.; Romano, E.; Zuhdi, N. Identification of α-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: Relevance to discordant xenografting in man. Transpl. Immunol. 1993, 1, 198–205. [Google Scholar] [CrossRef]
- Collins, B.H.; Cotterell, A.H.; McCurry, K.R.; Alvarado, C.G.; Magee, J.C.; Parker, W.; Platt, J.L. Cardiac xenografts between primate species provide evidence for the importance of the α-galactosyl. determinant in hyperacute rejection. J. Immunol. 1995, 154, 5500–5510. [Google Scholar] [PubMed]
- Galili, U. α1,3Galactosyltransferase knockout pigs produce the natural anti-Gal antibody and simulate the evolutionary appearance of this antibody in primates. Xenotransplantation 2013, 20, 267–276. [Google Scholar] [CrossRef]
- Galili, U. Biosynthesis of α-gal epitopes (Galα1-3Galβ1-4GlcNAc-R) and their unique potential in future α-gal therapies. Front. Mol. Biosci. 2021, 8, 746883. [Google Scholar] [CrossRef]
- Galili, U.; Wigglesworth, K.; Abdel-Motal, U.M. Accelerated healing of skin burns by anti-Gal/α-gal liposomes interaction. Burns 2010, 36, 239–251. [Google Scholar] [CrossRef]
- Wigglesworth, K.M.; Raski, W.J.; Mishra, R.; Szomolanyi-Tsuda, E.; Greiner, D.L.; Galili, U. Rapid recruitment and activation of macrophages by anti-Gal/α-Gal liposome interaction accelerates wound healing. J. Immunol. 2011, 186, 4422–4432. [Google Scholar] [CrossRef]
- Galili, U. α-Gal Nanoparticles in Wound and Burn Healing Acceleration. Adv. Wound Care 2017, 6, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Kaymakcalan, O.E.; Karinja, S.; Abadeer, A.; Dong, X.; Jin, J.L.; Galili, U.; Spector, J.A. Antigen-Mediated, Macrophage-Stimulated, Accelerated Wound Healing Using α-Gal Nanoparticles. Ann. Plast. Surg. 2018, 80 (Suppl. 4), S196–S203. [Google Scholar] [CrossRef]
- Kaymakcalan, O.E.; Abadeer, A.; Goldufsky, J.W.; Galili, U.; Karinja, S.J.; Dong, X.; Jin, J.L.; Samadi, A.; Spector, J.A. Topical α-gal nanoparticles accelerate diabetic wound healing. Exp. Dermatol. 2020, 29, 404–413. [Google Scholar] [CrossRef]
- Samadi, A.; Buro, J.; Dong, X.; Weinstein, A.; Lara, D.O.; Celie, K.B.; Wright, M.A.; Gadijko, M.A.; Galili, U.; Spector, J.A. Topical α-Gal Nanoparticles Enhance Wound Healing in Radiated Skin. Skin Pharmacol. Physiol. 2022, 35, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Galili, U. Profiling terminal N-acetyllactosamines of glycans on mammalian cells by an immuno-enzymatic assay. Glycoconj. J. 2006, 23, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. The Natural Anti-Gal Antibody as Foe Turned Friend in Medicine; Elsevier/Academic Press: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Reca, R.; Mastellos, D.; Majka, M.; Marquez, L.; Ratajczak, J.; Franchini, S.; Glodek, A.; Honczarenko, M.; Spruce, L.A.; Janowska-Wieczorek, A.; et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood 2003, 101, 3784–3793. [Google Scholar] [CrossRef]
- Brunstein, C.G.; McKenna, D.H.; DeFor, T.E.; Sumstad, D.; Paul, P.; Weisdorf, D.J.; Ratajczak, M.; Laughlin, M.J.; Wagner, J.E. Complement fragment 3a priming of umbilical cord blood progenitors: Safety profile. Biol. Blood Marrow Transplant. 2013, 19, 1474–1479. [Google Scholar] [CrossRef][Green Version]
- Bujko, K.; Rzeszotek, S.; Hoehlig, K.; Yan, J.; Vater, A.; Ratajczak, M.Z. Signaling of the Complement Cleavage Product Anaphylatoxin C5a Through C5aR (CD88) Contributes to Pharmacological Hematopoietic Stem Cell Mobilization. Stem. Cell Rev. Rep. 2017, 13, 793–800. [Google Scholar] [CrossRef]
- Adamiak, M.; Ratajczak, M.Z. Innate Immunity and Mobilization of Hematopoietic Stem Cells. Curr. Stem Cell Rep. 2017, 3, 172–180. [Google Scholar] [CrossRef][Green Version]
- Thall, A.D.; Malý, P.; Lowe, J.B. Oocyte Galα1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 1995, 270, 21437–21440. [Google Scholar] [CrossRef]
- Tearle, R.G.; Tange, M.J.; Zannettino, Z.L.; Katerelos, M.; Shinkel, T.A.; Van Denderen, B.J.; Lonie, A.J.; Lyons, I.; Nottle, M.B.; Cox, T.; et al. The α-1,3-galactosyltransferase knockout mouse. Implic. Xenotransplantation. Transplant. 1996, 61, 13–19. [Google Scholar]
- Lai, L.; Kolber-Simonds, D.; Park, K.W.; Cheong, H.T.; Greenstein, J.L.; Im, G.S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.J.; Koike, C.; Vaught, T.D.; Boone, J.; Wells, K.D.; Chen, S.H.; Ball, S.; Specht, S.M.; Polejaeva, I.A.; Monahan, J.A.; et al. Production of α1,3-galactosyltransferase-deficient pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, M.; Yin, D.; Chong, A.S.; Galili, U. Differential immune responses to α-gal epitopes on xenografts and allografts: Implications for accommodation in xenotransplantation. J. Clin. Investig. 2000, 105, 301–310. [Google Scholar] [CrossRef]
- Dor, F.J.; Tseng, Y.L.; Cheng, J.; Moran, K.; Sanderson, T.M.; Lancos, C.J.; Shimizu, A.; Yamada, K.; Awwad, M.; Sachs, D.H.; et al. α1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation 2004, 78, 15–20. [Google Scholar] [CrossRef]
- Fang, J.; Walters, A.; Hara, H.; Long, C.; Yeh, P.; Ayares, D.; Cooper, D.K.; Bianchi, J. Anti-gal antibodies in α1,3-galactosyltransferase gene knockout pigs. Xenotransplantation 2012, 19, 305–310. [Google Scholar] [CrossRef]
- Piccolo, M.T.; Wang, Y.; Sannomiya, P.; Piccolo, N.S.; Piccolo, M.S.; Hugli, T.E.; Ward, P.A.; Till, G.O. Chemotactic mediator requirements in lung injury following skin burns in rats. Exp. Mol. Pathol. 1999, 66, 220–226. [Google Scholar] [CrossRef]
- Heinrich, S.A.; Messingham, K.A.; Gregory, M.S.; Colantoni, A.; Ferreira, A.M.; Dipietro, L.A.; Kovacs, E.J. Elevated monocyte chemoattractant protein-1 levels following thermal injury precede monocyte recruitment to the wound site and are controlled, in part, by tumor necrosis factor-α. Wound Repair Regen. 2003, 11, 110–119. [Google Scholar] [CrossRef]
- Shukaliak, J.; Dorovini-Zis, K. Expression of the [β]-Chemokines RANTES and MIP-1β by Human Brain Microvessel Endothelial Cells in Primary Culture. J. Neuropathol. Exp. Neurol. 2000, 59, 339–352. [Google Scholar] [CrossRef]
- Franz, M.G.; Steed, D.L.; Robson, M.C. Optimizing healing of the acute wound by minimizing complications. Curr. Probl. Surg. 2007, 4, 691–763. [Google Scholar] [CrossRef]
- Shallo, H.; Plackett, T.P.; Heinrich, S.A.; Kovacs, E.J. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns 2003, 29, 641–647. [Google Scholar] [CrossRef]
- Galili, U. Avoiding detrimental human immune response against mammalian extracellular matrix implants. Tissue Eng. B 2015, 21, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A.; Koh, T.J. Macrophages and wound healing. Adv. Wound Care 2016, 2, 71–75. [Google Scholar]
- Hurwitz, Z.; Ignotz, R.; Lalikos, J.; Galili, U. Accelerated porcine wound healing with α-gal nanoparticles. Plast. Reconstr. Surg. 2012, 129, 242–251. [Google Scholar] [CrossRef]
- Liubaviciute, A.; Ivaskiene, T.; Biziuleviciene, G. Modulated mesenchymal stromal cells improve skin wound healing. Biologicals 2020, 67, 1–8. [Google Scholar] [CrossRef]
- Kiwanuka, E.; Junker, J.; Eriksson, E. Harnessing growth factors to influence wound healing. Clin. Plast. Surg. 2012, 39, 239–248. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galili, U.; Goldufsky, J.W.; Schaer, G.L. α-Gal Nanoparticles Mediated Homing of Endogenous Stem Cells for Repair and Regeneration of External and Internal Injuries by Localized Complement Activation and Macrophage Recruitment. Int. J. Mol. Sci. 2022, 23, 11490. https://doi.org/10.3390/ijms231911490
Galili U, Goldufsky JW, Schaer GL. α-Gal Nanoparticles Mediated Homing of Endogenous Stem Cells for Repair and Regeneration of External and Internal Injuries by Localized Complement Activation and Macrophage Recruitment. International Journal of Molecular Sciences. 2022; 23(19):11490. https://doi.org/10.3390/ijms231911490
Chicago/Turabian StyleGalili, Uri, Josef W. Goldufsky, and Gary L. Schaer. 2022. "α-Gal Nanoparticles Mediated Homing of Endogenous Stem Cells for Repair and Regeneration of External and Internal Injuries by Localized Complement Activation and Macrophage Recruitment" International Journal of Molecular Sciences 23, no. 19: 11490. https://doi.org/10.3390/ijms231911490
APA StyleGalili, U., Goldufsky, J. W., & Schaer, G. L. (2022). α-Gal Nanoparticles Mediated Homing of Endogenous Stem Cells for Repair and Regeneration of External and Internal Injuries by Localized Complement Activation and Macrophage Recruitment. International Journal of Molecular Sciences, 23(19), 11490. https://doi.org/10.3390/ijms231911490






