The Protective Effects of Sesamin against Cyclophosphamide-Induced Nephrotoxicity through Modulation of Oxidative Stress, Inflammatory-Cytokines and Apoptosis in Rats
Abstract
:1. Introduction
2. Results
2.1. Kidney Function Test
2.2. Oxidative Stress Markers
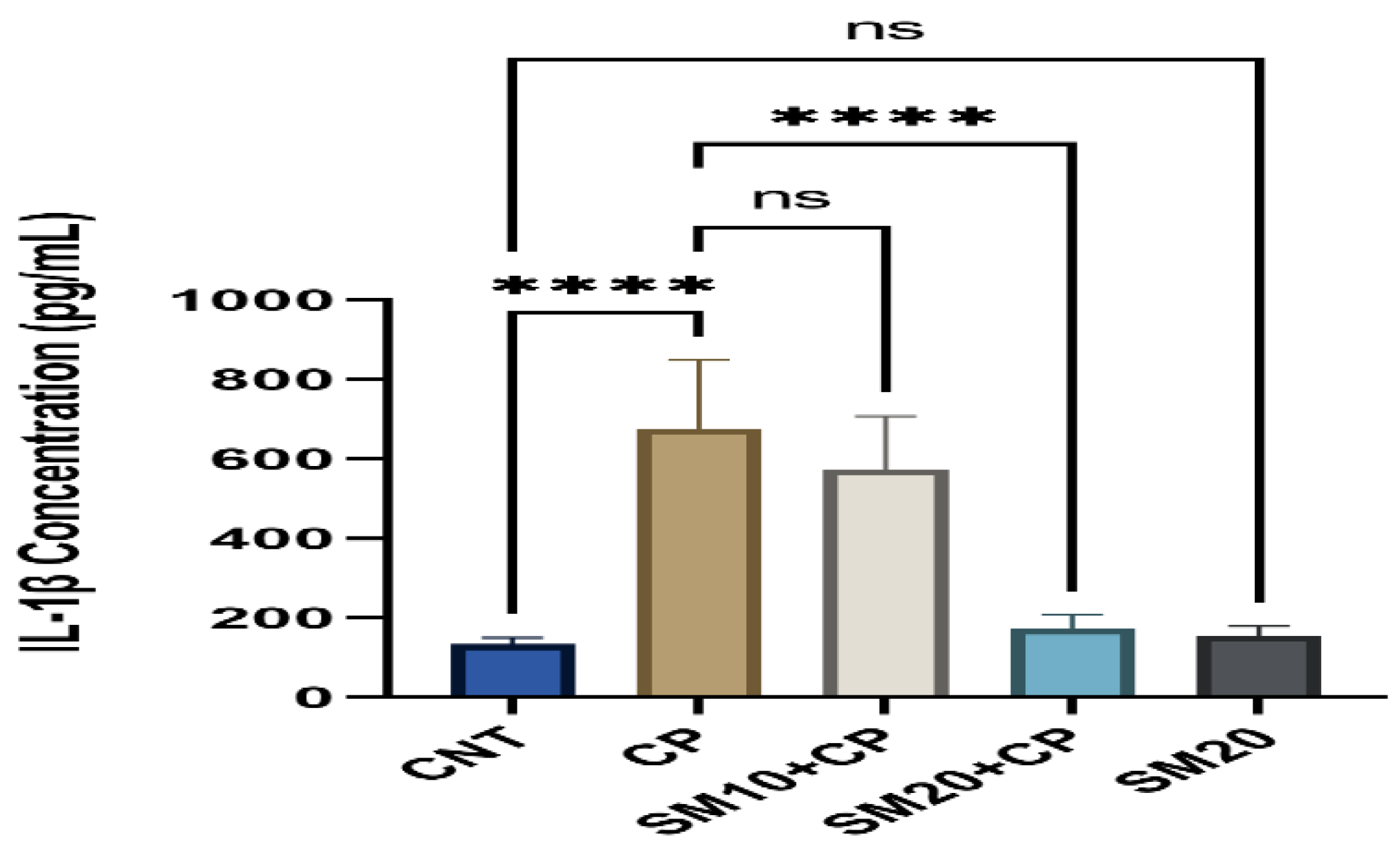
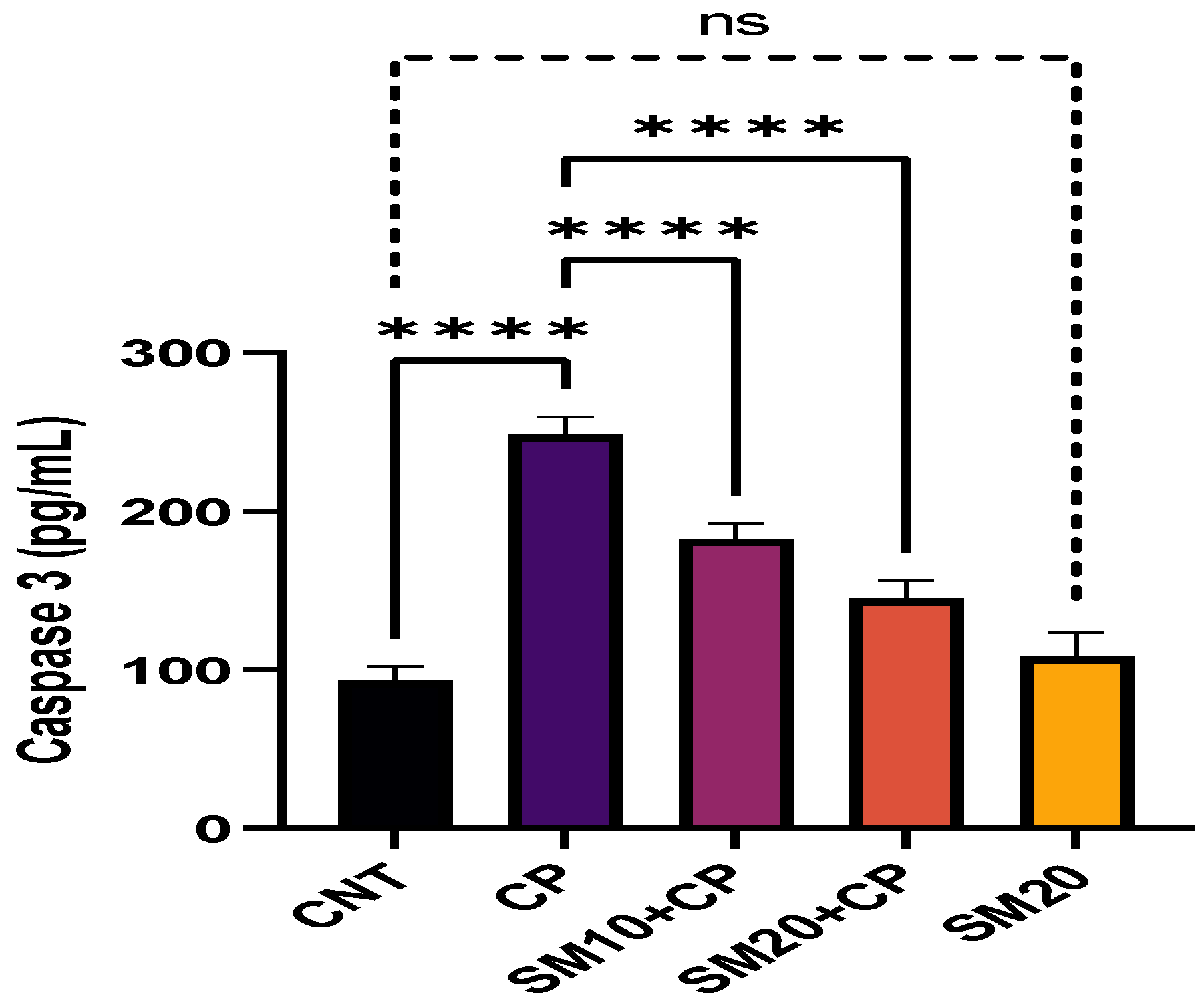
2.3. Inflammation and Apoptosis
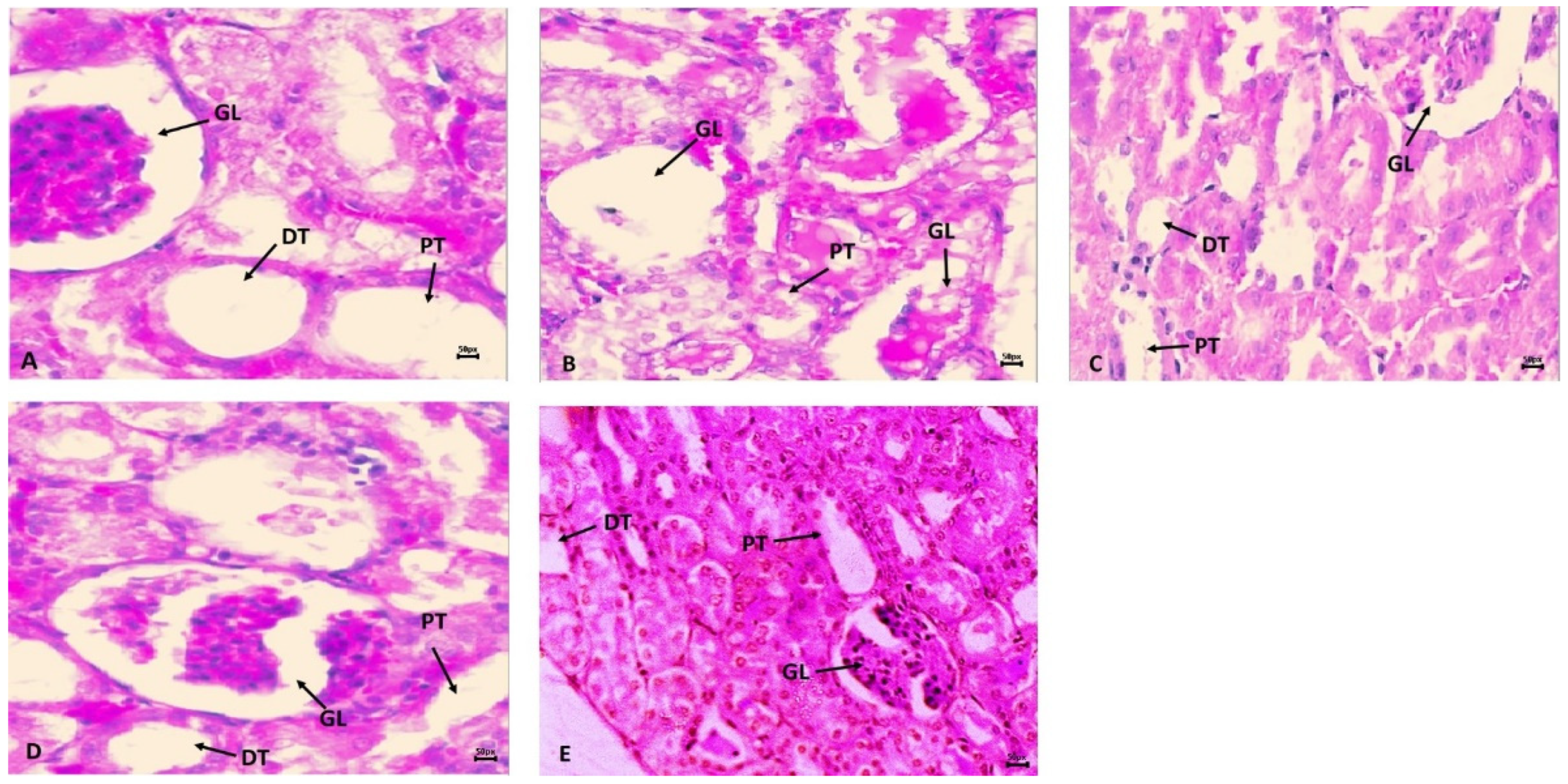
2.4. Histopathology
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Experimental Design
4.4. Assessment of Renal Function Test in Serum
4.5. Assessment of Oxidative Stress Markers
4.6. Assessment of Inflammation and Apoptosis
4.7. Histopathological Inspection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lameire, N. Nephrotoxicity of recent anti-cancer agents. Clin. Kidney J. 2014, 7, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.S.; Sundin, A.; Juhlin, C.; Wilander, E.; Öberg, K.; Eriksson, B. Cisplatin, teniposide, and cyclophosphamide combination in the treatment of recurrent or meta-static adrenocortical cancer. Med. Oncol. 2004, 21, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hales, F.B. Comparison of the mutagenicity and teratogenicity of cyclophosphamide and its active metabolites, 4-hydroxycyclophosphamide, phosphoramide mustard, and acrolein. Cancer Res. 1982, 42, 3016. [Google Scholar] [PubMed]
- Milsted, A.V.R.; Jarman, M. Metabolism of high doses of cyclophosphamide. Cancer Chemother. Pharmacol. 1982, 8, 311–313. [Google Scholar] [CrossRef]
- Barry, H. Free radicals, antioxidants, and human disease curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health, Current status and future prospects. J. Assoc. Phys. India 2004, 52, 794–804. [Google Scholar]
- Dumontet, C.; Drai, J.; Thieblemont, C.; Hequet, O.; Espinouse, D.; Bouafia, F.; Salles, G.; Coiffier, B. The superoxide dismutase content in erythrocytes predicts short-term toxicity of high-dose cyclophosphamide. Br. J. Haematol. 2001, 112, 405–409. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Neboh, E.E.; Ufelle, S.A. Myeloprotective activity of crude methanolic leaf extract of Cassia occidentalis in cyclophosphamideinduced bone marrow suppression in Wistar rats. Adv. Biomed. Res. 2015, 6, 4–5. [Google Scholar]
- Khan, M.S.A.; Ahmad, I. Chapter 1—Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine; Khan, M.S.A., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. ISBN 9780128146194. [Google Scholar] [CrossRef]
- Alshahrani, S.; Al Sreaya, A.A.; Mashyakhi, M.Y.; Alqahtani, S.; Sivakumar, S.M.; Alhazmi, H.A.; Rehman, Z.; Alam, F. Chemical characterization and antibacterial efficacy of Saudi sesame oil against human pathogenic bacteria. Environ. Conserv. J. 2020, 21, 19–29. [Google Scholar] [CrossRef]
- Fan, D.; Yang, Z.; Liu, F.; Jin, Y.-G.; Zhang, N.; Ni, J.; Yuan, Y.; Liao, H.-H.; Wu, Q.-Q.; Xu, M.; et al. Sesamin protects against cardiac remodeling via Sirt3/ROS Pathway. Cell Physiol. Biochem. 2020, 44, 2212–2227. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Yang, Z.; Yuan, Y.; Wu, Q.Q.; Xu, M.; Jin, Y.G.; Tang, Q.Z. Sesamin prevents apoptosis and inflammation after experimental myocardial infarction by JNK and NF-κB pathways. Food Funct. 2017, 8, 2875–2885. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Kinugawa, S.; Matsushima, S.; Takemoto, D.; Furihata, T.; Mizushima, W.; Fukushima, A.; Yokota, T.; Ono, Y.; Shibata, H.; et al. Sesamin prevents decline in exercise capacity and impairment of skeletal muscle mitochondrial function in mice high-fat diet-induced diabetes. Exp. Physiol. 2015, 100, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penalvo, J.L.; Hopia, A.; Adlercreutz, H. Effect of sesamin on serum cholesterol and triglycerides levels in LDL receptor-deficient mice. Eur. J. Nutr. 2006, 45, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, S.; Matsumura, Y.; Morimoto, S.; Akimoto, K.; Furuya, M.; Oka, N.; Tanaka, T. Antihypertensive effect of sesamin. II. Protection against two-kidney, one-clip renal hypertension and cardiovascular hypertrophy. Biol. Pharm. Bull. 1995, 18, 1283–1285. [Google Scholar] [CrossRef] [Green Version]
- Akimoto, K.; Kitagawa, Y.; Akamatsu, T.; Hirose, N.; Sugano, M.; Shimizu, S.; Yamada, H. Protective effects of sesamin against liver damage caused by alcohol or carbon tetrachloride in rodents. Ann. Nutr. Metab. 1993, 37, 218–224. [Google Scholar] [CrossRef]
- Li, L.C.; Piao, H.M.; Zheng, M.Y.; Lin, Z.H.; Li, G.; Yan, G.H. Sesamin attenuates mast cell-mediated allergic responses by suppressing the activation of p38 and nuclear factor-κB. Mol. Med. Rep. 2016, 13, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Za’abi, M.; Ashique, M.; Manoj, P.; Nemmar, A. Ameliorative effect of sesamin in cispla-tin-induced nephrotoxicity in rats by suppressing inflammation, oxidative/nitrosative stress, and cellular damage. Physiol. Res. 2020, 69, 61–72. [Google Scholar] [CrossRef]
- Sinanoglu, O.; Yener, A.; Ekici, S.; Midi, A.; Aksungar, F. The Protective effects of spirulina in cyclophosphamide induced nephrotoxicity and urotoxicity in rats. Urology 2012, 80, 1392–1396. [Google Scholar] [CrossRef]
- Lameire, N.; Kruse, V.; Rottey, S. Nephrotoxicity of anticancer drugs—An underestimated problem. Acta Clin. Belg. 2011, 66, 337–345. [Google Scholar]
- Cagle, C.S.; Appel, S.; Skelly, A.H.; Carter-Edwards, L. Mid-life African-American women with type 2 diabetes, influence on work and the multicare giver role. Ethn. Dis. 2002, 12, 555–566. [Google Scholar] [PubMed]
- Gines, S.; Seong, I.S.; Fossale, E.; Ivanova, E.; Trettel, F.; Gusella, J.F.; Wheeler, V.C.; Persichetti, F.; MacDonald, M.E. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington’s disease knock-in mice. Hum. Mol. Genet. 2003, 12, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Zhang, H.J.; Toth, P.T.; Zhang, Y.; Marsboom, G.; Hong, Z.; Salgia, R.; Husain, A.N.; Wietholt, C.; Archer, S.L. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012, 26, 2175–2186. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.C.; Hewitt, P. Biomarkers for drug-induced renal damage and nephrotoxicity-an overview for applied toxicology. AAPS J. 2011, 13, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, B.; Eirin, A.; Li, Z.; Zhu, X.; Zhang, X.; Lerman, A.; Textor, S.C.; Lerman, L.O. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS ONE 2013, 8, e67474. [Google Scholar] [CrossRef] [Green Version]
- Boddy, A.V.; Furtun, Y.; Sardas, S.; Sardas, O.; Idle, J.R. Individual variation in the activation and inactivation of metabolic pathways of cyclophosphamide. J. Nat. Cancer Inst. 1992, 84, 1744–1748. [Google Scholar] [CrossRef]
- Mirkes, P.E.; Greenaway, J.C.; Rogers, J.G.; Brundrett, R.B. Role of acrolein in cyclophosphamide teratogenicity in rat embryos in vitro. Toxicol. Appl. Pharmacol. 1984, 72, 281–291. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Yogeeta, S.K.; Subashini, R.; Devaki, T. Attenuation of cyclophosphamide induced toxicity by squalene in experimental rats. Chem. Biol. Interact. 2006, 160, 252–260. [Google Scholar] [CrossRef]
- Santos, M.L.C.; de Brito, B.B.; da Silva, F.A.F.; Botelho, A.C.D.S.; de Melo, F.F. Nephrotoxicity in cancer treatment, An overview. World J. Clin. Oncol. 2020, 11, 190–204. [Google Scholar] [CrossRef]
- Haque, R.; Bin-Hafeez, B.; Parvez, S.; Pandey, S. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide induced biochemical toxicity. Hum. Exp. Toxicol. 2003, 22, 473–480. [Google Scholar] [CrossRef]
- Manda, K.; Bhatia, A.L. Prophylactic action of melatonin against cyclophosphamide induced oxidative stress in mice. Cell Biol. Toxicol. 2003, 19, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, H.; Fu, G.; Chen, X.; Chen, F.; Xie, M. The relationship of antioxidant components and antioxidant activity of sesame seed oil. J. Sci. Food Agric. 2015, 95, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, É.M.H.; Chibli, L.A.; Yamamoto, C.H.; Pereira, M.C.S.; Vilela, F.M.P.; Rodarte, M.P.; De Oliveira Pinto, M.A.; Da Penha Henriques do Amaral, M.; Silvério, M.S.; De Matos Araújo, A.L.S.; et al. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 2014, 6, 1931–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Ying, X.; Chen, L.; Zhang, W.; Zhang, Y. Protective effects of sesamin on liver fibrosis through antioxidative and anti-inflammatory activities in rats. Immunopharmacol. Immunotoxicol. 2015, 37, 465–472. [Google Scholar] [CrossRef]
- Kiso, Y. Antioxidative roles of sesamin, a functional lignan in sesame seed, and it’s effect on lipid- and alcohol-metabolism in the liver: A DNA microarray study. Biofactors 2004, 21, 191–196. [Google Scholar] [CrossRef]
- Sakaki, T.; Yasuda, K.; Nishikawa, M.; Ikushiro, S. Metabolism of Sesamin and Drug-Sesamin Interaction. Yakugaku Zasshi 2018, 138, 357–363. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ito, S.; Nishio, N.; Tanaka, Y.; Chen, N.; Isobe, K.-I. Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol. Lett. 2014, 229, 384–392. [Google Scholar] [CrossRef]
- Mythili, Y.; Sudharsan, P.T.; Selvakumar, E.; Varalakshmi, P. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem. Biol. Interact. 2004, 151, 13–19. [Google Scholar] [CrossRef]
- El-Kholy, A.A.; Elkablawy, M.A.; El-Agamy, D.S. Lutein mitigates cyclophosphamide induced lung and liver injury via NF-κB/MAPK dependent mechanism. Biomed. Pharmacother. 2017, 92, 519–527. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-κB, its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Alhoshani, A.R.; Hafez, M.M.; Husain, S.; Al-sheikh, A.M.; Alotaibi, M.R.; Al Rejaie, S.S.; Alshammari, M.A.; Almutairi, M.M.; Al-Shabanah, O.A. Protective effect of rutin supplementation against cisplatin-induced Nephrotoxicity in rats. BMC Nephrol. 2017, 18, 194. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines, recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats, role of NFκB/MAPK pathway. Chem. Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, X.; Li, H.; Yuan, J.; Peng, Y.; Dong, L.; Dai, S. Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed. Pharmacother. 2016, 84, 1899–1905. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, C.Q.; Liu, L. Sesamin ameliorates oxidative liver injury induced by carbon tetrachloride in rat. Int. J. Clin. Exp. Pathol. 2015, 8, 5733–5738. [Google Scholar]
- Abraham, P.; Isaac, B. The effects of oral glutamine on cyclophosphamide-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2011, 30, 616–623. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, proteinbound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Claiborne, A.L. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; CRC: Boca Raton, FL, USA, 1985; Volume 1, pp. 283–284. [Google Scholar]
- Stevens, M.J.; Obrosova, I.; Cao, X.; Van Huysen, C.; Greene, D.A. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism and oxidative, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 2000, 49, 1006–1015. [Google Scholar] [CrossRef] [Green Version]

| Test Group | Blood Urea Nitrogen (mg/dL) | Uric Acid (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|
| CNT | 18.66 ± 2.58 | 4.57 ± 1.18 | 0.81 ± 0.08 |
| CP | 50.16 ± 3.97 a (168.81%) | 16.71 ± 2.64 b (265.64%) | 2.06 ± 0.44 c (154.32%) |
| SM10 + CP | 29.16 ± 4.71 d (−40.86%) | 9.36 ± 1.66 e (−43.98%) | 1.57 ± 0.78 f (−23.78%) |
| SM20 + CP | 21.16 ± 2.79 g (56.93%) | 5.63 ± 1.32 h (−66.30%) | 0.91 ± 0.1 i (−55.82%) |
| SM20 | 19.00 ± 3.03 j (1.82%) | 4.84 ± 1.47 k (5.90%) | 0.85 ± 0.04 l (4.93%) |
| Test Group | MDA (nmole/mg Tissue) | GSH (DTNB Conjugate Formed/mg Protein) | CAT (nmole of H2O2 Consumed/min/mg Protein | SOD (nmole Ephinephrine Protected from Oxidation/min/mg Protein |
|---|---|---|---|---|
| CNT | 9.07 ± 1.37 | 21.95 ± 3.39 | 15.01 ± 2.41 | 33.89 ± 1.07 |
| CP | 27.3 ± 3.65 ** | 6.23 ± 1.25 ## | 5.45 ± 1.30 a | 13.35 ± 3.45 $$ |
| SM10 + CP | 15.66 ± 2.98 *** | 12.89 ± 2.17 ### | 10.74 ± 1.76 b | 25.42 ± 4.35 $$$ |
| SM20 + CP | 10.50 ± 1.49 *** | 19.89 ± 2.43 ### | 12.98 ± 1.74 b | 33.22 ± 1.58 $$$ |
| SM20 | 10.12 ± 1.20 * | 21.027 ± 2.25 # | 14.15 ± 2.65 c | 34.04 ± 3.55 $ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshahrani, S.; Ali Thubab, H.M.; Ali Zaeri, A.M.; Anwer, T.; Ahmed, R.A.; Jali, A.M.; Qadri, M.; Nomier, Y.; Moni, S.S.; Alam, M.F. The Protective Effects of Sesamin against Cyclophosphamide-Induced Nephrotoxicity through Modulation of Oxidative Stress, Inflammatory-Cytokines and Apoptosis in Rats. Int. J. Mol. Sci. 2022, 23, 11615. https://doi.org/10.3390/ijms231911615
Alshahrani S, Ali Thubab HM, Ali Zaeri AM, Anwer T, Ahmed RA, Jali AM, Qadri M, Nomier Y, Moni SS, Alam MF. The Protective Effects of Sesamin against Cyclophosphamide-Induced Nephrotoxicity through Modulation of Oxidative Stress, Inflammatory-Cytokines and Apoptosis in Rats. International Journal of Molecular Sciences. 2022; 23(19):11615. https://doi.org/10.3390/ijms231911615
Chicago/Turabian StyleAlshahrani, Saeed, Hani M. Ali Thubab, Abdulrahman M. Ali Zaeri, Tarique Anwer, Rayan A. Ahmed, Abdulmajeed M. Jali, Marwa Qadri, Yousra Nomier, Sivakumar S. Moni, and Mohammad F. Alam. 2022. "The Protective Effects of Sesamin against Cyclophosphamide-Induced Nephrotoxicity through Modulation of Oxidative Stress, Inflammatory-Cytokines and Apoptosis in Rats" International Journal of Molecular Sciences 23, no. 19: 11615. https://doi.org/10.3390/ijms231911615






