Receptor for Advanced Glycation End-Products Promotes Activation of Alveolar Macrophages through the NLRP3 Inflammasome/TXNIP Axis in Acute Lung Injury
Abstract
1. Introduction
2. Results
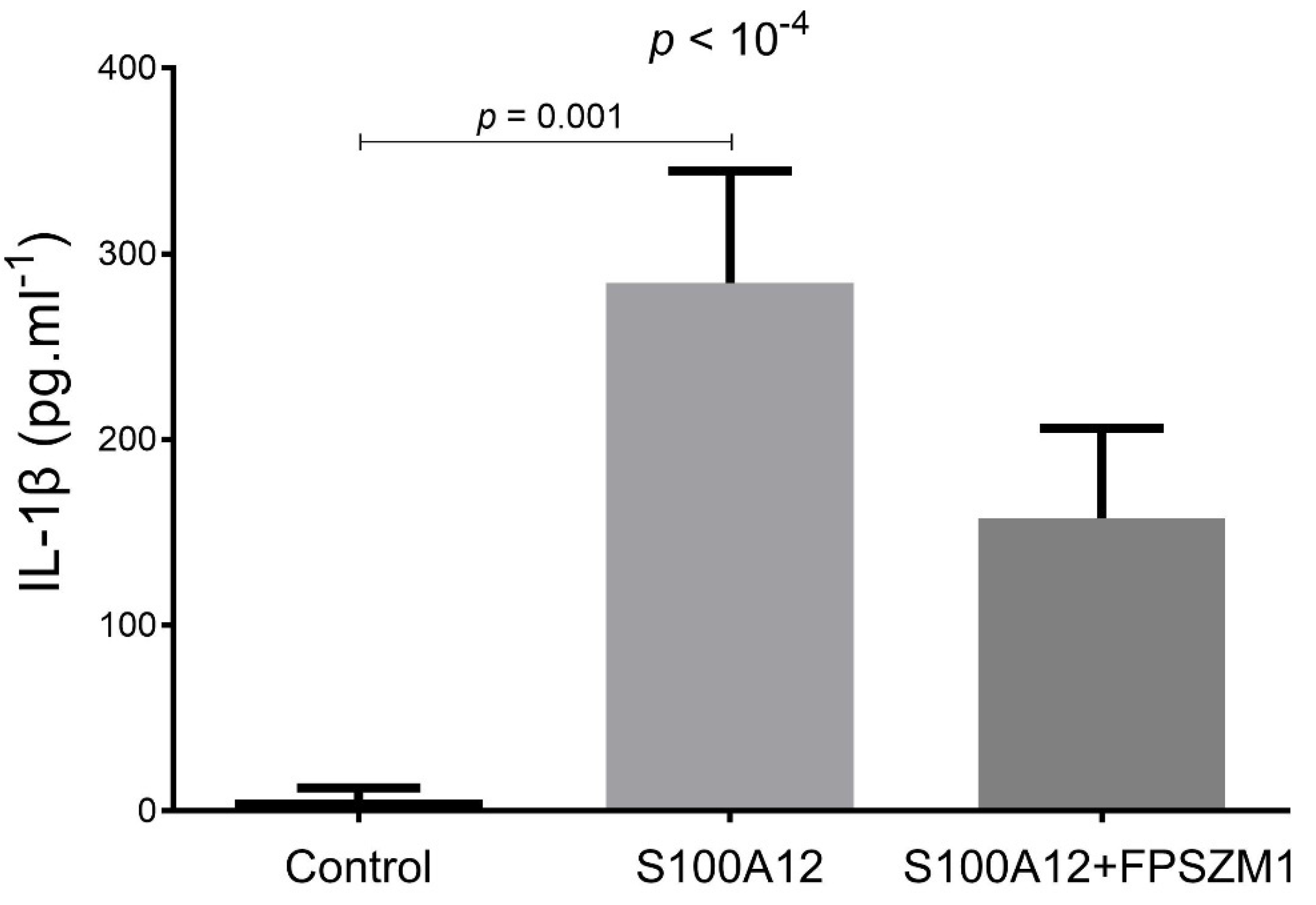
2.1. S100A12 Stimulates ROS Production and IL-1β Secretion in a RAGE-Dependent Manner in THP-1 Cells
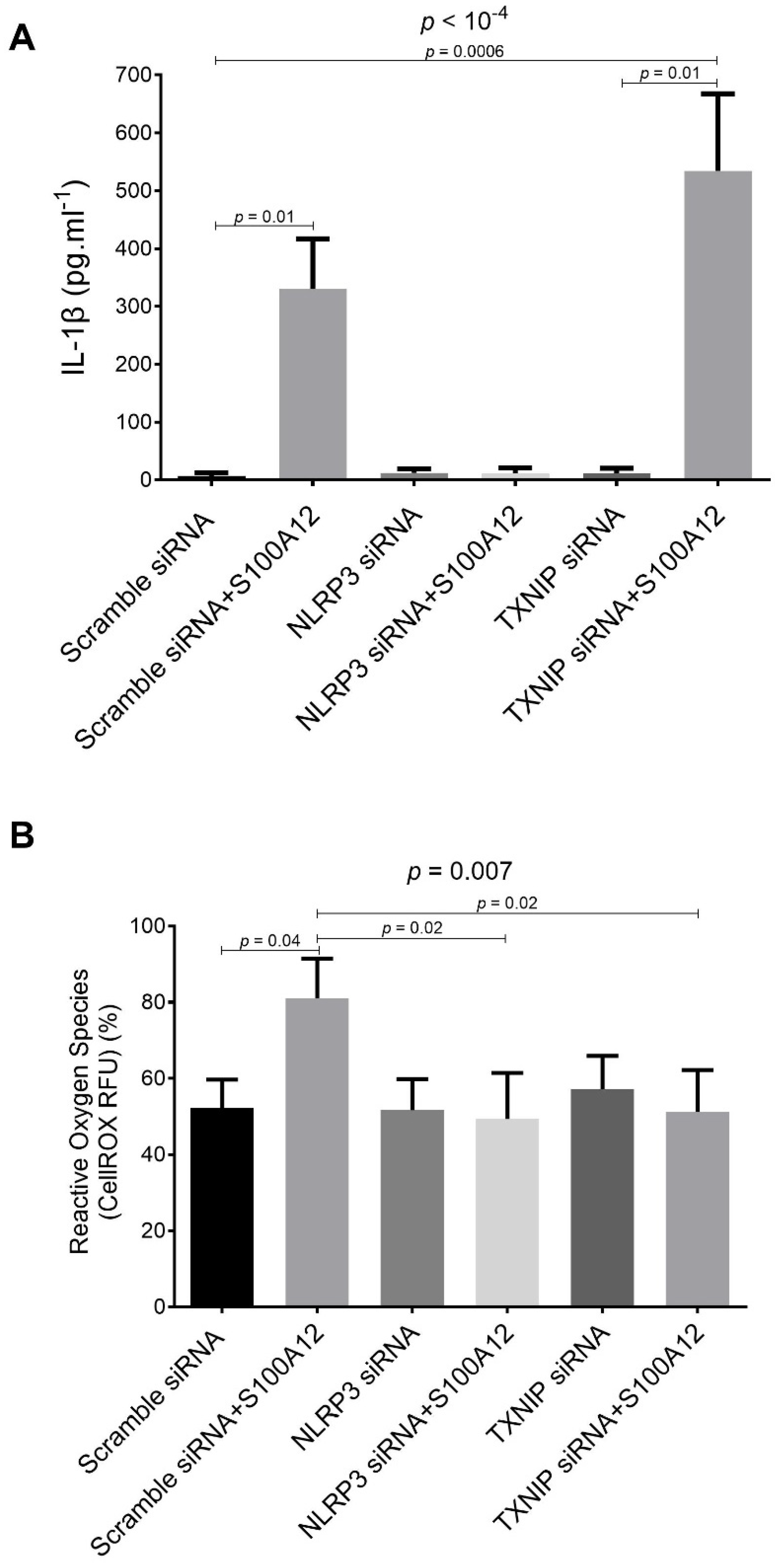
2.2. Effects of siRNA-Targeted TXNIP and NLRP3 Knockdown on S100A12-Induced IL-1β Secretion and ROS Production by THP-1 Cells
2.3. RAGE-Dependent Regulation of Gene Expression in S100A12-Activated THP-1 Cells
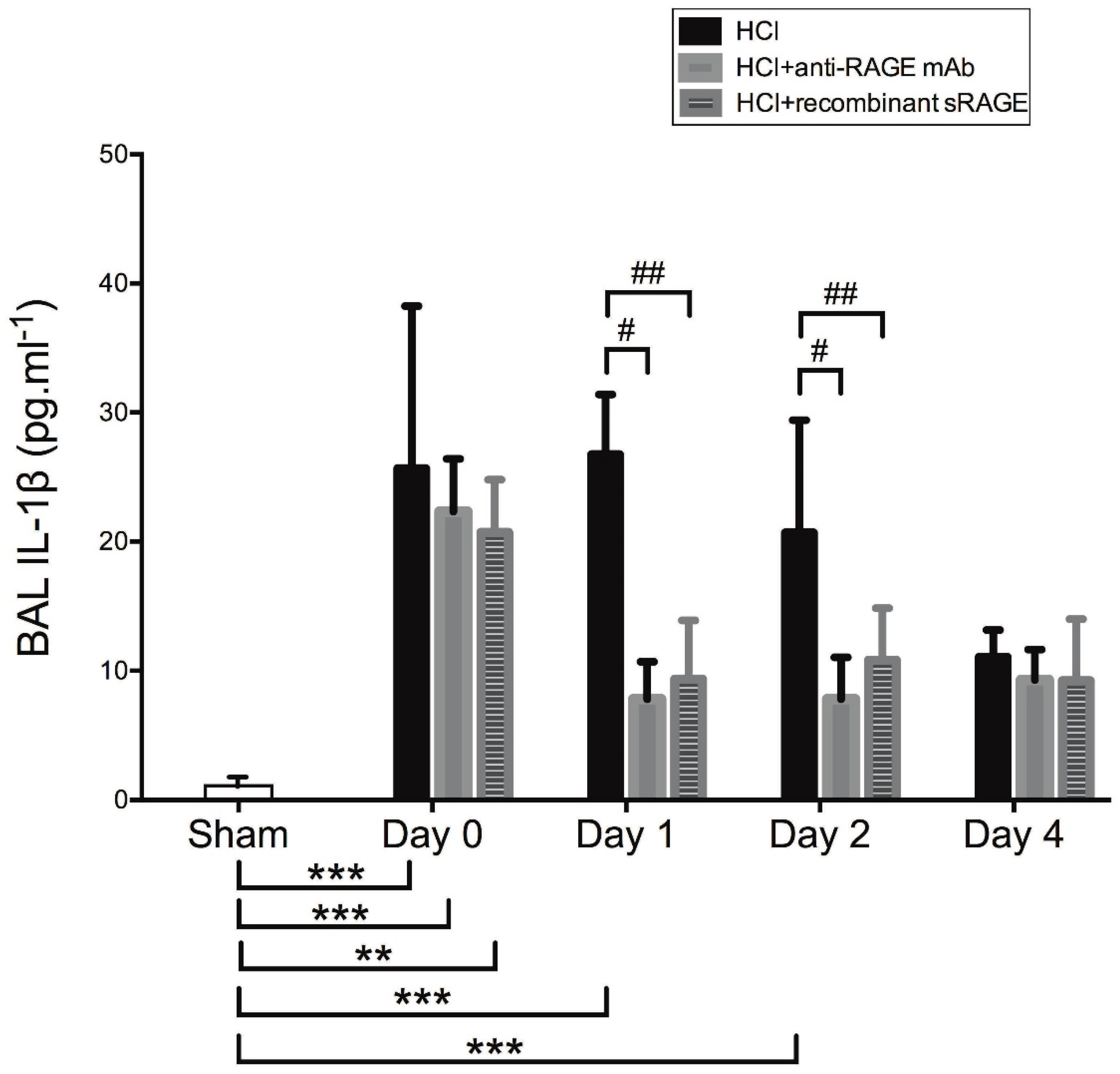
2.4. Anti-RAGE mAb and Recombinant sRAGE Prevent HCl Injury-Induced Upregulation of Lung NLRP3 and TXNIP In Vivo
2.5. Effects of Acid-Induced Injury and RAGE Modulation on Alveolar Levels of IL-1β
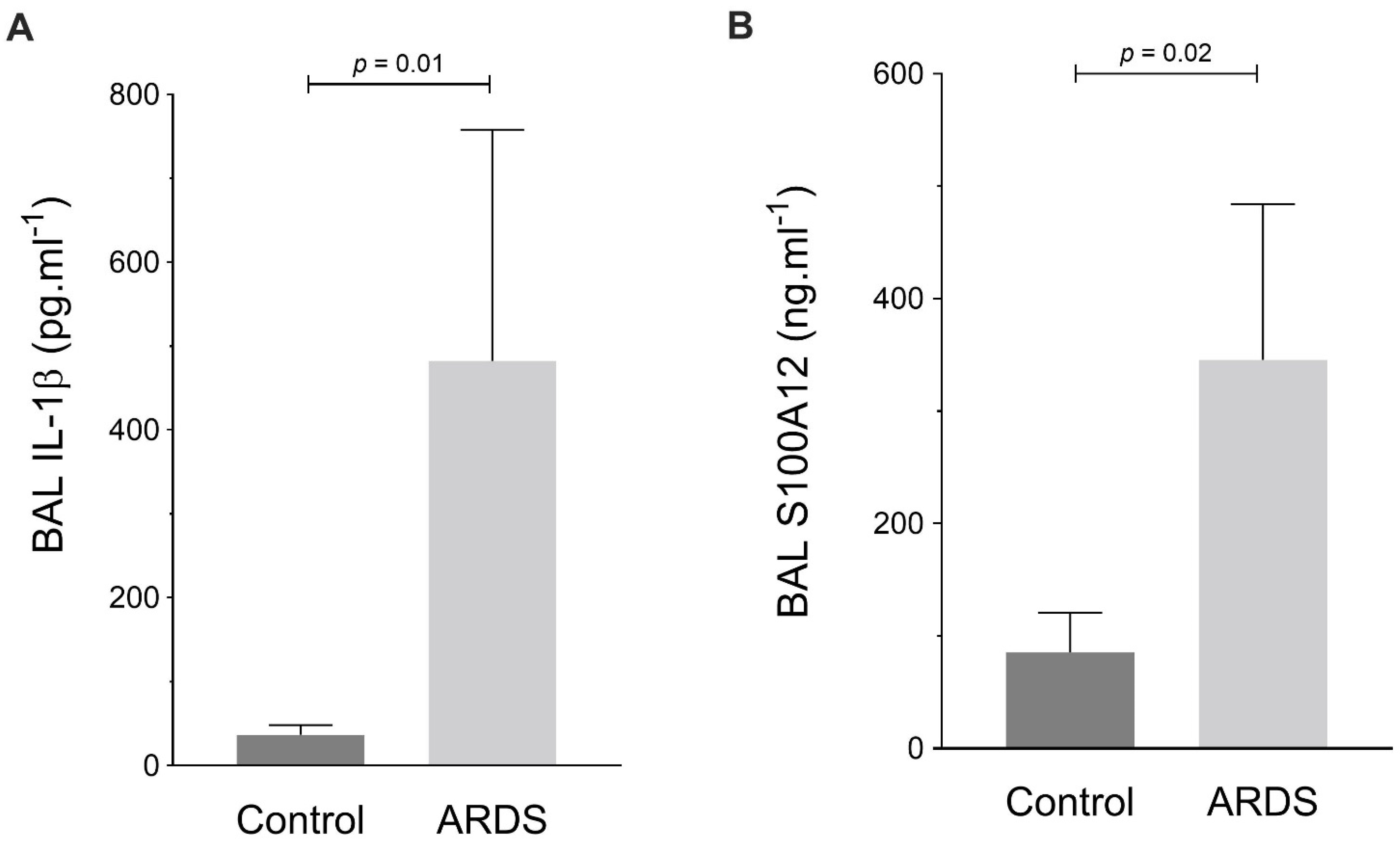
2.6. RAGE, TXNIP and NLRP3 Expression by Human Alveolar Macrophages during ARDS
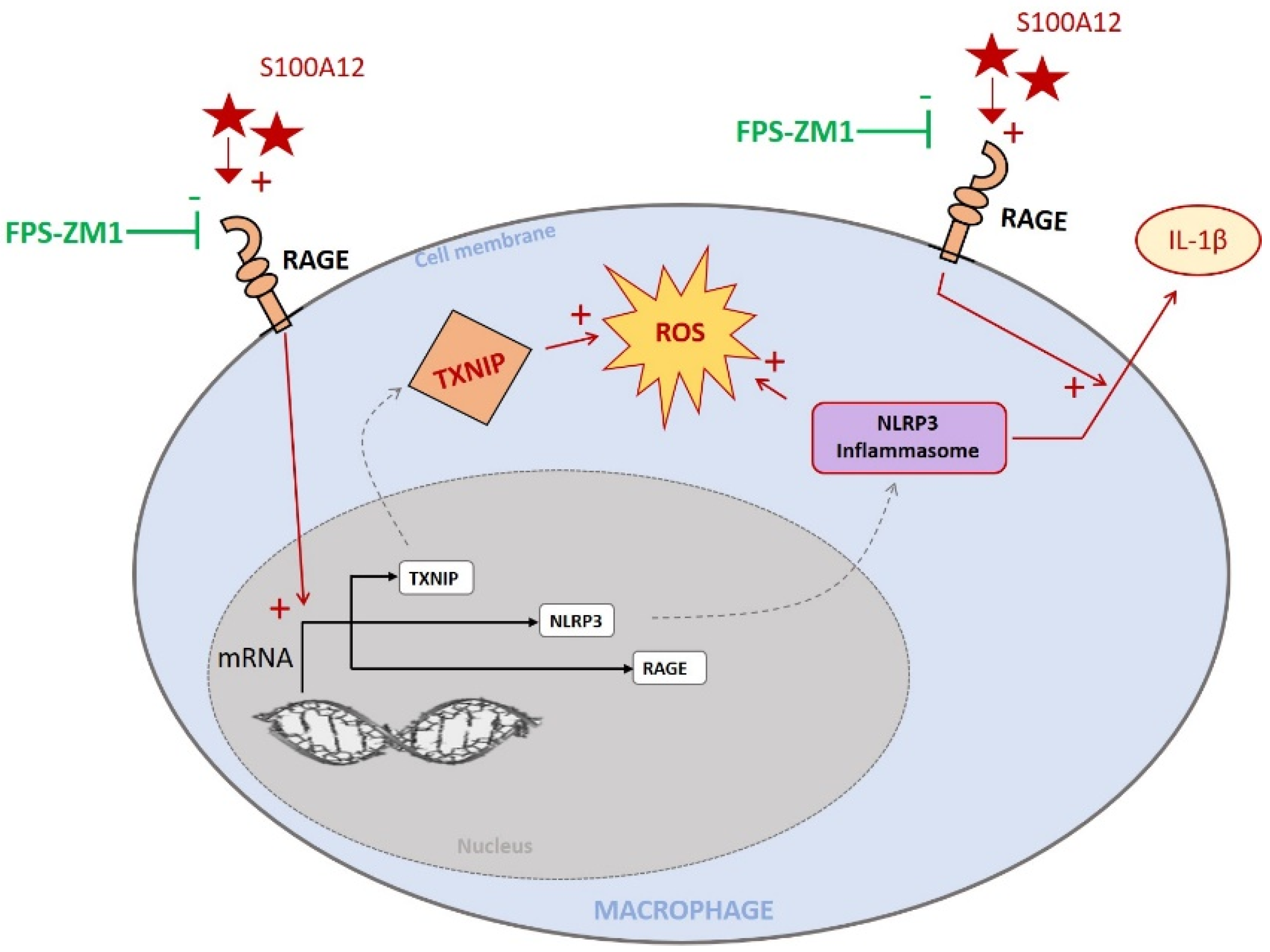
3. Discussion
4. Materials and Methods
4.1. Cell Experiments
4.2. Animal Studies
4.3. Human Study
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.W.; Berg, N.K.; Reskallah, A.; Yuan, X.; Eltzschig, H.K. Acute Respiratory Distress Syndrome. Anesthesiology 2021, 134, 270–282. [Google Scholar] [CrossRef]
- Guo, W.A.; Knight, P.R.; Raghavendran, K. The Receptor for Advanced Glycation End Products and Acute Lung Injury/acute Respiratory Distress Syndrome. Intensive Care Med. 2012, 38, 1588–1598. [Google Scholar] [CrossRef]
- Li, J.; Wang, K.; Huang, B.; Li, R.; Wang, X.; Zhang, H.; Tang, H.; Chen, X. The Receptor for Advanced Glycation End Products Mediates Dysfunction of Airway Epithelial Barrier in a Lipopolysaccharides-Induced Murine Acute Lung Injury Model. Int. Immunopharmacol. 2021, 93, 107419. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Roszyk, L.; Bouvier, D.; Audard, J.; Clairefond, G.; Fournier, M.; Marceau, G.; Déchelotte, P.; Pereira, B.; et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2015, 192, 191–199. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Laffey, J.G.; Ware, L.B.; Heijnen, N.F.L.; Sinha, P.; Patel, B.; Jabaudon, M.; Bastarache, J.A.; McAuley, D.F.; Summers, C.; et al. Towards a Biological Definition of ARDS: Are Treatable Traits the Solution? Intensive Care Med. Exp. 2022, 10, 8. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The Biology of the Receptor for Advanced Glycation End Products and Its Ligands. Biochim. Biophys. Acta 2000, 1498, 99–111. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The Multiligand Receptor RAGE as a Progression Factor Amplifying Immune and Inflammatory Responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Stern, D.M. Receptor for Age (RAGE) Is a Gene within the Major Histocompatibility Class III Region: Implications for Host Response Mechanisms in Homeostasis and Chronic Disease. Front. Biosci. 2001, 6, D1151–D1160. [Google Scholar] [PubMed]
- Uchida, T.; Shirasawa, M.; Ware, L.B.; Kojima, K.; Hata, Y.; Makita, K.; Mednick, G.; Matthay, Z.A.; Matthay, M.A. Receptor for Advanced Glycation End-Products Is a Marker of Type I Cell Injury in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2006, 173, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Jabaudon, M.; Blondonnet, R.; Roszyk, L.; Pereira, B.; Guérin, R.; Perbet, S.; Cayot, S.; Bouvier, D.; Blanchon, L.; Sapin, V.; et al. Soluble Forms and Ligands of the Receptor for Advanced Glycation End-Products in Patients with Acute Respiratory Distress Syndrome: An Observational Prospective Study. PLoS ONE 2015, 10, e0135857. [Google Scholar] [CrossRef] [PubMed]
- Jabaudon, M.; Futier, E.; Roszyk, L.; Chalus, E.; Guerin, R.; Petit, A.; Mrozek, S.; Perbet, S.; Cayot-Constantin, S.; Chartier, C.; et al. Soluble Form of the Receptor for Advanced Glycation End Products Is a Marker of Acute Lung Injury but Not of Severe Sepsis in Critically Ill Patients. Crit. Care Med. 2011, 39, 480–488. [Google Scholar] [CrossRef]
- Jabaudon, M.; Hamroun, N.; Roszyk, L.; Guérin, R.; Bazin, J.-E.; Sapin, V.; Pereira, B.; Constantin, J.-M. Effects of a Recruitment Maneuver on Plasma Levels of Soluble RAGE in Patients with Diffuse Acute Respiratory Distress Syndrome: A Prospective Randomized Crossover Study. Intensive Care Med. 2015, 41, 846–855. [Google Scholar] [CrossRef]
- Jabaudon, M.; Pereira, B.; Laroche, E.; Roszyk, L.; Blondonnet, R.; Audard, J.; Godet, T.; Futier, E.; Bazin, J.-E.; Sapin, V.; et al. Changes in Plasma Soluble Receptor for Advanced Glycation End-Products Are Associated with Survival in Patients with Acute Respiratory Distress Syndrome. J. Clin. Med. Res. 2021, 10, 2076. [Google Scholar] [CrossRef]
- Lim, A.; Radujkovic, A.; Weigand, M.A.; Merle, U. Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Biomarker of COVID-19 Disease Severity and Indicator of the Need for Mechanical Ventilation, ARDS and Mortality. Ann. Intensive Care 2021, 11, 50. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Ware, L.B. Biomarkers in Acute Respiratory Distress Syndrome. Curr. Opin. Crit. Care 2021, 27, 46–54. [Google Scholar] [CrossRef]
- Zhang, L.; Bukulin, M.; Kojro, E.; Roth, A.; Metz, V.V.; Fahrenholz, F.; Nawroth, P.P.; Bierhaus, A.; Postina, R. Receptor for Advanced Glycation End Products Is Subjected to Protein Ectodomain Shedding by Metalloproteinases. J. Biol. Chem. 2008, 283, 35507–35516. [Google Scholar] [CrossRef]
- Herold, K.; Moser, B.; Chen, Y.; Zeng, S.; Yan, S.F.; Ramasamy, R.; Emond, J.; Clynes, R.; Schmidt, A.M. Receptor for Advanced Glycation End Products (RAGE) in a Dash to the Rescue: Inflammatory Signals Gone Awry in the Primal Response to Stress. J. Leukoc. Biol. 2007, 82, 204–212. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE Mediates a Novel Proinflammatory Axis: A Central Cell Surface Receptor for S100/calgranulin Polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef]
- Abraham, E.; Arcaroli, J.; Carmody, A.; Wang, H.; Tracey, K.J. HMG-1 as a Mediator of Acute Lung Inflammation. J. Immunol. 2000, 165, 2950–2954. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yao, T.; Zhou, Z. ’e; Zhu, J.; Zhang, S.; Hu, W.; Shen, C. Advanced Glycation End Products Enhance Macrophages Polarization into M1 Phenotype through Activating RAGE/NF-κB Pathway. Biomed Res. Int. 2015, 2015, 732450. [Google Scholar] [CrossRef] [PubMed]
- Motoyoshi, S.; Yamamoto, Y.; Munesue, S.; Igawa, H.; Harashima, A.; Saito, H.; Han, D.; Watanabe, T.; Sato, H.; Yamamoto, H. cAMP Ameliorates Inflammation by Modulation of Macrophage Receptor for Advanced Glycation End-Products. Biochem. J 2014, 463, 75–82. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Taming the Inflammasome. Nat. Med. 2015, 21, 213–215. [Google Scholar] [CrossRef]
- Lee, S.; Suh, G.-Y.; Ryter, S.W.; Choi, A.M.K. Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 151–160. [Google Scholar] [CrossRef]
- Grailer, J.J.; Canning, B.A.; Kalbitz, M.; Haggadone, M.D.; Dhond, R.M.; Andjelkovic, A.V.; Zetoune, F.S.; Ward, P.A. Critical Role for the NLRP3 Inflammasome during Acute Lung Injury. J. Immunol. 2014, 192, 5974–5983. [Google Scholar] [CrossRef]
- Hosseinian, N.; Cho, Y.; Lockey, R.F.; Kolliputi, N. The Role of the NLRP3 Inflammasome in Pulmonary Diseases. Ther. Adv. Respir. Dis. 2015, 4, 188–197. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and Regulation of the Inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- van Zoelen, M.A.D.; Yang, H.; Florquin, S.; Meijers, J.C.M.; Akira, S.; Arnold, B.; Nawroth, P.P.; Bierhaus, A.; Tracey, K.J.; van der Poll, T. Role of Toll-like Receptors 2 and 4, and the Receptor for Advanced Glycation End Products in High-Mobility Group Box 1-Induced Inflammation in Vivo. Shock 2009, 31, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of NADPH Oxidase by AGE Links Oxidant Stress to Altered Gene Expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef]
- Zhong, Y.; Cheng, C.-F.; Luo, Y.-Z.; Tian, C.-W.; Yang, H.; Liu, B.-R.; Chen, M.-S.; Chen, Y.-F.; Liu, S.-M. C-Reactive Protein Stimulates RAGE Expression in Human Coronary Artery Endothelial Cells in Vitro via ROS Generation and ERK/NF-κB Activation. Acta Pharmacol. Sin. 2015, 36, 440–447. [Google Scholar] [CrossRef]
- He, Q.; You, H.; Li, X.-M.; Liu, T.-H.; Wang, P.; Wang, B.-E. HMGB1 Promotes the Synthesis of pro-IL-1β and pro-IL-18 by Activation of p38 MAPK and NF-κB through Receptors for Advanced Glycation End-Products in Macrophages. Asian Pac. J. Cancer Prev. 2012, 13, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-Interacting Protein Links Oxidative Stress to Inflammasome Activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 Inflammasome: A Sensor for Metabolic Danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Schulze, P.C.; Yoshioka, J.; Takahashi, T.; He, Z.; King, G.L.; Lee, R.T. Hyperglycemia Promotes Oxidative Stress through Inhibition of Thioredoxin Function by Thioredoxin-Interacting Protein. J. Biol. Chem. 2004, 279, 30369–30374. [Google Scholar] [CrossRef]
- Gao, P.; Meng, X.-F.; Su, H.; He, F.-F.; Chen, S.; Tang, H.; Tian, X.-J.; Fan, D.; Wang, Y.-M.; Liu, J.-S.; et al. Thioredoxin-Interacting Protein Mediates NALP3 Inflammasome Activation in Podocytes during Diabetic Nephropathy. Biochim. Biophys. Acta 2014, 1843, 2448–2460. [Google Scholar] [CrossRef]
- Xiang, M.; Shi, X.; Li, Y.; Xu, J.; Yin, L.; Xiao, G.; Scott, M.J.; Billiar, T.R.; Wilson, M.A.; Fan, J. Hemorrhagic Shock Activation of NLRP3 Inflammasome in Lung Endothelial Cells. J. Immunol. 2011, 187, 4809–4817. [Google Scholar] [CrossRef]
- Kelleher, Z.T.; Sha, Y.; Foster, M.W.; Foster, W.M.; Forrester, M.T.; Marshall, H.E. Thioredoxin-Mediated Denitrosylation Regulates Cytokine-Induced Nuclear Factor κB (NF-κB) Activation. J. Biol. Chem. 2013, 289, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Tipple, T.E.; Welty, S.E.; Nelin, L.D.; Hansen, J.M.; Rogers, L.K. Alterations of the Thioredoxin System by Hyperoxia: Implications for Alveolar Development. Am. J. Respir. Cell Mol. Biol. 2009, 41, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, R.; Jiang, W.; Wang, H.; Xu, A.; Lu, G.; Ren, Y.; Xu, Y.; Song, Y.; Yong, S.; et al. Protective Effect of Astragaloside IV Against Paraquat-Induced Lung Injury in Mice by Suppressing Rho Signaling. Inflammation 2016, 39, 483–492. [Google Scholar] [CrossRef]
- Han, S.; Cai, W.; Yang, X.; Jia, Y.; Zheng, Z.; Wang, H.; Li, J.; Li, Y.; Gao, J.; Fan, L.; et al. ROS-Mediated NLRP3 Inflammasome Activity Is Essential for Burn-Induced Acute Lung Injury. Mediators Inflamm. 2015, 2015, 720457. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Shirasuna, K.; Usui, F.; Karasawa, T.; Kawashima, A.; Kimura, H.; Kobayashi, M.; Komada, T.; Inoue, Y.; Mato, N.; et al. NLRP3 Protein Deficiency Exacerbates Hyperoxia-Induced Lethality through Stat3 Protein Signaling Independent of Interleukin-1β. J. Biol. Chem. 2015, 290, 5065–5077. [Google Scholar] [CrossRef]
- Dolinay, T.; Kim, Y.S.; Howrylak, J.; Hunninghake, G.M.; An, C.H.; Fredenburgh, L.; Massaro, A.F.; Rogers, A.; Gazourian, L.; Nakahira, K.; et al. Inflammasome-Regulated Cytokines Are Critical Mediators of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1225–1234. [Google Scholar] [CrossRef]
- Nakahira, K.; Kyung, S.-Y.; Rogers, A.J.; Gazourian, L.; Youn, S.; Massaro, A.F.; Quintana, C.; Osorio, J.C.; Wang, Z.; Zhao, Y.; et al. Circulating Mitochondrial DNA in Patients in the ICU as a Marker of Mortality: Derivation and Validation. PLoS Med. 2013, 10, e1001577. [Google Scholar] [CrossRef]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of Thioredoxin-Binding Protein-2/vitamin D(3) up-Regulated Protein 1 as a Negative Regulator of Thioredoxin Function and Expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef]
- Yoshihara, E.; Masaki, S.; Matsuo, Y.; Chen, Z.; Tian, H.; Yodoi, J. Thioredoxin/Txnip: Redoxisome, as a Redox Switch for the Pathogenesis of Diseases. Front. Immunol. 2014, 4, 514. [Google Scholar] [CrossRef]
- Mohamed, I.N.; Hafez, S.S.; Fairaq, A.; Ergul, A.; Imig, J.D.; El-Remessy, A.B. Thioredoxin-Interacting Protein Is Required for Endothelial NLRP3 Inflammasome Activation and Cell Death in a Rat Model of High-Fat Diet. Diabetologia 2014, 57, 413–423. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Li, G.; Chen, Y.; Conley, S.M.; Gehr, T.W.B.; Boini, K.M.; Li, P.-L. Nod-like Receptor Protein 3 (NLRP3) Inflammasome Activation and Podocyte Injury via Thioredoxin-Interacting Protein (TXNIP) during Hyperhomocysteinemia. J. Biol. Chem. 2014, 289, 27159–27168. [Google Scholar] [CrossRef] [PubMed]
- Sbai, O.; Devi, T.S.; Melone, M.A.B.; Feron, F.; Khrestchatisky, M.; Singh, L.P.; Perrone, L. RAGE-TXNIP Axis Is Required for S100B-Promoted Schwann Cell Migration, Fibronectin Expression and Cytokine Secretion. J. Cell Sci. 2010, 123, 4332–4339. [Google Scholar] [CrossRef]
- Johnston, L.K.; Rims, C.R.; Gill, S.E.; McGuire, J.K.; Manicone, A.M. Pulmonary Macrophage Subpopulations in the Induction and Resolution of Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2012, 47, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 Differentiation Is Required for the Detection of Responses to Weak Stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kiryushko, D.; Novitskaya, V.; Soroka, V.; Klingelhofer, J.; Lukanidin, E.; Berezin, V.; Bock, E. Molecular Mechanisms of Ca(2+) Signaling in Neurons Induced by the S100A4 Protein. Mol. Cell. Biol. 2006, 26, 3625–3638. [Google Scholar] [CrossRef]
- Wittkowski, H.; Sturrock, A.; van Zoelen, M.A.D.; Viemann, D.; van der Poll, T.; Hoidal, J.R.; Roth, J.; Foell, D. Neutrophil-Derived S100A12 in Acute Lung Injury and Respiratory Distress Syndrome. Crit. Care Med. 2007, 35, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A Multimodal RAGE-Specific Inhibitor Reduces Amyloid β-Mediated Brain Disorder in a Mouse Model of Alzheimer Disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef]
- Patel, B.V.; Wilson, M.R.; Takata, M. Resolution of Acute Lung Injury and Inflammation: A Translational Mouse Model. Eur. Respir. J. 2012, 39, 1162–1170. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. Acute Lung Injury in Animals Study Group An Official American Thoracic Society Workshop Report: Features and Measurements of Experimental Acute Lung Injury in Animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- Aeffner, F.; Traylor, Z.P.; Yu, E.N.Z.; Davis, I.C. Double-Stranded RNA Induces Similar Pulmonary Dysfunction to Respiratory Syncytial Virus in BALB/c Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L99–L109. [Google Scholar] [CrossRef]
- Wolk, K.E.; Lazarowski, E.R.; Traylor, Z.P.; Yu, E.N.Z.; Jewell, N.A.; Durbin, R.K.; Durbin, J.E.; Davis, I.C. Influenza A Virus Inhibits Alveolar Fluid Clearance in BALB/c Mice. Am. J. Respir. Crit. Care Med. 2008, 178, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Lutterloh, E.C.; Opal, S.M.; Pittman, D.D.; Keith, J.C., Jr.; Tan, X.-Y.; Clancy, B.M.; Palmer, H.; Milarski, K.; Sun, Y.; Palardy, J.E.; et al. Inhibition of the RAGE Products Increases Survival in Experimental Models of Severe Sepsis and Systemic Infection. Crit. Care 2007, 11, R122. [Google Scholar] [CrossRef] [PubMed]
- Blondonnet, R.; Audard, J.; Belville, C.; Clairefond, G.; Lutz, J.; Bouvier, D.; Roszyk, L.; Gross, C.; Lavergne, M.; Fournet, M.; et al. RAGE Inhibition Reduces Acute Lung Injury in Mice. Sci. Rep. 2017, 7, 7208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tasaka, S.; Shiraishi, Y.; Fukunaga, K.; Yamada, W.; Seki, H.; Ogawa, Y.; Miyamoto, K.; Nakano, Y.; Hasegawa, N.; et al. Role of Soluble Receptor for Advanced Glycation End Products on Endotoxin-Induced Lung Injury. Am. J. Respir. Crit. Care Med. 2008, 178, 356–362. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]


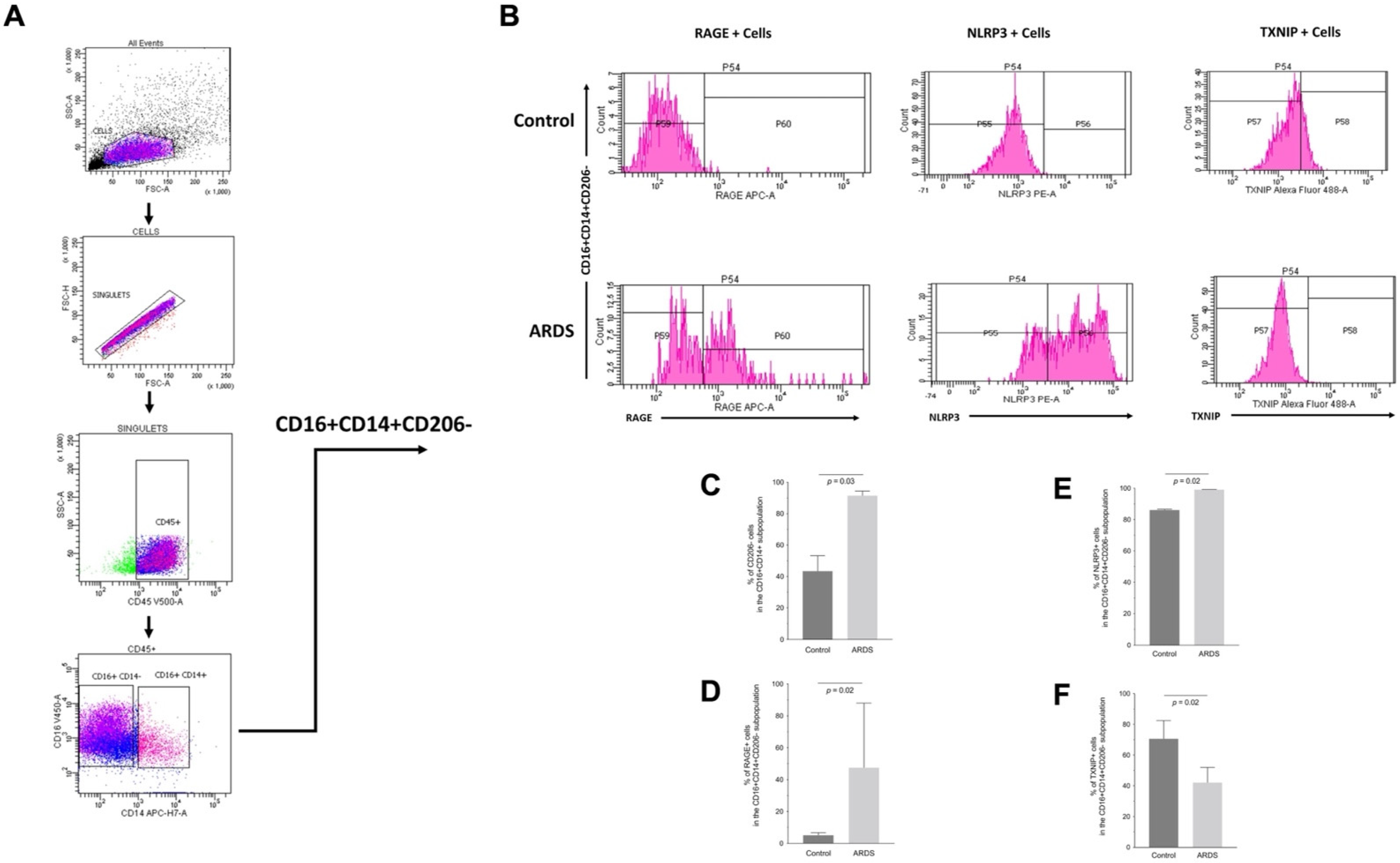
| Characteristic | Control (n = 5) | ARDS (n = 5) | p-Value |
|---|---|---|---|
| Demographics | |||
| Male sex, n (%) | 3 (60) | 3 (60) | 1.0 |
| Age, years | 61 ± 11 | 69 ± 3 | 0.5 |
| Body mass index, kg·m−2 | 28 ± 1 | 26 ± 1 | 0.4 |
| Coexisting Conditions, n (%) | |||
| Arterial hypertension | 1 (20) | 1 (20) | 1.0 |
| Type 2 diabetes | 1 (20) | 1 (20) | 1.0 |
| Chronic obstructive pulmonary disease | 0 (0) | 0 (0) | -- |
| Current smoking | 1 (20) | 1 (20) | 1.0 |
| Chronic kidney disease | 1 (20) | 0 (0) | 0.3 |
| Hematologic neoplasm | 0 (0) | 0 (0) | -- |
| Solid cancer | 0 (0) | 2 (40) | 0.1 |
| Indication for ICU Admission, n (%) | 0.4 | ||
| Septic shock of digestive origin | 0 (0) | 1 (20) | |
| Hemorrhagic shock | 3 (0) | 0 (0) | |
| Coma (intoxication) | 1 (20) | 0 (0) | |
| Acute respiratory failure | 0 (0) | 4 (80) | |
| Status epilepticus | 1 (20) | 0 (0) | |
| Baseline Respiratory Variables | |||
| PEEP, cmH2O | 8 [7–8] | 14 [10–14] | 0.02 |
| Tidal volume, mL·kg−1 PBW | 6.9 [6.6–7.7] | 6.2 [5.9–6.6] | 0.06 |
| PaO2/FiO2, mmHg | 164 [162–333] | 166 [153–167] | 0.6 |
| PaCO2 mmHg | 34 [31–37] | 43 [40–53] | 0.06 |
| Inspiratory plateau pressure, cmH2O | 15 [13–16] | 29 [24–30] | 0.04 |
| Baseline Hemodynamic Status | |||
| Mean arterial blood pressure, mmHg | 89 ± 18 | 82 ± 9 | 0.6 |
| Heart rate, per min | 97 ± 23 | 97 ± 14 | 0.8 |
| Need for norepinephrine, n (%) | 1 (20) | 3 (75) | 0.2 |
| Serum creatinine, μmol·L−1 | 87 ± 53 | 118 ± 69 | 0.5 |
| Sequential Organ Failure Assessment score | 6.6 ± 2.9 | 8.2 ± 3.8 | 0.4 |
| Clinical Outcomes | 0.5 | ||
| Death at day 28, n (%) | 1 (20) | 3 (60) | |
| Ventilator-free days at day 28 | 25 [24–27] | 0 [0–21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenga Ma Bonda, W.; Fournet, M.; Zhai, R.; Lutz, J.; Blondonnet, R.; Bourgne, C.; Leclaire, C.; Saint-Béat, C.; Theilliere, C.; Belville, C.; et al. Receptor for Advanced Glycation End-Products Promotes Activation of Alveolar Macrophages through the NLRP3 Inflammasome/TXNIP Axis in Acute Lung Injury. Int. J. Mol. Sci. 2022, 23, 11659. https://doi.org/10.3390/ijms231911659
Lenga Ma Bonda W, Fournet M, Zhai R, Lutz J, Blondonnet R, Bourgne C, Leclaire C, Saint-Béat C, Theilliere C, Belville C, et al. Receptor for Advanced Glycation End-Products Promotes Activation of Alveolar Macrophages through the NLRP3 Inflammasome/TXNIP Axis in Acute Lung Injury. International Journal of Molecular Sciences. 2022; 23(19):11659. https://doi.org/10.3390/ijms231911659
Chicago/Turabian StyleLenga Ma Bonda, Woodys, Marianne Fournet, Ruoyang Zhai, Jean Lutz, Raiko Blondonnet, Céline Bourgne, Charlotte Leclaire, Cécile Saint-Béat, Camille Theilliere, Corinne Belville, and et al. 2022. "Receptor for Advanced Glycation End-Products Promotes Activation of Alveolar Macrophages through the NLRP3 Inflammasome/TXNIP Axis in Acute Lung Injury" International Journal of Molecular Sciences 23, no. 19: 11659. https://doi.org/10.3390/ijms231911659
APA StyleLenga Ma Bonda, W., Fournet, M., Zhai, R., Lutz, J., Blondonnet, R., Bourgne, C., Leclaire, C., Saint-Béat, C., Theilliere, C., Belville, C., Bouvier, D., Blanchon, L., Berger, M., Sapin, V., & Jabaudon, M. (2022). Receptor for Advanced Glycation End-Products Promotes Activation of Alveolar Macrophages through the NLRP3 Inflammasome/TXNIP Axis in Acute Lung Injury. International Journal of Molecular Sciences, 23(19), 11659. https://doi.org/10.3390/ijms231911659








