Study of Biological Activities and ADMET-Related Properties of Salicylanilide-Based Peptidomimetics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Physicochemical Properties
2.2. In Vitro Antimicrobial Activity
2.3. In Vitro Cell Viability
2.4. Structure–Activity Relationships
3. Materials and Methods
3.1. General Methods
3.2. Synthesis
3.2.1. General Procedure for Synthesis of (benzyloxy) Trifluoromethylbenzamides 2
3.2.2. General Procedure for Synthesis of (hydroxy) Trifluoromethylbenzamides 3
3.3. Lipophilicity Determination by HPLC
3.4. In Vitro Antibacterial Evaluation
3.5. Determination of Minimum Bactericidal Concentrations
3.6. MTT Assay
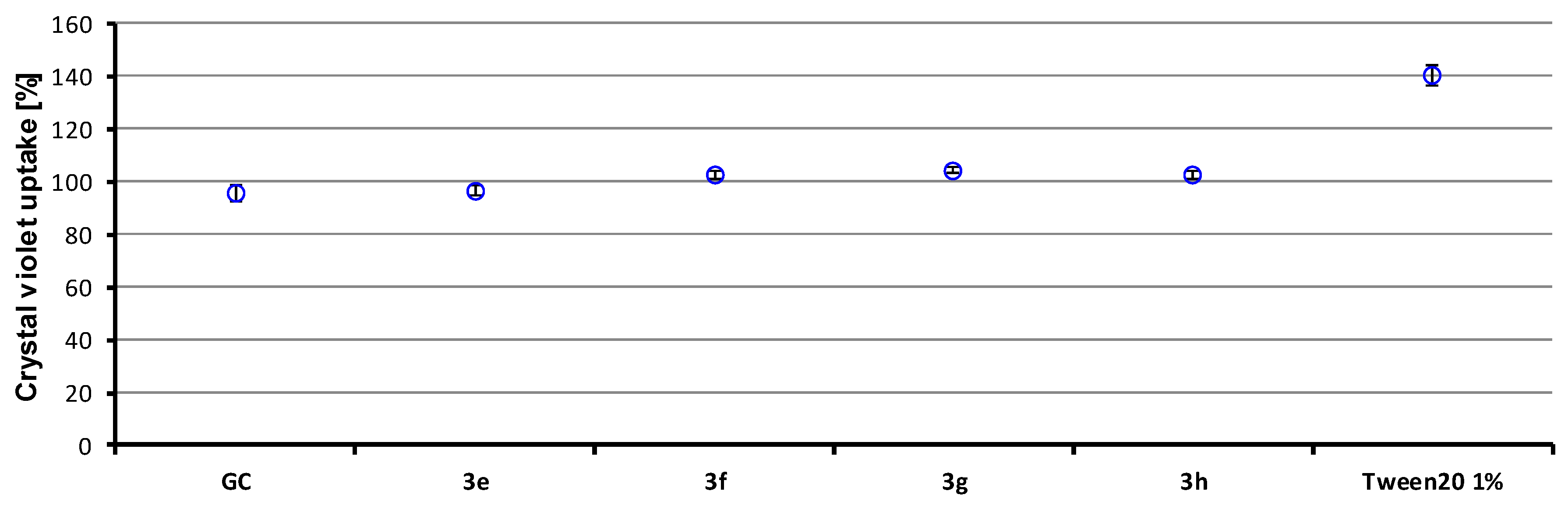
3.7. Crystal Violet Uptake
3.8. In Vitro Antimycobacterial Evaluation
3.9. In Vitro Cell Viability Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Salicylic Acid, DrugBank. Available online: https://go.drugbank.com/drugs/DB00936 (accessed on 2 September 2022).
- Aspirin, DrugBank. Available online: https://go.drugbank.com/drugs/DB00945 (accessed on 2 September 2022).
- WHO Model List of Essential Medicines-22nd List. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 2 September 2022).
- Somasundaram, S.; Sigthorsson, G.; Simpson, R.J.; Watts, J.; Jacob, M.; Tavares, I.A.; Rafi, S.; Roseth, A.; Foster, R.; Price, A.B.; et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment. Pharmacol. Ther. 2000, 14, 639–650. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; de Boer, V.C.J. Mitochondrial and glycolytic extracellular flux analysis optimization for isolated pig intestinal epithelial cells. Sci. Rep. 2021, 11, 19961. [Google Scholar] [CrossRef] [PubMed]
- Paul-Clark, M.J.; van Cao, T.; Moradi-Bidhendi, N.; Cooper, D.; Gilroy, D.W. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J. Exp. Med. 2004, 200, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ausina, P.; Branco, J.R.; Demaria, T.M.; Esteves, A.M.; Leandro, J.G.B.; Ochioni, A.C.; Mendonca, A.P.M.; Palhano, F.L.; Oliveira, M.F.; Abou-Kheir, W.; et al. Acetylsalicylic acid and salicylic acid present anticancer properties against melanoma by promoting nitric oxide-dependent endoplasmic reticulum stress and apoptosis. Sci. Rep. 2020, 10, 19617. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basilio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Nath, N.; Chattopadhyay, M.; Rodes, D.B.; Nazarenko, A.; Kodela, R.; Kashfi, K. Nitric oxide-releasing aspirin suppresses NF-κB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Molecules 2015, 20, 12481–12499. [Google Scholar] [CrossRef] [Green Version]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; Green, K.A.; Mustard, K.J.; et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 2012, 336, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, W.; Kim, D.; Jeong, S.; Jung, Y. Mesalazine activates adenosine monophosphate-activated protein kinase: Implication in the anti-inflammatory activity of this anti-colitic drug. Curr. Mol. Pharmacol. 2019, 12, 272–280. [Google Scholar] [CrossRef]
- Alfonso, L.F.; Srivenugopal, K.S.; Bhat, G.J. Does aspirin acetylate multiple cellular proteins? Mol. Med. Rep. 2009, 2, 533–537. [Google Scholar] [PubMed] [Green Version]
- Christensen, D.G.; Xie, X.; Basisty, N.; Byrnes, J.; McSweeney, S.; Schilling, B.; Wolfe, A.J. Post-translational protein acetylation: An elegant mechanism for bacteria to dynamically regulate metabolic functions. Front. Microbiol. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angom, R.S.; Zhu, J.; Wu, A.T.H.; Sumitra, M.R.; Pham, V.; Dutta, S.; Wang, E.; Madamsetty, V.S.; Perez-Cordero, G.D.; Huang, H.S.; et al. LCC-09, a novel salicylanilide derivative, exerts anti-inflammatory effect in vascular endothelial cells. J. Inflamm. Res. 2021, 14, 4551–4565. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J. Salicylanilide ester prodrugs as potential antimicrobial agents—A review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Marrugal-Lorenzo, J.A.; Serna-Gallego, A.; Berastegui-Cabrera, J.; Pachon, J.; Sanchez-Cespedes, J. Repositioning salicylanilide anthelmintic drugs to treat adenovirus infections. Sci. Rep. 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Monreal-Ferriz, J.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus. Eur. J. Pharm. Sci. 2015, 77, 197–207. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kollar, P.; Ferreira, A.L.; Palma, D.; Duarte, A.; Lopes, M.M.; Bartos, M.; Pauk, K.; Imramovsky, A.; Jampilek, J. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J. Appl. Biomed. 2016, 14, 125–130. [Google Scholar] [CrossRef]
- Copp, J.N.; Pletzer, D.; Brown, A.S.; van der Heijden, J.; Miton, C.M.; Edgar, R.J.; Rich, M.H.; Little, R.F.; Williams, E.M.; Hancock, R.E.W.; et al. Mechanistic understandingenables the rational design of salicylanilide combination therapies for Gram-negative infections. mBio 2020, 11, e02068-20. [Google Scholar] [CrossRef]
- Imramovsky, A.; Stepankova, S.; Vanco, J.; Pauk, K.; Monreal-Ferriz, J.; Vinsova, J.; Jampilek, J. Acetylcholinesterase-inhibiting activity of salicylanilide N-alkylcarbamates and their molecular docking. Molecules 2012, 17, 10142–10158. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Stepankova, S.; Houngbedji, N.H.; Vosatka, R.; Vorcakova, K.; Vinsova, J. 2-Hydroxy-N-phenylbenzamides and their esters inhibit acetylcholinesterase and butyrylcholinesterase. Biomolecules 2019, 9, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabocchi, A. Principles and applications of small molecule peptidomimetics. In Small Molecule Drug Discovery; Trabocchi, A., Lenci, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–195. [Google Scholar]
- Ghosh, A.K.; Brindisi, M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matosevic, A.; Bosak, A. Carbamate group as structural motif in drugs: A review of carbamate derivatives used as therapeutic agents. Arch. Ind. Hyg. Toxicol. 2020, 71, 285–299. [Google Scholar]
- Makhoba, X.H.; Viegas, C.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des. Dev. Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.A.; Wenzel, M. Multitarget approaches against multiresistant superbugs. ACS Infect. Dis. 2020, 6, 1346–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules 2011, 16, 2414–2430. [Google Scholar] [CrossRef] [Green Version]
- Pauk, K.; Zadrazilova, I.; Imramovsky, A.; Vinsova, J.; Pokorna, M.; Masarikova, M.; Cizek, A.; Jampilek, J. New derivatives of salicylamides: Preparation and antimicrobial activity against various bacterial species. Bioorg. Med. Chem. 2013, 21, 6574–6581. [Google Scholar] [CrossRef]
- Zadrazilova, I.; Pospisilova, S.; Pauk, K.; Imramovsky, A.; Vinsova, J.; Cizek, A.; Jampilek, J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA. BioMed Res. Int. 2015, 2015, 349534. [Google Scholar] [CrossRef] [Green Version]
- Imramovsky, A.; Jorda, R.; Pauk, K.; Reznickova, E.; Dusek, J.; Hanusek, J.; Krystof, V. Substituted 2-hydroxy-N-(arylalkyl)benzamides induce apoptosis in cancer cell lines. Eur. J. Med. Chem. 2013, 68, 253–259. [Google Scholar] [CrossRef]
- Dusek, J.; Imramovsky, A.; Pauk, K.; Jorda, R.; Reznickova, E.; Krystof, V. Synthesis and antiproliferative activities of novel O-benzyl salicylamide derivatives. Lett. Drug Des. Discov. 2017, 14, 662–671. [Google Scholar] [CrossRef]
- Jorda, R.; Dusek, J.; Reznickova, E.; Pauk, K.; Magar, P.; Imramovsky, A.; Krystof, V. Synthesis and antiproteasomal activity of novel O-benzyl salicylamide-based inhibitors built from leucine and phenylalanine. Eur. J. Med. Chem. 2017, 135, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Magar, P.; Hendrychova, D.; Pauk, K.; Dibus, M.; Pilarova, E.; Imramovsky, A.; Krystof, V. Novel modified leucine and phenylalanine dipeptides modulate viability and attachment of cancer cells. Eur. J. Med. Chem. 2020, 188, 112036. [Google Scholar] [CrossRef] [PubMed]
- Felicio, M.R.; Silva, O.N.; Goncalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thankappan, B.; Sivakumar, J.; Asokan, S.; Ramasamy, M.; Pillai, M.M.; Selvakumar, R.; Angayarkanni, J. Dual antimicrobial and anticancer activity of a novel synthetic α-helical antimicrobial peptide. Eur. J. Pharm. Sci. 2021, 161, 105784. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.P. Antibacterial and anticancer activity of a series of novel peptides incorporating cyclic tetra-substituted Cα amino acids. Bioorg. Med. Chem. 2016, 24, 4056–4065. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, S.; Chadha, B.S.; Kaur, R.; Kaur, M.; Kaur, S. Anticancer and antimicrobial potential of enterocin 12a from Enterococcus faecium. BMC Microbiol. 2021, 21, 39. [Google Scholar] [CrossRef]
- Parchebafi, A.; Tamanaee, F.; Ehteram, H.; Ahmad, E.; Nikzad, H.; Kashani, H.H. The dual interaction of antimicrobial peptides on bacteria and cancer cells; mechanism of action and therapeutic strategies of nanostructures. Microb. Cell Fact. 2022, 21, 118. [Google Scholar] [CrossRef]
- Garner, A.L.; Gloeckner, C.; Tricoche, N.; Zakhari, J.S.; Samje, M.; Cho-Ngwa, F.; Lustigman, S.; Janda, K.D. Design, synthesis, and biological activities of closantel analogues: Structural promiscuity and its impact on Onchocerca volvulus. J. Med. Chem. 2011, 54, 3963–3972. [Google Scholar] [CrossRef]
- Fomovska, A.; Wood, R.D.; Mui, E.; Dubey, J.P.; Ferreira, L.R.; Hickman, M.R.; Lee, P.J.; Leed, S.E.; Auschwitz, J.M.; Welsh, W.J.; et al. Salicylanilide inhibitors of toxoplasma gondii. J. Med. Chem. 2012, 55, 8375–8391. [Google Scholar] [CrossRef] [Green Version]
- Laudisi, F.; Maronek, M.; Di Grazia, A.; Monteleone, G.; Stolfi, C. Repositioning of anthelmintic drugs for the treatment of cancers of the digestive system. Int. J. Mol. Sci. 2020, 21, 4957. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Drug repurposing to overcome microbial resistance. Drug Discov. Today 2022, 27, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J.; Novotna, E.; Mandikova, J.; Trejtnar, F.; Stolarikova, J. Antibacterial activity of salicylanilide 4-(trifluoromethyl)-benzoates. Molecules 2013, 18, 3674–3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratky, M.; Volkova, M.; Novotna, E.; Trejtnar, F.; Stolarikova, J.; Vinsova, J. Synthesis and biological activity of new salicylanilide N,N-disubstituted carbamates and thiocarbamates. Bioorg. Med. Chem. 2014, 22, 4073–4082. [Google Scholar] [CrossRef]
- Molchanova, N.; Nielsen, J.E.; Sorensen, K.B.; Prabhala, B.K.; Hansen, P.R.; Lund, R.; Barron, A.E.; Jenssen, H. Halogenation as a tool to tune antimicrobial activity of peptoids. Sci. Rep. 2020, 10, 14805. [Google Scholar] [CrossRef]
- Arnot, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Kerns, E.H.; Di, L. Drug-like Properties: Concepts. Structure Design and Methods: From ADME to Toxicity Optimization; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Wermuth, C.; Aldous, D.; Raboisson, P.; Rognan, D. The Practice of Medicinal Chemistry, 4th ed.; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ertl, P.; Schuffenhauer, A. Estimation of synthetic accessibility score of drug-like molecules based on molecular complexity and fragment contributions. J. Cheminform. 2009, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Oravcova, V.; Zurek, L.; Townsend, A.; Clark, A.B.; Ellis, J.C.; Cizek, A. American crows as carriers of vancomycin-resistant enterococci with vanA gene. Environ. Microbiol. 2014, 16, 939–949. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Nubel, U.; Dordel, J.; Kurt, K.; Strommenger, B.; Westh, H.; Shukla, S.K.; Zemlickova, H.; Leblois, R.; Wirth, T.; Jombart, T.; et al. A timescale for evolution, population expansion, and spatial spread of an emerging clone of methicillin-resistant Staphylococcus aureus. PLoS Pathog. 2010, 6, e1000855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Measuring Cell Viability/Cytotoxicity. Dojindo EU GmbH, Munich, Germany. Available online: https://www.dojindo.eu.com/Protocol/Dojindo-Cell-Proliferation-Protocol.pdf (accessed on 2 September 2022).
- Grela, E.; Kozlowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Permana, A.D.; Djide, N.J.N.; Anjani, Q.K.; Utami, R.N.; Rumata, N.R.; Zhang, J.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical approaches on antimicrobial resistance: Prospects and challenges. Antibiotics 2021, 10, 981. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, J.I.; Szczepski, K.; Scano, A.; Casu, C.; Fais, S.; Orru, G.; Pisano, B.; Piras, M.; Jaremko, M. The best peptidomimetic strategies to undercover antibacterial peptides. Int. J. Mol. Sci. 2020, 21, 7349. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Vaara, M.; Vaara, T. Outer membrane permeability barrier disruption by polymyxinin polymyxin-susceptible and-resistant Salmonella typhimurium. Antimicrob. Agents Chemother. 1981, 19, 578–583. [Google Scholar] [CrossRef] [Green Version]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07; NCCLS: Wayne, PA, USA, 2018. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.; Duran, N.; Nakazato, G.; Kobayashi, R.K. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef]
- Guimaraes, A.C.; Meireles, L.M.; Lemos, M.F.; Guimaraes, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Kos, J.; Kozik, V.; Pindjakova, D.; Jankech, T.; Smolinski, A.; Stepankova, S.; Hosek, J.; Oravec, M.; Jampilek, J.; Bak, A. Synthesis and hybrid SAR property modeling of novel cholinesterase inhibitors. Int. J. Mol. Sci. 2021, 22, 3444. [Google Scholar] [CrossRef]



| No. | R1 | R2 | log k | log D6.5 | log D7.4 | log P a | log P b | Clog P b | MW a | HBD a | HBA a | RB a | MV a [cm3] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
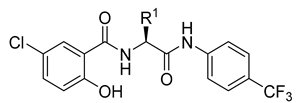 | |||||||||||||
| 3a | Me | – | 0.866 | 0.804 | 0.759 | 4.51 | 3.27 | 5.004 | 386.75 | 3 | 5 | 5 | 30.79 |
| 3b | Pr | – | 1.141 | 1.060 | 1.016 | 5.14 | 4.18 | 6.062 | 414.81 | 3 | 5 | 7 | 63.80 |
| 3c | iPr | – | 1.081 | 0.973 | 0.934 | 4.98 | 4.16 | 5.932 | 414.81 | 3 | 5 | 6 | 64.18 |
| 3d | Bu | – | 1.322 | 1.247 | 1.213 | 5.30 | 4.59 | 6.591 | 428.83 | 3 | 5 | 8 | 80.31 |
| 3e | S-Bu | – | 1.076 | 1.021 | 0.963 | 5.20 | 3.61 | 5.152 | 446.87 | 3 | 5 | 8 | 77.77 |
| 3f | iBu | – | 1.266 | 1.256 | 1.161 | 5.32 | 4.51 | 6.461 | 428.83 | 3 | 5 | 7 | 80.68 |
| 3g | Me-cHex | – | 1.679 | 1.615 | 1.576 | 6.37 | 5.26 | 7.654 | 468.89 | 3 | 5 | 7 | 113.26 |
| 3h | Bn | – | 1.192 | 1.148 | 1.043 | 5.80 | 4.95 | 6.422 | 462.84 | 3 | 5 | 7 | 91.49 |
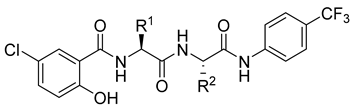 | |||||||||||||
| 6a | iBu | Bn | 1.560 | 1.510 | 1.493 | 6.38 | 5.52 | 7.621 | 576.01 | 4 | 7 | 11 | 172.17 |
| 6b | Bn | iBu | 1.547 | 1.528 | 1.453 | 6.29 | 5.52 | 7.621 | 576.01 | 4 | 7 | 11 | 172.17 |
| 6c | Bn | Bn | 1.587 | 1.548 | 1.495 | 7.56 | 5.97 | 7.582 | 610.02 | 4 | 7 | 11 | 182.98 |
| Ro5 | – | – | – | <5 | <5 | – | <500 | <5 | <10 | – | – | ||
| No. | R1 R2 | MIC (µM) MBC (µM) | IC50 (µM) THP-1@10% FBS 24 h | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | MRSA1 | MRSA2 | MRSA3 | EF | VRE1 | VRE2 | VRE3 | MT | MS | |||
| 3a | Me – | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | >10 |
| 3b | Pr – | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | 7.3 ± 1.2 |
| 3c | iPr – | 2.41 4.82 | 4.82 4.82 | 4.82 4.82 | 4.82 9.64 | 38.6 38.6 | 38.6 38.6 | 38.6 38.6 | 38.6 38.6 | 77.1 n.d. | 77.1 n.d. | >10 |
| 3d | Bu – | 0.583 1.17 | 2.33 2.33 | 2.33 4.66 | 4.66 4.66 | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | 4.5 ± 1.2 |
| 3e | S-Bu – | 2.24 2.24 | 8.95 8.95 | 4.48 8.95 | 8.95 8.95 | 17.9 35.8 | 35.8 35.8 | 17.9 35.8 | 35.8 35.8 | 35.8 n.d. | 35.8 n.d. | >10 |
| 3f | iBu – | 1.17 1.17 | 1.17 1.17 | 1.17 1.17 | 0.070 0.070 | 4.66 9.33 | 4.66 18.7 | 9.33 18.7 | 4.66 37.3 | 18.7 n.d. | 18.7 n.d. | 1.9 ± 1.1 |
| 3g | Me-cHex – | 1.07 1.07 | 1.07 1.07 | 1.07 2.13 | 2.13 2.13 | 546 n.d. | 546 n.d. | 546 n.d. | 546 n.d. | n.a. n.d. | n.a. n.d. | 1.4 ± 1.1 |
| 3h | Bn – | 1.08 2.16 | 2.16 2.16 | 1.08 2.16 | 0.270 0.270 | 277 n.d. | 277 n.d. | 277 n.d. | 277 n.d. | n.a. n.d. | n.a. n.d. | 3.3 ± 1.0 |
| 6a | iBu Bn | 222 n.d. | 444 n.d. | 444 n.d. | 444 n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | 2.2 ± 1.1 |
| 6b | Bn iBu | 55.6 112 | 55.6 n.d. | 55.6 444 | 444 n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | 1.6 ± 1.0 |
| 6c | Bn Bn | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | n.a. n.d. | 1.3 ± 1.1 |
| APC | – | 5.72 >5.72 | 45.8 >45.8 | 45.8 >45.8 | 45.8 >45.8 | 2.81 2.81 | 11.5 11.5 | 11.5 11.5 | 11.5 11.5 | – | – | – |
| INH | – | – | – | – | – | – | – | – | – | 36.6 – | 117 – | – |
| No. | Conc. | S. aureus Viability Inhibition (%) |
|---|---|---|
| 3e | 2× MIC (2× MBC) | 94.6 |
| 3f | 1× MIC (1× MBC) | 95.3 |
| 3g | 0.5× MIC (0.5× MBC) | 95.2 |
| 3h | 2× MIC (2× MBC) | 94.0 |
| APC | 8× MIC (>8× MBC) | 90.0 |
| CPX | 32× MIC (32× MBC) | 92.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pindjakova, D.; Pilarova, E.; Pauk, K.; Michnova, H.; Hosek, J.; Magar, P.; Cizek, A.; Imramovsky, A.; Jampilek, J. Study of Biological Activities and ADMET-Related Properties of Salicylanilide-Based Peptidomimetics. Int. J. Mol. Sci. 2022, 23, 11648. https://doi.org/10.3390/ijms231911648
Pindjakova D, Pilarova E, Pauk K, Michnova H, Hosek J, Magar P, Cizek A, Imramovsky A, Jampilek J. Study of Biological Activities and ADMET-Related Properties of Salicylanilide-Based Peptidomimetics. International Journal of Molecular Sciences. 2022; 23(19):11648. https://doi.org/10.3390/ijms231911648
Chicago/Turabian StylePindjakova, Dominika, Eliska Pilarova, Karel Pauk, Hana Michnova, Jan Hosek, Pratibha Magar, Alois Cizek, Ales Imramovsky, and Josef Jampilek. 2022. "Study of Biological Activities and ADMET-Related Properties of Salicylanilide-Based Peptidomimetics" International Journal of Molecular Sciences 23, no. 19: 11648. https://doi.org/10.3390/ijms231911648
APA StylePindjakova, D., Pilarova, E., Pauk, K., Michnova, H., Hosek, J., Magar, P., Cizek, A., Imramovsky, A., & Jampilek, J. (2022). Study of Biological Activities and ADMET-Related Properties of Salicylanilide-Based Peptidomimetics. International Journal of Molecular Sciences, 23(19), 11648. https://doi.org/10.3390/ijms231911648







