The Changes in Canine Parvovirus Variants over the Years
Abstract
1. Introduction
2. Results
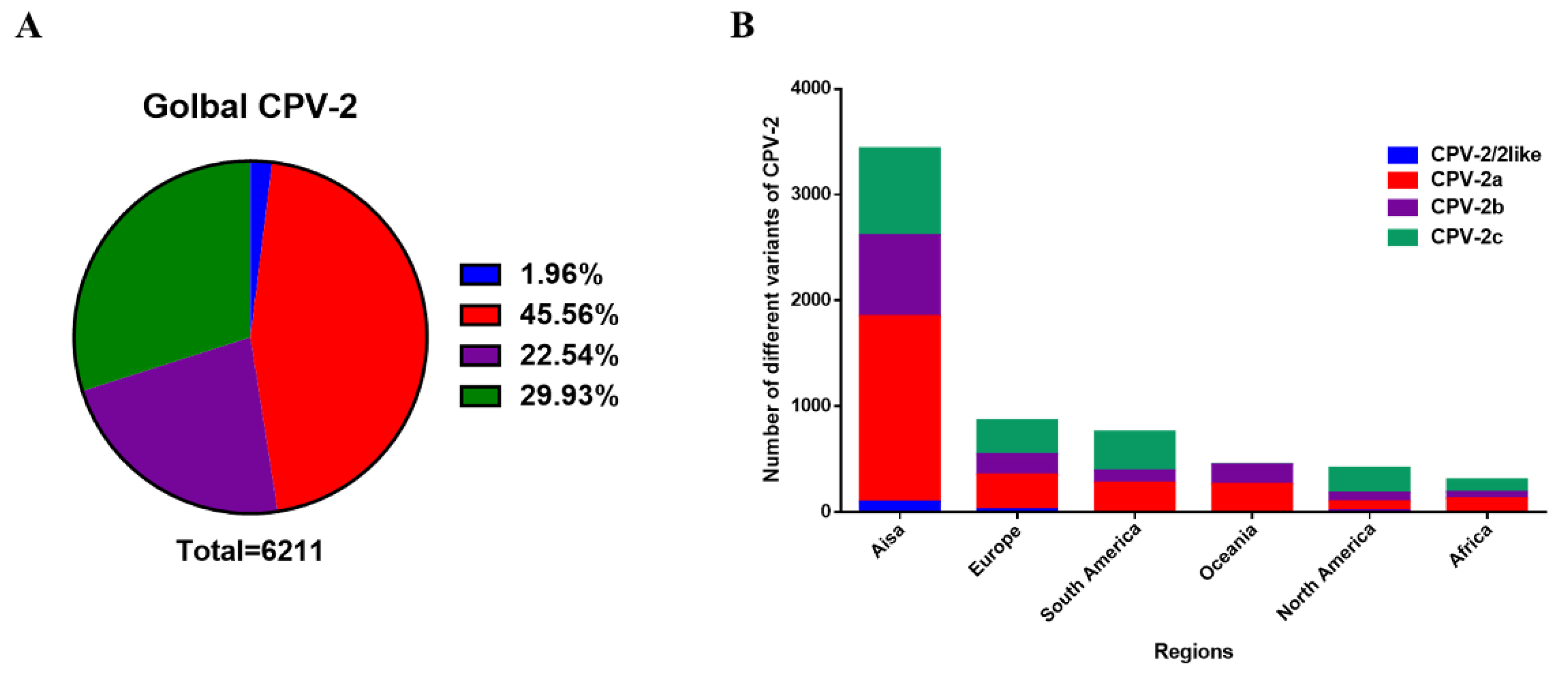
2.1. Global Epidemic of CPV-2
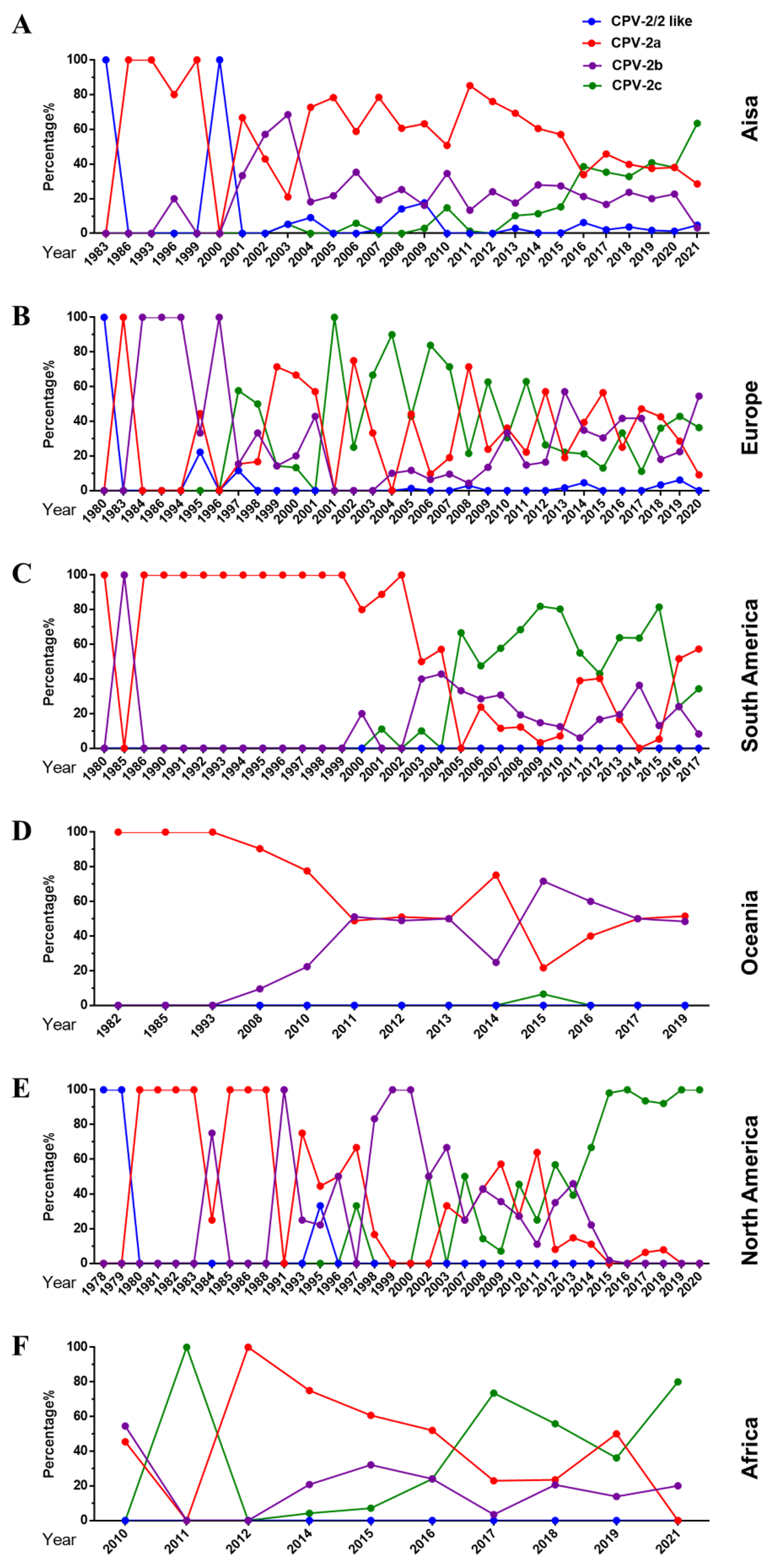
2.2. Distribution of CPV-2 Variants
2.2.1. Statistics of the Number of Variants of CPV-2a/2b/2c
2.2.2. Overall Change of CPV-2a/2b/2c Globally
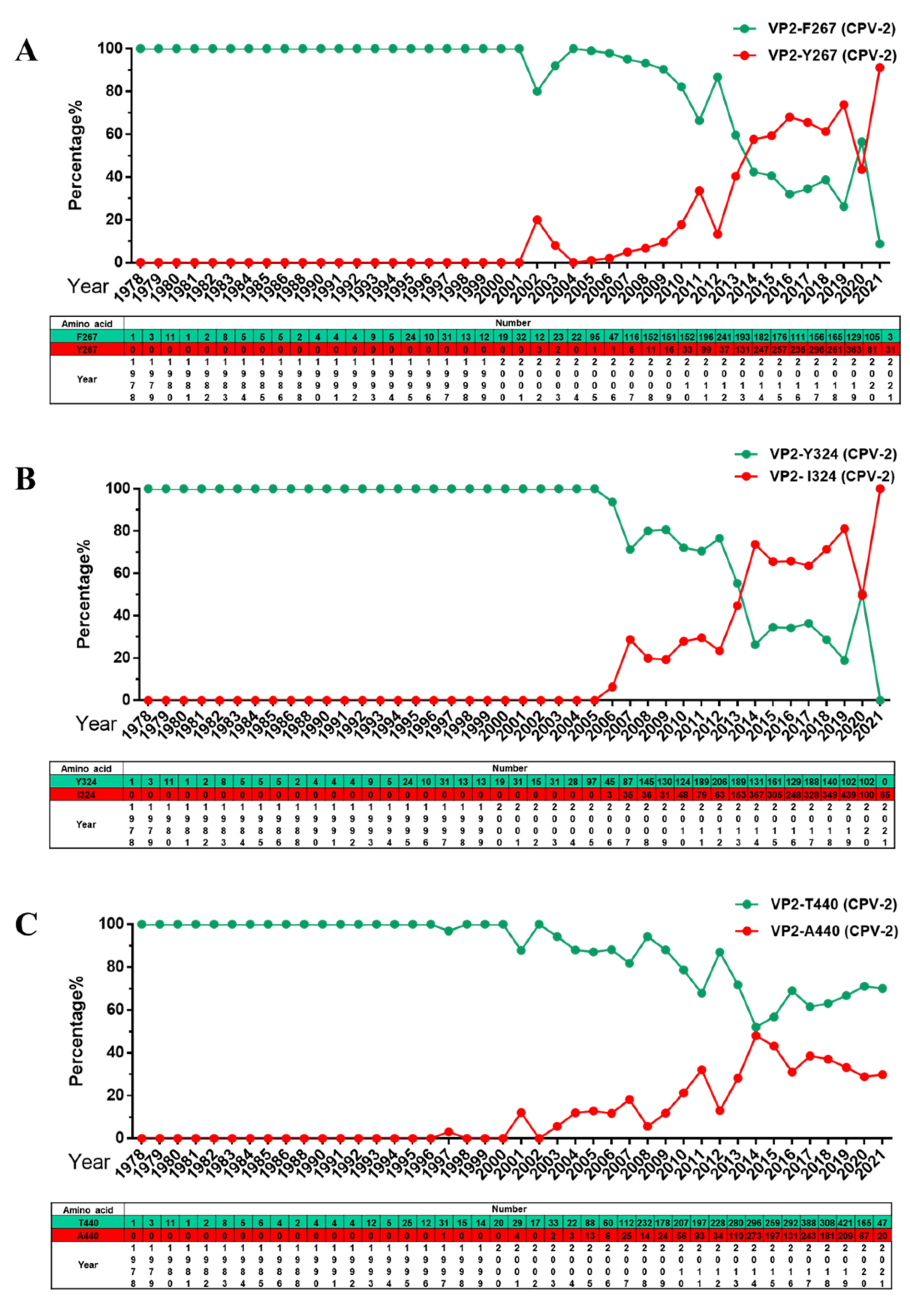
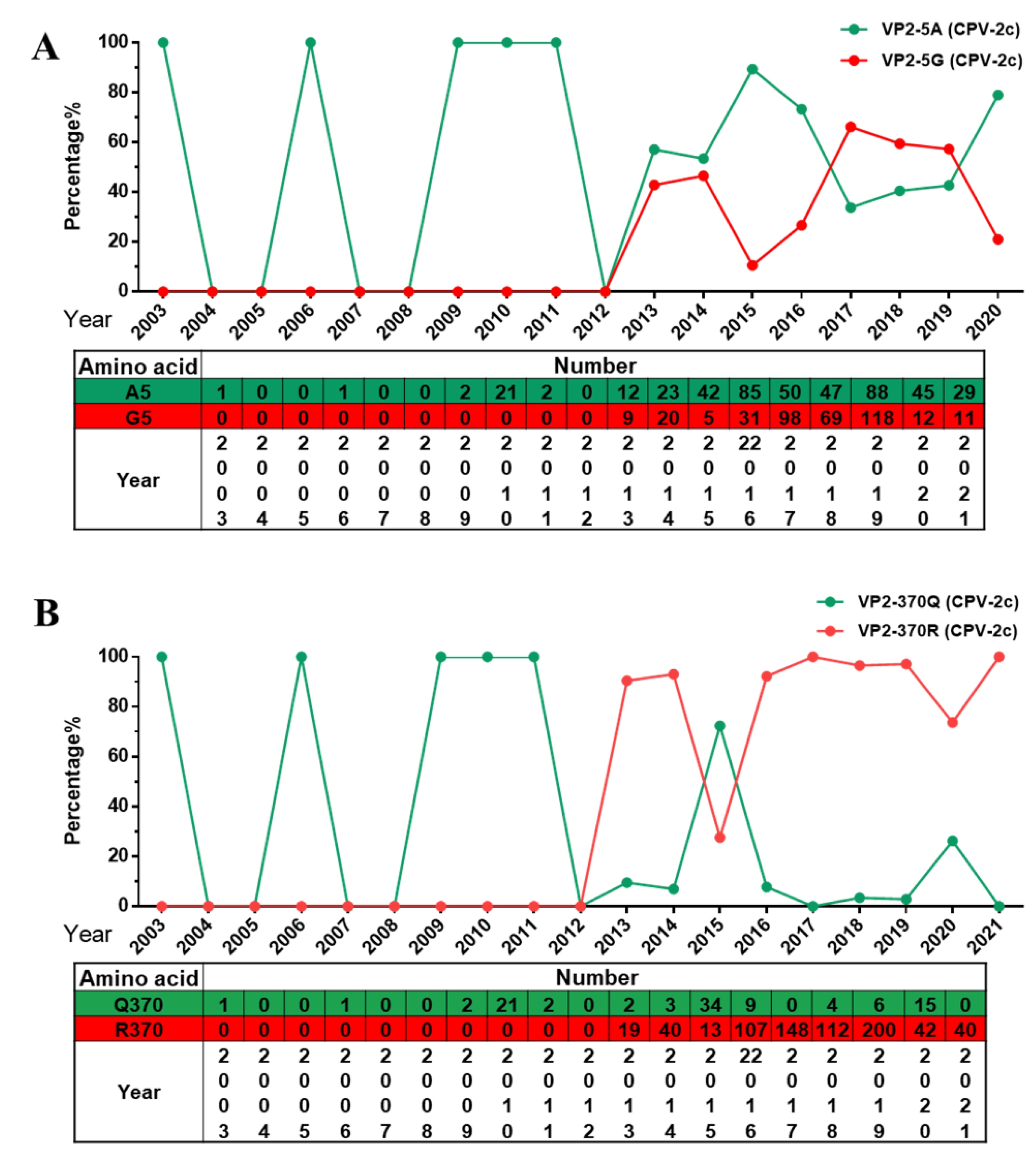
2.3. Analysis of Specific Amino Acid Mutation Sites in the VP2 Gene
2.4. The Q370R Locus on the Surface of the VP2 Protein Pocket
3. Discussion
4. Materials and Methods
4.1. Sequence Data Collection
4.2. Sequences and Amino Acid Analysis
4.3. Graph
4.4. D Modeling Prediction of the VP2 Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parrish, C.R. Pathogenesis of feline panleukopenia virus and canine parvovirus. Bailliere's Clin. Haematol. 1995, 8, 57–71. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Penzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.P.; Jones, E.V.; Miller, T.J. Nucleotide sequence and genome organization of canine parvovirus. J. Virol. 1988, 62, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.; Chen, S.; Zhang, X.; Ma, P.; Ma, M.; Chen, C.; Zhang, X.; Chang, L.; Du, Q.; Huang, Y.; et al. T598 and T601 phosphorylation sites of canine parvovirus NS1 are crucial for viral replication and pathogenicity. Vet. Microbiol. 2022, 264, 109301. [Google Scholar] [CrossRef]
- Saxena, L.; Kumar, G.R.; Saxena, S.; Chaturvedi, U.; Sahoo, A.P.; Singh, L.V.; Santra, L.; Palia, S.K.; Desai, G.S.; Tiwari, A.K. Apoptosis induced by NS1 gene of Canine Parvovirus-2 is caspase dependent and p53 independent. Virus Res. 2013, 173, 426–430. [Google Scholar] [CrossRef]
- Arora, R.; Malla, W.A.; Tyagi, A.; Mahajan, S.; Sajjanar, B.; Tiwari, A.K. Canine Parvovirus and Its Non-Structural Gene 1 as Oncolytic Agents: Mechanism of Action and Induction of Anti-Tumor Immune Response. Front. Oncol. 2021, 11, 1290. [Google Scholar] [CrossRef]
- Llamas-Saiz, A.L.; Agbandje-McKenna, M.; Parker, J.S.; Wahid, A.T.; Parrish, C.R.; Rossmann, M.G. Structural analysis of a mutation in canine parvovirus which controls antigenicity and host range. Virology 1996, 225, 65–71. [Google Scholar] [CrossRef]
- Organtini, L.J.; Allison, A.B.; Lukk, T.; Parrish, C.R.; Hafenstein, S. Global displacement of canine parvovirus by a host-adapted variant: Structural comparison between pandemic viruses with distinct host ranges. J. Virol. 2015, 89, 1909–1912. [Google Scholar] [CrossRef]
- Truyen, U. Emergence and recent evolution of canine parvovirus. Vet. Microbiol. 1999, 69, 47–50. [Google Scholar] [CrossRef]
- Zhou, P.; Zeng, W.; Zhang, X.; Li, S. The genetic evolution of canine parvovirus—A new perspective. PLoS ONE 2017, 12, e175035. [Google Scholar] [CrossRef]
- Carmichael, L.E.; Binn, L.N. New enteric viruses in the dog. Adv. Vet. Sci. Comp. Med. 1981, 25, 1–37. [Google Scholar] [PubMed]
- Yip, H.; Peaston, A.; Woolford, L.; Khuu, S.J.; Wallace, G.; Kumar, R.S.; Patel, K.; Ahani, A.A.; Akbarzadeh, M.; Sharifian, M.; et al. Diagnostic Challenges in Canine Parvovirus 2c in Vaccine Failure Cases. Viruses 2020, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Woolford, L.; Crocker, P.; Bobrowski, H.; Baker, T.; Hemmatzadeh, F. Detection of the Canine Parvovirus 2c Subtype in Australian Dogs. Viral Immunol. 2017, 30, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Thompson, G. Canine parvovirus in vaccinated dogs: A field study. Vet. Rec. 2016, 178, 397. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Buonavoglia, C. Canine parvovirus—A review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; He, Y.; Wang, C.; Xiao, W.; Liu, R.; Xiao, X.; Zhou, P.; Li, S. The increasing prevalence of CPV-2c in domestic dogs in China. PeerJ 2020, 8, e9869. [Google Scholar] [CrossRef]
- Chang, S.F.; Sgro, J.Y.; Parrish, C.R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J. Virol. 1992, 66, 6858–6867. [Google Scholar] [CrossRef]
- Allison, A.B.; Kohler, D.J.; Ortega, A.; Hoover, E.A.; Grove, D.M.; Holmes, E.C.; Parrish, C.R. Host-specific parvovirus evolution in nature is recapitulated by in vitro adaptation to different carnivore species. PLoS Pathog. 2014, 10, e1004475. [Google Scholar] [CrossRef]
- Mittal, M.; Chakravarti, S.; Mohapatra, J.K.; Chug, P.K.; Dubey, R.; Upmanuyu, V.; Narwal, P.S.; Kumar, A.; Churamani, C.P.; Kanwar, N.S. Molecular typing of canine parvovirus strains circulating from 2008 to 2012 in an organized kennel in India reveals the possibility of vaccination failure. Infect. Genet. Evol. 2014, 23, 1–6. [Google Scholar] [CrossRef]
- Balboni, A.; Niculae, M.; Di Vito, S.; Urbani, L.; Terrusi, A.; Muresan, C.; Battilani, M. The detection of canine parvovirus type 2c of Asian origin in dogs in Romania evidenced its progressive worldwide diffusion. BMC Vet. Res. 2021, 17, 206. [Google Scholar] [CrossRef]
- Tion, M.T.; Shima, F.K.; Ogbu, K.I.; Omobowale, T.O.; Amine, A.A.; Nguetyo, S.A.; Igoh, F.A.; Oochi, J.O.; Fotina, H.A.; Saganuwan, S.A.; et al. Genetic diversity of canine parvovirus variants circulating in Nigeria. Infect. Genet. Evol. 2021, 94, 104996. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.L.; Chen, S.J.; Zhang, Z.; Wang, C.; Hou, R.; Ren, Y.; Wen, X.; Cao, S.; Guo, W.; et al. Identification of canine parvovirus with the Q370R point mutation in the VP2 gene from a giant panda (Ailuropoda melanoleuca). Virol. J. 2013, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhao, J.; Guo, D.; Sun, D. A Mini-Review on the Epidemiology of Canine Parvovirus in China. Front. Vet. Sci. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Guo, D.; Li, C.; Wang, E.; Wei, S.; Wang, Z.; Yao, S.; Zhao, X.; Su, M.; Wang, X.; et al. Co-Circulation of the Rare CPV-2c with Unique Gln370Arg Substitution, New CPV-2b with Unique Thr440Ala Substitution, and New CPV-2a with High Prevalence and Variation in Heilongjiang Province, Northeast China. PLoS ONE 2015, 10, e137288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, P.; Zhao, H.; Cheng, Y.; Jiang, Z.; Zhu, H.; Wu, H.; Cheng, S. Continuing evolution of canine parvovirus in China: Isolation of novel variants with an Ala5Gly mutation in the VP2 protein. Infect. Genet. Evol. 2016, 38, 73–78. [Google Scholar] [CrossRef]
- Carmichael, L.E.; Joubert, J.C.; Pollock, R.V. Hemagglutination by canine parvovirus: Serologic studies and diagnostic applications. Am. J. Vet. Res. 1980, 41, 784–791. [Google Scholar]
- Cavalli, A.; Martella, V.; Desario, C.; Camero, M.; Lanave, G.; Barrs, V.R.; Decaro, N.; Buonavoglia, C. Modified haemagglutination inhibition assay for the detection of canine parvovirus type 2 antibodies in dog sera. Vet. J. 2021, 274, 105709. [Google Scholar] [CrossRef]
- Zhuang, Q.Y.; Qiu, Y.; Pan, Z.H.; Wang, S.C.; Wang, B.; Wu, W.K.; Yu, J.M.; Yi, Y.; Sun, F.L.; Wang, K.C. Genome sequence characterization of canine parvoviruses prevalent in the Sichuan province of China. Transbound. Emerg. Dis. 2019, 66, 897–907. [Google Scholar] [CrossRef]
- MaCartney, L.; MaCartney, C.M. Canine parvovirus: Development of immunofluorescence and immunoperoxidase techniques. Res. Vet. Sci. 1986, 40, 201–208. [Google Scholar] [CrossRef]
- Oh, J.S.; Ha, G.W.; Cho, Y.S.; Kim, M.J.; An, D.J.; Hwang, K.K.; Lim, Y.K.; Park, B.K.; Kang, B.; Song, D.S. One-step immunochromatography assay kit for detecting antibodies to canine parvovirus. Clin. Vaccine Immunol. 2006, 13, 520–524. [Google Scholar] [CrossRef]
- Elia, G.; Desario, C.; Pezzoni, G.; Camero, M.; Brocchi, E.; Decaro, N.; Martella, V.; Buonavoglia, C. Recombinant ELISA using baculovirus-expressed VP2 for detection of antibodies against canine parvovirus. J. Virol. Methods 2012, 184, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Elia, G.; Martella, V.; Campolo, M.; Desario, C.; Camero, M.; Cirone, F.; Lorusso, E.; Lucente, M.S.; Narcisi, D.; et al. Characterisation of the canine parvovirus type 2 variants using minor groove binder probe technology. J. Virol. Methods 2006, 133, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bingga, G.; Zhang, C.; Shao, J.; Shen, H.; Sun, J.; Zhang, J. Application of Duplex Fluorescence Melting Curve Analysis (FMCA) to Identify Canine Parvovirus Type 2 Variants. Front. Microbiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.; Wu, H.Y.; Lien, Y.X.; Chiou, M.T.; Lin, C.N. A SimpleProbe((R)) real-time PCR assay for differentiating the canine parvovirus type 2 genotype. J. Clin. Lab. Anal. 2019, 33, e22654. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Y.; Lin, P.; Yi, L.; Tong, M.; Cao, Z.; Wang, G.; Li, S.; Cheng, S.; Yuan, W.; et al. A multiplex TaqMan real-time PCR for detection and differentiation of four antigenic types of canine parvovirus in China. Mol. Cell. Probes 2018, 38, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bingga, G.; Liu, Z.; Zhang, J.; Zhu, Y.; Lin, L.; Ding, S.; Guo, P. High resolution melting curve analysis as a new tool for rapid identification of canine parvovirus type 2 strains. Mol. Cell. Probes 2014, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Chakravarti, S.; Gupta, V.; Nandi, S.; Singh, M.; Badasara, S.K.; Sharma, C.; Mittal, M.; Dandapat, S.; Gupta, V.K. Multiplex Amplification Refractory Mutation System PCR (ARMS-PCR) provides sequencing independent typing of canine parvovirus. Infect. Genet. Evol. 2016, 46, 59–64. [Google Scholar] [CrossRef]
- Hernandez-Blanco, B.; Catala-Lopez, F. Are licensed canine parvovirus (CPV2 and CPV2b) vaccines able to elicit protection against CPV2c subtype in puppies?: A systematic review of controlled clinical trials. Vet. Microbiol. 2015, 180, 1–9. [Google Scholar] [CrossRef]
- Hao, X.; Li, Y.; Chen, H.; Chen, B.; Liu, R.; Wu, Y.; Xiao, X.; Zhou, P.; Li, S. Canine Circovirus Suppresses the Type I Interferon Response and Protein Expression but Promotes CPV-2 Replication. Int. J. Mol. Sci. 2022, 23, 6382. [Google Scholar] [CrossRef]
- Kotsias, F.; Bucafusco, D.; Nunez, D.A.; Lago, B.L.; Rodriguez, M.; Bratanich, A.C. Genomic characterization of canine circovirus associated with fatal disease in dogs in South America. PLoS ONE 2019, 14, e218735. [Google Scholar] [CrossRef]
- Anderson, A.; Hartmann, K.; Leutenegger, C.M.; Proksch, A.L.; Mueller, R.S.; Unterer, S. Role of canine circovirus in dogs with acute haemorrhagic diarrhoea. Vet. Rec. 2017, 180, 542. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Buonavoglia, C.; Barrs, V.R. Canine parvovirus vaccination and immunisation failures: Are we far from disease eradication? Vet. Microbiol. 2020, 247, 108760. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Li, Y.; Xiao, X.; Chen, B.; Zhou, P.; Li, S. The Changes in Canine Parvovirus Variants over the Years. Int. J. Mol. Sci. 2022, 23, 11540. https://doi.org/10.3390/ijms231911540
Hao X, Li Y, Xiao X, Chen B, Zhou P, Li S. The Changes in Canine Parvovirus Variants over the Years. International Journal of Molecular Sciences. 2022; 23(19):11540. https://doi.org/10.3390/ijms231911540
Chicago/Turabian StyleHao, Xiangqi, Yanchao Li, Xiangyu Xiao, Bo Chen, Pei Zhou, and Shoujun Li. 2022. "The Changes in Canine Parvovirus Variants over the Years" International Journal of Molecular Sciences 23, no. 19: 11540. https://doi.org/10.3390/ijms231911540
APA StyleHao, X., Li, Y., Xiao, X., Chen, B., Zhou, P., & Li, S. (2022). The Changes in Canine Parvovirus Variants over the Years. International Journal of Molecular Sciences, 23(19), 11540. https://doi.org/10.3390/ijms231911540






