Revealing and Tuning the Photophysics of C=N Containing Photothermal Molecules: Excited State Dynamics Simulations
Abstract
1. Introduction
2. Computational Details
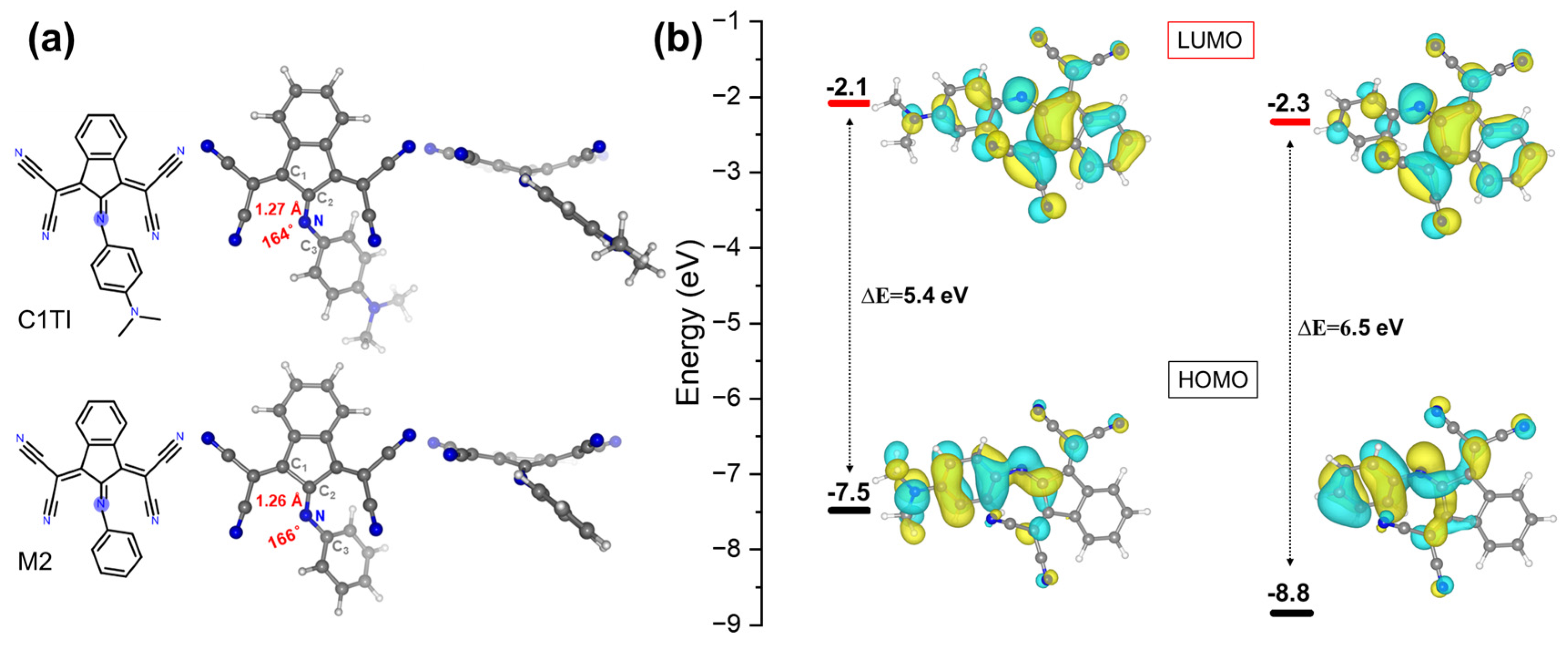
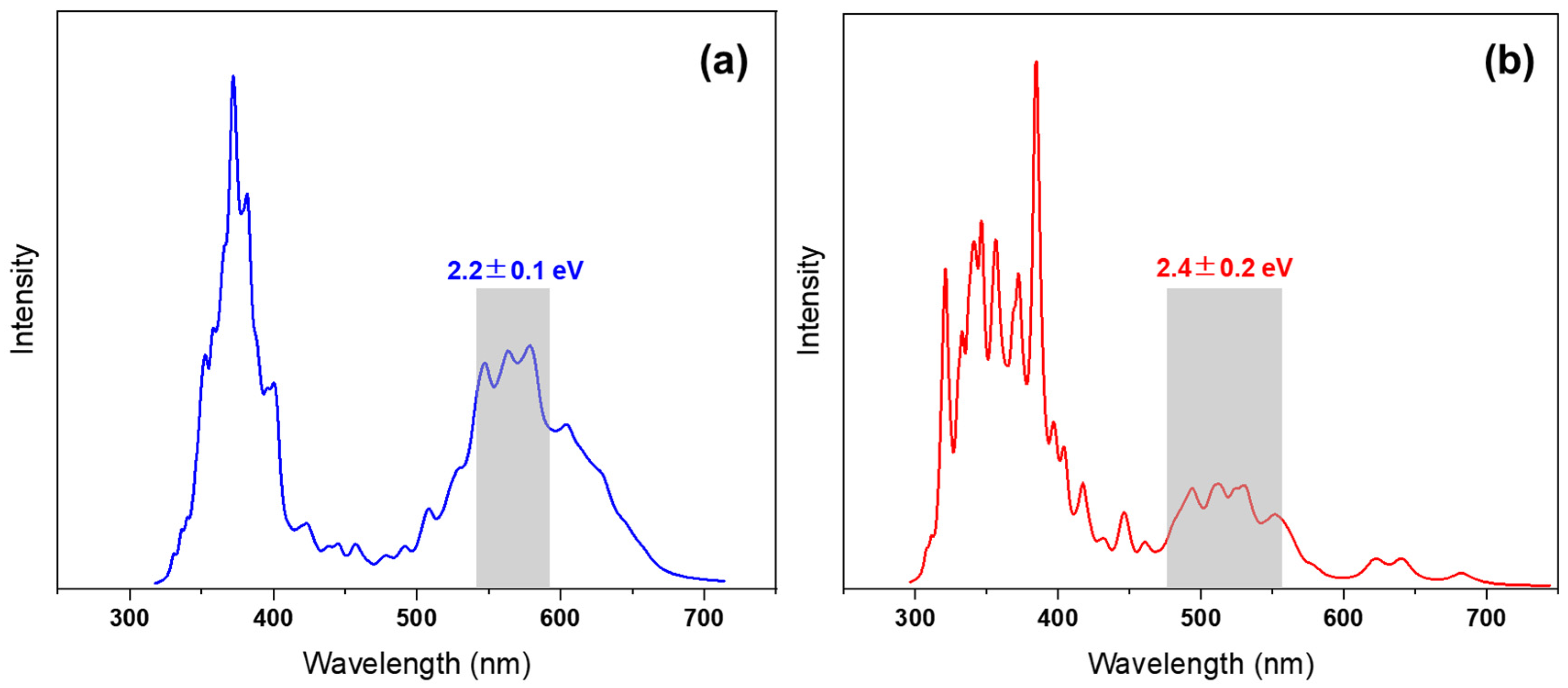
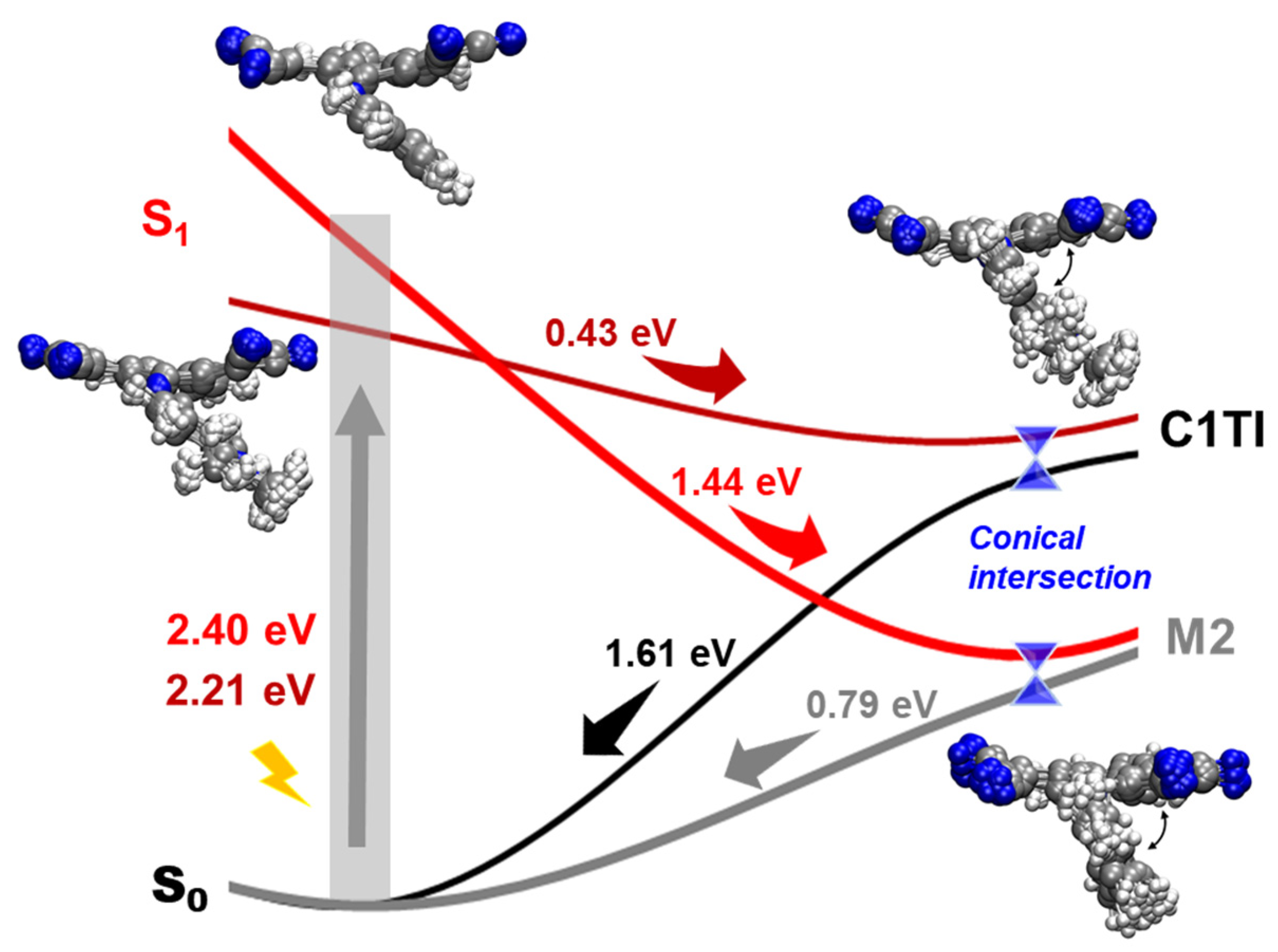
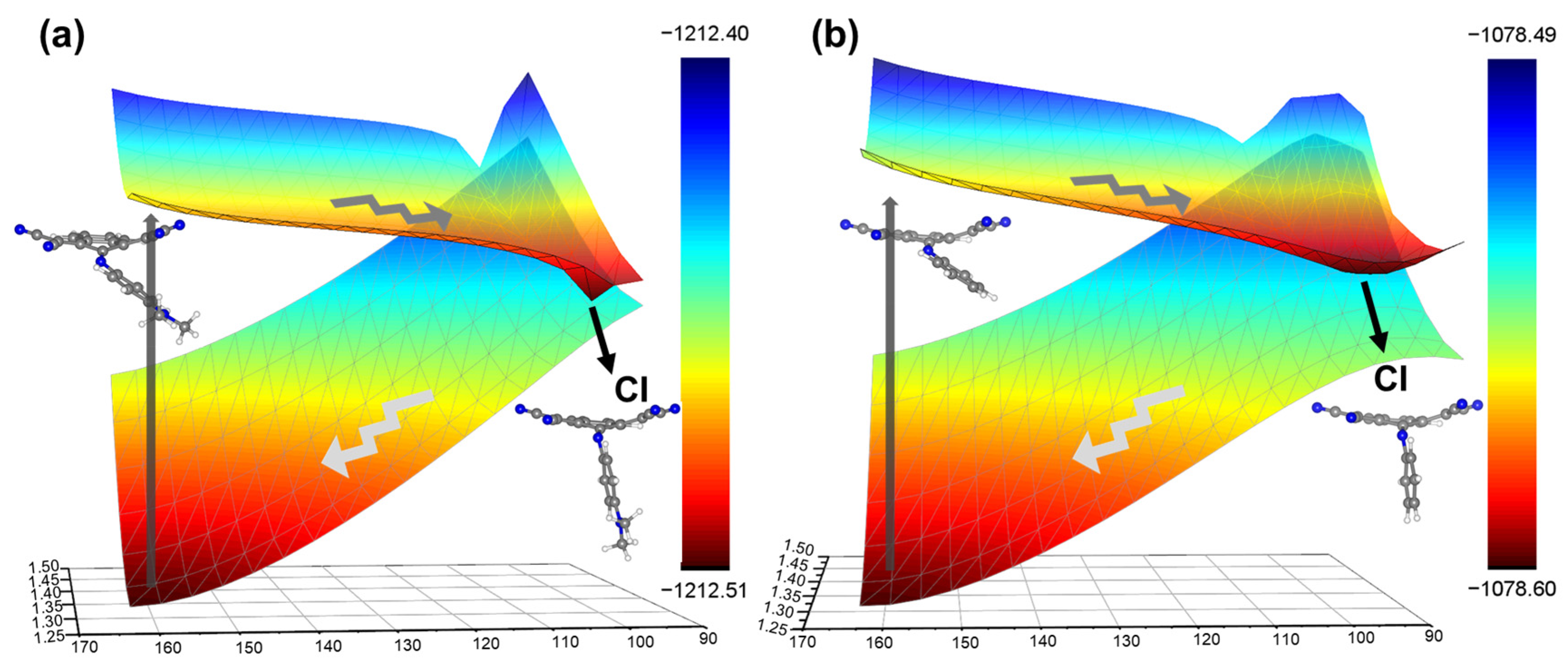
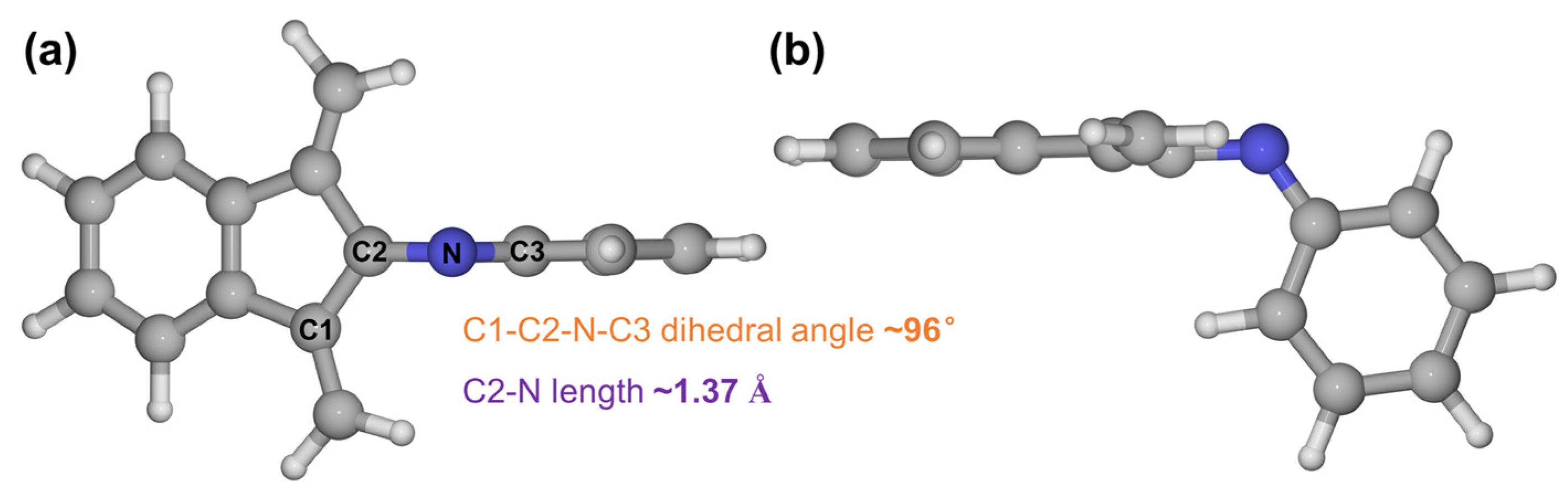
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic Molecule-Based Photothermal Agents: An Expanding Photothermal Therapy Universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.-L.; Liu, J.-Y. Strategies for Maximizing Photothermal Conversion Efficiency Based on Organic Dyes. Drug Discov. Today 2021, 26, 2045–2052. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second Near-Infrared Photothermal Materials for Combinational Nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal Therapy and Photoacoustic Imaging via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological Photothermal Nanodots Based on Self-Assembly of Peptide–Porphyrin Conjugates for Antitumor Therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.-H.; Zheng, B.-D.; Tang, J.; Zhao, Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. New Application of Phthalocyanine Molecules: From Photodynamic Therapy to Photothermal Therapy by Means of Structural Regulation Rather than Formation of Aggregates. Chem. Sci. 2018, 9, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Miao, X.; Tao, H.; Baev, A.; Ren, C.; Fan, Q.; He, T.; Huang, W.; Prasad, P.N. Manipulating Nonradiative Decay Channel by Intermolecular Charge Transfer for Exceptionally Improved Photothermal Conversion. ACS Nano 2019, 13, 12006–12014. [Google Scholar] [CrossRef]
- Guo, M.; Huang, J.; Deng, Y.; Shen, H.; Ma, Y.; Zhang, M.; Zhu, A.; Li, Y.; Hui, H.; Wang, Y.; et al. PH-Responsive Cyanine-Grafted Graphene Oxide for Fluorescence Resonance Energy Transfer-Enhanced Photothermal Therapy. Adv. Funct. Mater. 2015, 25, 59–67. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Wu, W.; Wang, F.; Du, L.; Zhang, X.; Xiong, Y.; He, X.; Cai, Y.; Kwok, R.T.K.; et al. Highly Efficient Photothermal Nanoagent Achieved by Harvesting Energy via Excited-State Intramolecular Motion within Nanoparticles. Nat. Commun. 2019, 10, 768. [Google Scholar] [CrossRef]
- Xu, C.; Ye, R.; Shen, H.; Lam, J.W.Y.; Zhao, Z.; Zhong Tang, B. Molecular Motion and Nonradiative Decay: Towards Efficient Photothermal and Photoacoustic Systems. Angew. Chem. Int. Ed. 2022, 61, e202204604. [Google Scholar]
- Ni, J.-S.; Zhang, X.; Yang, G.; Kang, T.; Lin, X.; Zha, M.; Li, Y.; Wang, L.; Li, K. A Photoinduced Nonadiabatic Decay-Guided Molecular Motor Triggers Effective Photothermal Conversion for Cancer Therapy. Angew. Chem. Int. Ed. 2020, 59, 11298–11302. [Google Scholar] [CrossRef]
- Roke, D.; Wezenberg, S.J.; Feringa, B.L. Molecular Rotary Motors: Unidirectional Motion around Double Bonds. Proc. Natl. Acad. Sci. USA 2018, 115, 9423–9431. [Google Scholar] [CrossRef]
- Greb, L.; Lehn, J.-M. Light-Driven Molecular Motors: Imines as Four-Step or Two-Step Unidirectional Rotors. J. Am. Chem. Soc. 2014, 136, 13114–13117. [Google Scholar] [CrossRef]
- Hoorens, M.W.H.; Medved’, M.; Laurent, A.D.; Di Donato, M.; Fanetti, S.; Slappendel, L.; Hilbers, M.; Feringa, B.L.; Jan Buma, W.; Szymanski, W. Iminothioindoxyl as a Molecular Photoswitch with 100 nm Band Separation in the Visible Range. Nat. Commun. 2019, 10, 2390. [Google Scholar] [CrossRef] [PubMed]
- Crespi, S.; Simeth, N.A.; Di Donato, M.; Doria, S.; Stindt, C.N.; Hilbers, M.F.; Kiss, F.L.; Toyoda, R.; Wesseling, S.; Buma, W.J.; et al. Phenylimino Indolinone: A Green-Light-Responsive T-Type Photoswitch Exhibiting Negative Photochromism. Angew. Chem. Int. Ed. 2021, 60, 25290–25295. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.E.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Barbatti, M.; Ruckenbauer, M.; Plasser, F.; Pittner, J.; Granucci, G.; Persico, M.; Lischka, H. Newton-X: A Surface-Hopping Program for Nonadiabatic Molecular Dynamics. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 26–33. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Blancafort, L. Photochemistry and Photophysics at Extended Seams of Conical Intersection. ChemPhysChem 2014, 15, 3166–3181. [Google Scholar] [CrossRef]
- Chen, S.; Ullah, N.; Zhao, Y.; Zhang, R. Nonradiative Excited-State Decay via Conical Intersection in Graphene Nanostructures. ChemPhysChem 2019, 20, 2754–2758. [Google Scholar] [CrossRef] [PubMed]
- Fazzi, D.; Barbatti, M.; Thiel, W. Modeling Ultrafast Exciton Deactivation in Oligothiophenes via Nonadiabatic Dynamics. Phys. Chem. Chem. Phys. 2015, 17, 7787–7799. [Google Scholar] [CrossRef] [PubMed]
- Barbatti, M. Photorelaxation Induced by Water–Chromophore Electron Transfer. J. Am. Chem. Soc. 2014, 136, 10246–10249. [Google Scholar] [CrossRef]
- Roos, B.O.; Taylor, P.R.; Sigbahn, P.E. A Complete Active Space SCF Method (CASSCF) Using a Density Matrix Formulated Super-CI Approach. Chem. Phys. 1980, 48, 157–173. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31g* Basis Set for Third-Row Atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Chen, S.; Ullah, N.; Zhang, R.-Q. Exciton Self-Trapping in sp2 Carbon Nanostructures Induced by Edge Ether Groups. J. Phys. Chem. Lett. 2018, 9, 4857–4864. [Google Scholar] [CrossRef]
- Hosoya, H. Cross-Conjugation at the Heart of Understanding the Electronic Theory of Organic Chemistry. Curr. Org. Chem. 2015, 19, 293–310. [Google Scholar] [CrossRef]
- Gu, J.; Wu, W.; Stuyver, T.; Danovich, D.; Hoffmann, R.; Tsuji, Y.; Shaik, S. Cross Conjugation in Polyenes and Related Hydrocarbons: What Can Be Learned from Valence Bond Theory about Single-Molecule Conductance? J. Am. Chem. Soc. 2019, 141, 6030–6047. [Google Scholar] [CrossRef]
- Bai, S.; Mansour, R.; Stojanović, L.; Toldo, J.M.; Barbatti, M. On the Origin of the Shift between Vertical Excitation and Band Maximum in Molecular Photoabsorption. J. Mol. Model. 2020, 26, 107. [Google Scholar] [CrossRef] [PubMed]
- Barbatti, M. Nonadiabatic Dynamics with Trajectory Surface Hopping Method. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 620–633. [Google Scholar] [CrossRef]
- Su, Q.; Li, Y.; Wang, B.; Liu, M.; Wang, H.; Wang, W.; Liu, F. Combining the Advantages of Alkene and Azo E–Z Photoisomerizations: Mechanistic Insights into Ketoimine Photoswitches. J. Phys. Chem. A 2017, 121, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Y.; Li, D.; Liu, Y. A Theoretical Study of the Potential Energy Surfaces for the Double Proton Transfer Reaction of Model DNA Base Pairs. Phys. Chem. Chem. Phys. 2017, 19, 4802–4808. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, H.; Li, Y.; Chen, T.; Liu, H.; Han, X. Revealing and Tuning the Photophysics of C=N Containing Photothermal Molecules: Excited State Dynamics Simulations. Int. J. Mol. Sci. 2022, 23, 11779. https://doi.org/10.3390/ijms231911779
Chen S, Zhang H, Li Y, Chen T, Liu H, Han X. Revealing and Tuning the Photophysics of C=N Containing Photothermal Molecules: Excited State Dynamics Simulations. International Journal of Molecular Sciences. 2022; 23(19):11779. https://doi.org/10.3390/ijms231911779
Chicago/Turabian StyleChen, Shunwei, Huajing Zhang, Yi Li, Tingfeng Chen, Hao Liu, and Xiujun Han. 2022. "Revealing and Tuning the Photophysics of C=N Containing Photothermal Molecules: Excited State Dynamics Simulations" International Journal of Molecular Sciences 23, no. 19: 11779. https://doi.org/10.3390/ijms231911779
APA StyleChen, S., Zhang, H., Li, Y., Chen, T., Liu, H., & Han, X. (2022). Revealing and Tuning the Photophysics of C=N Containing Photothermal Molecules: Excited State Dynamics Simulations. International Journal of Molecular Sciences, 23(19), 11779. https://doi.org/10.3390/ijms231911779






