Event-Related Potential Study of Recovery of Consciousness during Forced Awakening from Slow-Wave Sleep and Rapid Eye Movement Sleep
Abstract
1. Introduction
“… consciousness is generally equated with the waking state, and the abilities to perceive, interact and communicate with the environment and with others in the integrated manner which wakefulness normally implies. Consciousness in this sense is a matter of degree: a range of conscious states extends from waking through sleep into coma”[3] (p. 1265)
2. Results
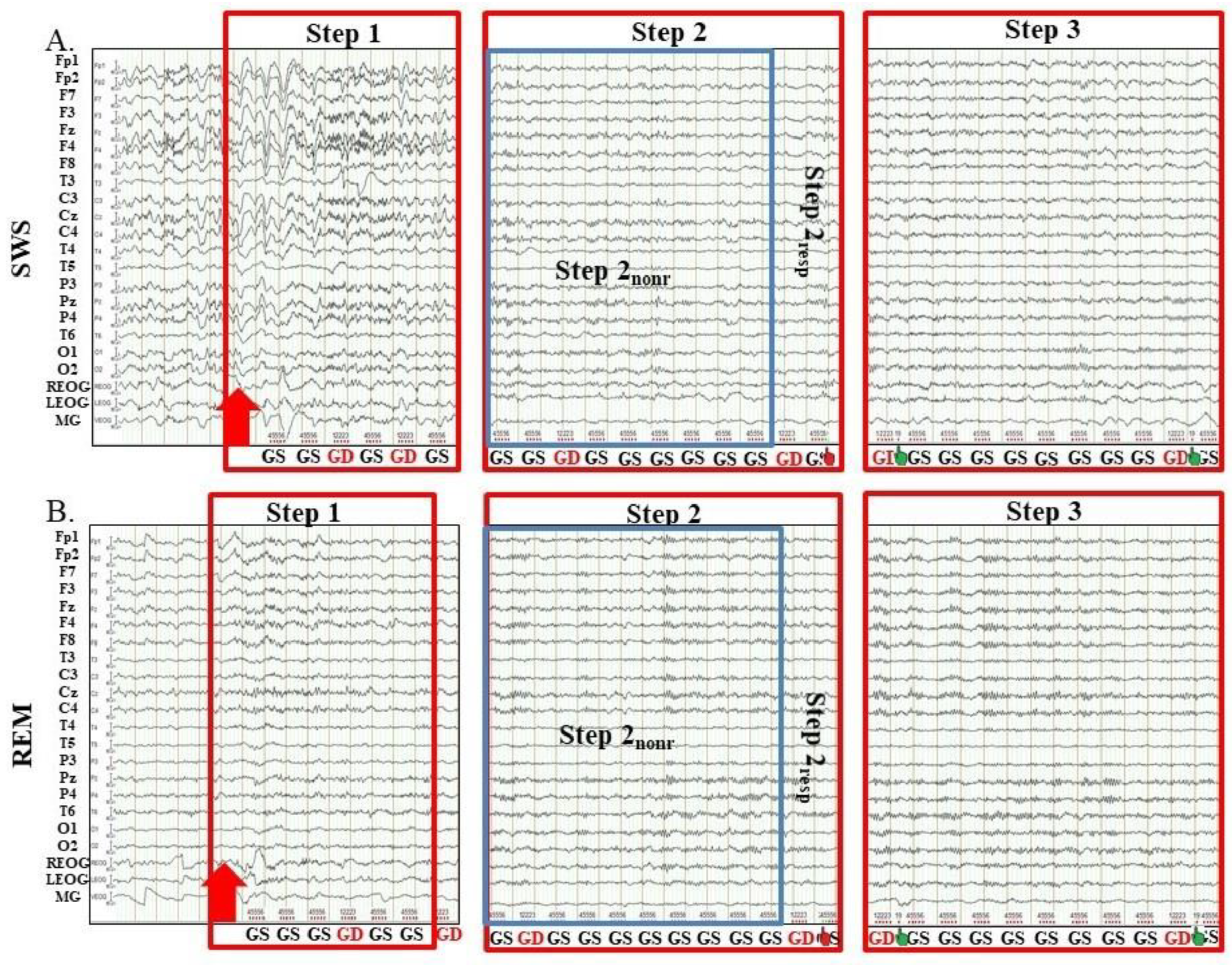
2.1. Polysomnographic Data
2.2. Criteria for Division of Forced Awakenings from SWS and from REM on Consecutive Steps
2.3. Comparison of EEG and Behavioral Characteristics of Forced Awakenings from SWS and REM
2.3.1. Types of Awakenings from Sleep
2.3.2. Difference in Latency of Each Step of Awakening
2.3.3. Difference in Latency of MR Recovery on Local and Global Irregularities
2.3.4. Circadian Factor
2.4. Event-Related Potentials Study
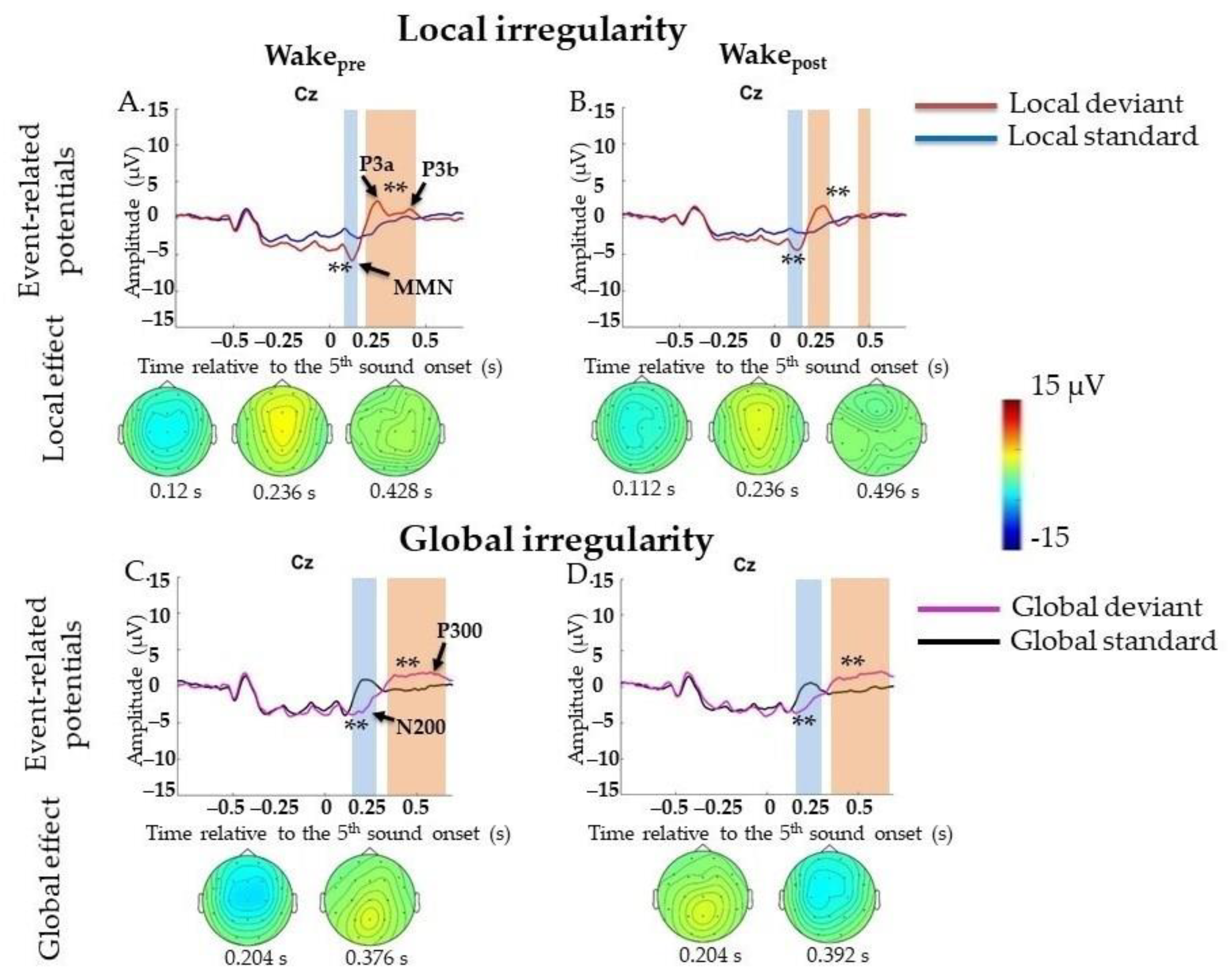
2.4.1. Event-Related Potentials during Wakefulness
Local Irregularities
Global Irregularities
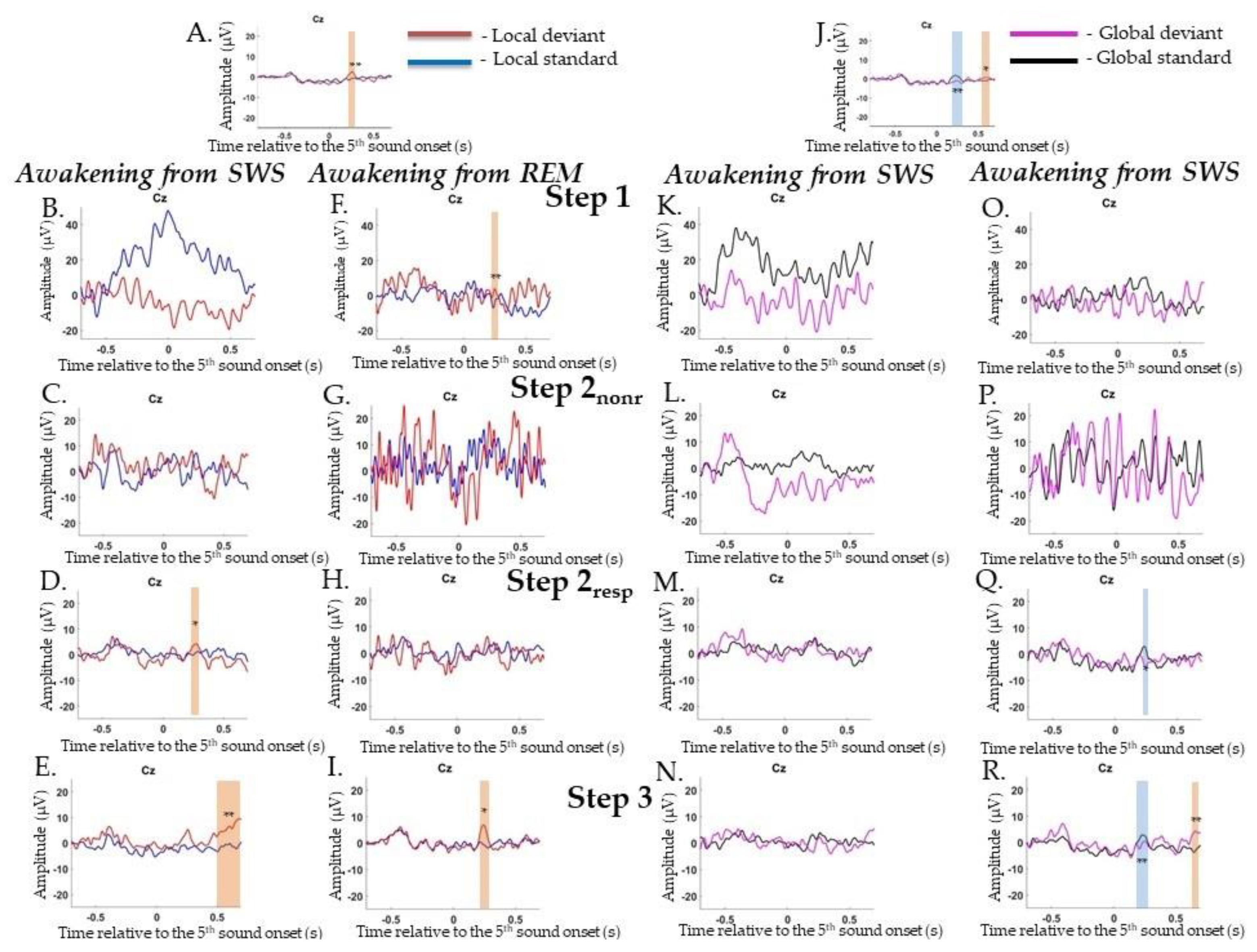
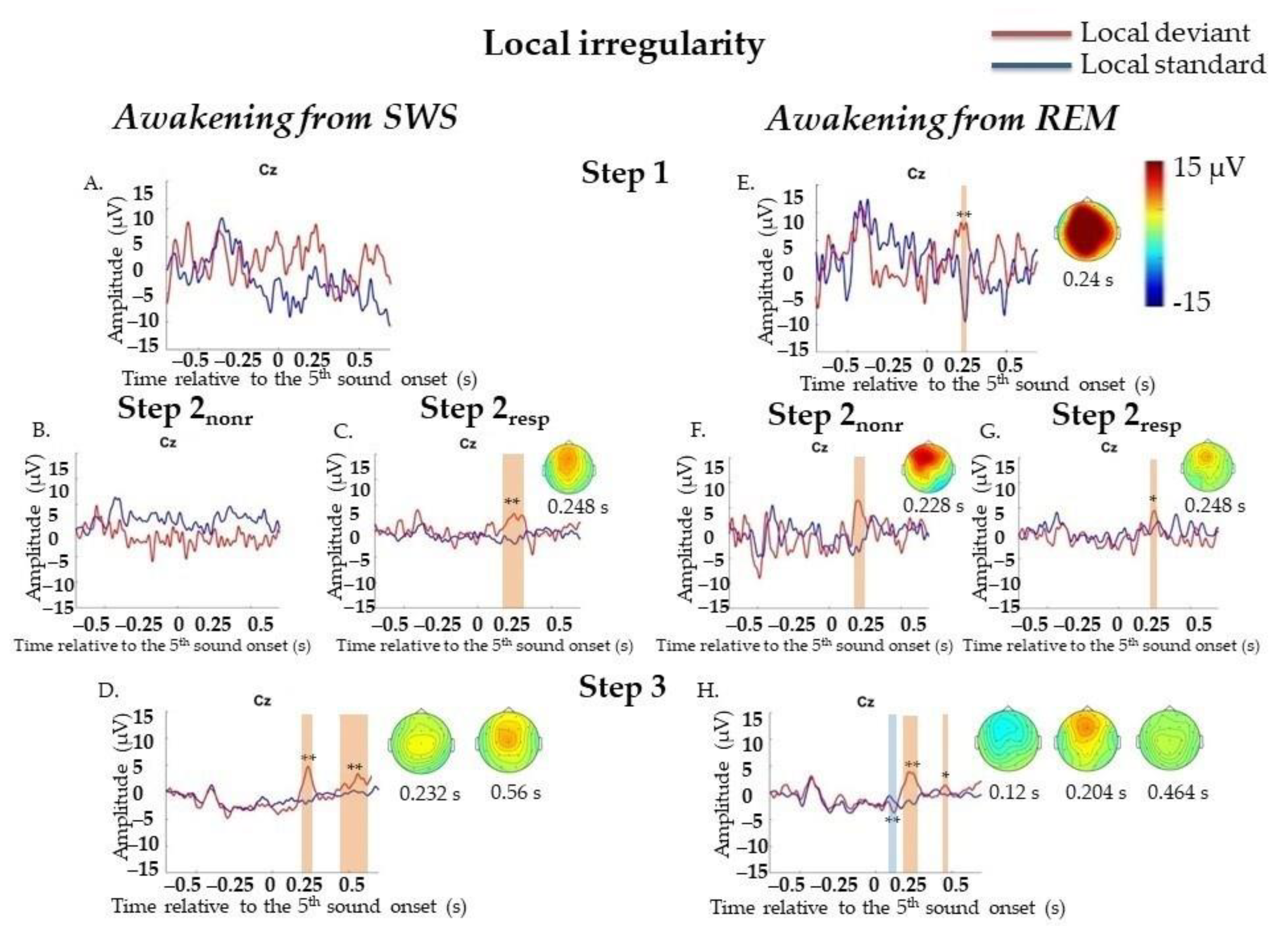
2.4.2. Event-Related Potentials during Awakenings from SWS
Local Irregularities
Global Irregularities
Target Irregularities Versus Nontarget Irregularities
2.4.3. Event-Related Potentials during Awakenings from REM
Local Irregularities
Global Irregularities
Target Irregularities Versus Nontarget Irregularities
3. Discussion
4. Limitations
5. Conclusions
6. Materials and Methods
6.1. Participants
6.2. Procedure
6.3. Materials
6.3.1. Test Instruments for Vigilance and Sleep Assessment
6.3.2. Auditory Paradigms and Stimuli
6.3.3. Data Acquisition and Polysomnography
6.4. Preprocessing and Statistical Data Analyses
6.4.1. Reaction Time
6.4.2. Event-Related Potentials
6.4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sleep Parameter | Session with Awakening from SWS (n = 26) a | Session with Awakening from REM (n = 22) | p |
|---|---|---|---|
| TST, min | 387.83 ± 128.63 | 407.39 ± 43.78 | 0.583 |
| SPT, min | 438.74 ± 134.83 | 479.53 ± 52.6 | 0.789 |
| NREM1, min | 18.4 ± 9.6 | 19.53 ± 11.24 | 0.8 |
| NREM2, min | 185.67 ± 56.28 | 197.72 ± 42.75 | 0.662 |
| SWS, min | 89.24 ± 48.34 | 102.5 ± 24.24 | 0.242 |
| REM, min | 86.36 ± 41.46 | 75.78 ± 24.68 | 0.271 |
| Wake, min | 50.9 ± 36.25 | 72.14 ± 46.77 | 0.147 |
| SOL, min | 10.4 ± 5.73 | 20.36 ± 27.8 | 0.124 |
| SWS latency, min | 14.33 ± 18.81 | 13.92 ± 12.6 | 0.746 |
| REM latency, min | 90.19 ± 34.86 | 91.14 ± 48.66 | 0.622 |
| SE, % | 87.73 ± 7.93 | 85.42 ± 8.55 | 0.304 |
Appendix B
| Session with Awakening from SWS (N = 77) | Session with Awakening from REM (N = 86) | p | |
|---|---|---|---|
| No awakenings | 29 (37.7) | 17 (19.8) | 0.023 |
| Partial awakenings | 20 (25.9) | 15(17.4) | 0.252 |
| Full awakenings | 28 (36.4) | 54 (62.8) | 0.001 |
Appendix C
| Session with Awakening from SWS (N = 77) | Session with Awakening from REM (N = 86) | p | |
|---|---|---|---|
| Part 1 (23:00–03:30) | 48 (62.3) | 30 (34.9) | <0.001 |
| Part 2 (03:30–08:00) | 29 (37.7) | 56 (65.1) |
Appendix D
| Parameter | Wakepre in the Session with Awakening from SWS (n = 25) a | p (SWS) | Wakepost in the Session with Awakening from SWS (n = 25) a | Wakepre in the Session with Awakening from REM (n = 22) | p (REM) | Wakepost in the Session with Awakening from REM (n = 22) | p (Wakepre SWS vs. REM) | p (Wakepost SWS vs. REM) |
|---|---|---|---|---|---|---|---|---|
| VASS, mm | 6.14 ± 1.85 | <0.001 | 3.93 ± 1.46 | 5.69 ± 1.99 | 0.002 | 3.46 ± 1.45 | 0.332 | 0.277 |
| SSS | 4.08 ± 1.26 | 0.01 | 3.16 ± 1 | 4.14 ± 1.13 | <0.001 | 2.77 ± 0.87 | 0.848 | 0.216 |
Appendix E
| Performance before and after Sleep | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deviants | Standards | Stimuli | Participant | Interaction | d.f. error | |||||||||
| d.f. | η2p | F | p | d.f. | η2p | F | p | d.f. | η2p | |||||
| Wakepre | MMN | 3.26 ± 1.72 | −0.85 ± 0.88 | 1 | 0.6 | 104.82 | <0.001 | 7 | 0.3 | 4.19 | <0.001 | 7 | 0.33 | 70 |
| P3a | 7.28 ± 4.31 | 2.09 ± 1.22 | 1 | 0.53 | 79.43 | <0.001 | 7 | 0.49 | 9.57 | <0.001 | 7 | 0.27 | ||
| P3b | 8.36 ± 3.25 | 4.29 ± 1.74 | 1 | 0.57 | 93.39 | <0.001 | 7 | 0.55 | 12.22 | <0.001 | 7 | 0.19 | ||
| Wakepost | MMN | −3.07 ± 1.85 | −0.95 ± 0.61 | 1 | 0.49 | 63.24 | <0.001 | 7 | 0.31 | 4.21 | <0.001 | 7 | 0.2 | 66 |
| P3a | 6.04 ± 3.72 | 1.35 ± 0.85 | 1 | 0.61 | 103.25 | <0.001 | 7 | 0.57 | 12.31 | <0.001 | 7 | 0.51 | ||
| P3b | 7.01 ± 3.41 | 3.69 ± 1.68 | 1 | 0.46 | 56.07 | <0.001 | 7 | 0.6 | 14.12 | <0.001 | 7 | 0.17 | ||
| SWS | ||||||||||||||
| Deviants | Standards | Stimuli | Participant | Interaction | d.f. error | |||||||||
| d.f. | η2p | F | p | d.f. | η2p | F | p | d.f. | η2p | |||||
| Step 1 | P3a | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Step 2 | P3a | 7.75 ± 7.63 | 1.65 ± 4.69 | 1 | 0.13 | 8.35 | 0.005 | 7 | 0.09 | 0.86 | 0.543 | 7 | 0.15 | 58 |
| Step 2nonr | P3a | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Step 2resp | P3a | 10.42 ± 7.89 | 2.88 ± 3.7 | 1 | 0.29 | 15.35 | <0.001 | 5 | 0.18 | 1.72 | 0.154 | 5 | 0.19 | 38 |
| Step 3 | MMN | - | - | - | - | - | - | - | - | - | - | - | - | −56 |
| P3a | 9.93 ± 6.05 | 3.57 ± 2.74 | 1 | 0.15 | 9.49 | 0.003 | 6 | 0.13 | 1.36 | 0.248 | 6 | 0.22 | ||
| P3b | 10.54 ± 5.03 | 5.77 ± 2.23 | 1 | 0.25 | 18.67 | <0.001 | 6 | 0.15 | 1.61 | 0.163 | 6 | 0.06 | ||
| REM | ||||||||||||||
| Deviants | Standards | Stimuli | Participant | Interaction | d.f. error | |||||||||
| d.f. | η2p | F | p | d.f. | η2p | F | p | d.f. | η2p | |||||
| Step 1 | P3a | 14.45 ± 8.36 | 1.51 ± 7.72 | 1 | 0.34 | 5.23 | 0.045 | 1 | 0.02 | 0.29 | 0.601 | 1 | 0.04 | 10 |
| Step 2 | P3a | 11.56 ± 7.66 | 5.00 ± 5.37 | 1 | 0.13 | 5.95 | 0.019 | 7 | 0.35 | 3.03 | 0.012 | 7 | 0.29 | 40 |
| Step 2nonr | P3a | 13.97 ± 9.82 | 2.72 ± 5.45 | 1 | 0.22 | 2.8 | 0.125 | 5 | 0.34 | 1.04 | 0.447 | 5 | 0.32 | 10 |
| Step 2resp | P3a | 9.69 ± 7.73 | 4.77 ± 3.8 | 1 | 0.31 | 8.81 | 0.008 | 6 | 0.52 | 3.68 | 0.013 | 6 | 0.37 | 20 |
| Step 3 | MMN | −4.5 ± 3.78 | −1.65 ± 2.36 | 1 | 0.2 | 10.27 | 0.003 | 5 | 0.35 | 4.48 | 0.002 | 5 | 0.08 | 42 |
| P3a | 9.8 ± 5.6 | 3.16 ± 2.19 | 1 | 0.19 | 9.59 | 0.004 | 5 | 0.42 | 6.2 | <0.001 | 5 | 0.46 | ||
| P3b | 4.92 ± 3.69 | 3.47 ± 2.28 | 1 | 0.12 | 5.57 | 0.023 | 5 | 0.22 | 2.37 | 0.056 | 5 | 0.11 | ||
Appendix F
| Performance before and after Sleep | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deviants | Standards | Stimuli | Participant | Interaction | d.f. error | |||||||||
| d.f. | η2p | F | p | d.f. | η2p | F | p | d.f. | η2p | |||||
| Wakepre | N200 | −1.68 ± 1.45 | −0.43 ± 1.22 | 1 | 0.16 | 13.77 | <0.001 | 7 | 0.16 | 1.93 | 0.077 | 7 | 0.06 | 70 |
| P300 | 7.51 ± 3.05 | 4.87 ± 1.63 | 1 | 0.44 | 54.69 | <0.001 | 7 | 0.61 | 15.91 | <0.001 | 7 | 0.25 | ||
| Wakepost | N200 | −1.65 ± 1.68 | −0.48 ± 1.65 | 1 | 0.18 | 14.16 | <0.001 | 7 | 0.48 | 8.66 | <0.001 | 7 | 0.09 | 66 |
| P300 | 7.45 ± 3.82 | 4.53 ± 1.82 | 1 | 0.34 | 34.71 | <0.001 | 7 | 0.5 | 9.25 | <0.001 | 7 | 0.2 | ||
| SWS | ||||||||||||||
| Deviants | Standards | Stimuli | Participant | Intersection | d.f. error | |||||||||
| d.f. | η2p | F | p | d.f. | η2p | F | p | d.f. | η2p | |||||
| Step 1 | N200 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Step 2 | N200 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Step2nonr | N200 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Step2resp | N200 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Step 3 | N200 | −3.64 ± 6.83 | −0.4 ± 3.33 | 1 | 0.02 | 1.84 | 0.18 | 6 | 0.07 | 0.91 | 0.49 | 6 | 0.04 | 72 |
| P300 | 11.69 ± 5.21 | 6.1 ± 2.75 | 1 | 0.23 | 22.07 | <0.001 | 6 | 0.23 | 3.57 | 0.004 | 6 | 0.09 | ||
| REM | ||||||||||||||
| Deviants | Standards | Stimuli | Participant | Interaction | d.f. error | |||||||||
| d.f. | η2p | F | p | d.f. | η2p | F | p | d.f. | η2p | |||||
| Step 1 | N200 | −3.4 ± 8.86 | 4.91 ± 10.65 | 1 | 0.17 | 3.65 | 0.72 | 3 | 0.25 | 1.97 | 0.155 | 3 | 0.08 | 18 |
| Step 2 | N200 | −6.91 ± 5.62 | −1.95 ± 4.55 | 1 | 0.24 | 16.61 | <0.001 | 7 | 0.17 | 1.56 | 0.168 | 7 | 0.1 | 52 |
| Step2nonr | N200 | −3.52 ± 9.29 | 1.27 ± 5.46 | 1 | 0.52 | 6.39 | 0.045 | 5 | 0.74 | 3.57 | 0.077 | 5 | 0.57 | 6 |
| Step2resp | N200 | −5.39 ± 4.55 | −0.71 ± 3.64 | 1 | 0.29 | 14.01 | <0.001 | 7 | 0.12 | 0.64 | 0.722 | 7 | 0.18 | 34 |
| Step 3 | N200 | −3.06 ± 2.33 | −0.73 ± 1.98 | 1 | 0.19 | 10.78 | 0.002 | 6 | 0.24 | 2.46 | 0.038 | 6 | 0.06 | 46 |
| P300 | 10.12 ± 3.13 | 5.95 ± 2.03 | 1 | 0.39 | 29.84 | <0.001 | 6 | 0.21 | 2.02 | 0.083 | 6 | 0.08 | ||
Appendix G
Appendix G.1. Local Irregularities
Appendix G.2. Global Irregularities

Appendix H
Appendix H.1. Local Irregularities
Appendix H.2. Global Irregularities
References
- Fabbro, F.; Cantone, D.; Feruglio, S.; Crescentini, C. Chapter 12—Origin and Evolution of Human Consciousness. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 317–343. ISBN 9780444643179. [Google Scholar] [CrossRef]
- Anokhin, K.V. Cognitome: In search of fundamental neuroscience theory of consciousness. Zhurnal Vyss. Nervn. Deyatel’nosti Im I P Pavlov. 2021, 71, 39–71. (In Russian) [Google Scholar]
- Zeman, A. Consciousness. Brain 2001, 124, 1263–1289. [Google Scholar] [CrossRef]
- Owen, A.M.; Coleman, M.R.; Boly, M.; Davis, M.H.; Laureys, S.; Pickard, J.D. Detecting Awareness in the Vegetative State. Science 2006, 313, 1402. [Google Scholar] [CrossRef]
- Huntley, A. Documenting Level of Consciousness. Nursing 2008, 38, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S. The Neural Correlate of (Un)Awareness: Lessons from the Vegetative State. Trends. Cogn. Sci. 2005, 9, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Manuello, J.; Mancuso, L.; Liloia, D.; Costa, T.; Cauda, F. The Neural Correlates of Consciousness and Attention: Two Sister Processes of the Brain. Front. Neurosci. 2019, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G. What Is Consciousness? A Tridimensional View and Neural Predispositions of Consciousness (NPC). Neuropsychoanalysis 2013, 15, 59–62. [Google Scholar] [CrossRef]
- Stickgold, R.; Malia, A.; Fosse, R.; Propper, R.; Hobson, J.A. Brain-Mind States: I. Longitudinal Field Study of Sleep/Wake Factors Influencing Mentation Report Length. Sleep 2001, 24, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Siclari, F.; Bernardi, G.; Cataldi, J.; Tononi, G. Dreaming in NREM Sleep: A High-Density EEG Study of Slow Waves and Spindles. J. Neurosci. 2018, 38, 9175–9185. [Google Scholar] [CrossRef]
- Langford, G.W.; Meddis, R.; Pearson, A.J.D. Awakening Latency from Sleep for Meaningful and Non-Meaningful Stimuli. Psychophysiology 1974, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.S.; Webb, W.B. The Perception of Wakefulness within Sleep. Sleep 1981, 4, 177–183. [Google Scholar] [CrossRef]
- Dehaene, S.; Changeux, J.-P. Experimental and Theoretical Approaches to Conscious Processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G. Integrated Information Theory of Consciousness: An Updated Account. Arch. Ital. De Biol. 2012, 150, 56–90. [Google Scholar] [CrossRef]
- Rosenthal, D.M. Consciousness and the Mind. Iyyun Jerus. Philos. Q. 2002, 51, 227–251. [Google Scholar]
- Rees, G.; Kreiman, G.; Koch, C. Neural Correlates of Consciousness in Humans. Nat. Rev. Neurosci. 2002, 3, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Akeju, O.; Loggia, M.L.; Catana, C.; Pavone, K.J.; Vazquez, R.; Rhee, J.; Contreras Ramirez, V.; Chonde, D.B.; Izquierdo-Garcia, D.; Arabasz, G.; et al. Disruption of Thalamic Functional Connectivity Is a Neural Correlate of Dexmedetomidine-Induced Unconsciousness. eLife 2014, 3, e04499. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.I.; Krause, B.M.; Endemann, C.M.; Campbell, D.I.; Kovach, C.K.; Dyken, M.E.; Kawasaki, H.; Nourski, K.V. Cortical Functional Connectivity Indexes Arousal State during Sleep and Anesthesia. NeuroImage 2020, 211, 116627. [Google Scholar] [CrossRef]
- Sadaghiani, S.; Scheeringa, R.; Lehongre, K.; Morillon, B.; Giraud, A.-L.; D’Esposito, M.; Kleinschmidt, A. Alpha-Band Phase Synchrony Is Related to Activity in the Fronto-Parietal Adaptive Control Network. J. Neurosci. 2012, 32, 14305–14310. [Google Scholar] [CrossRef] [PubMed]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associated Events. Rules Terminol. Tech. Specif. Darien Ill. Am. Acad. Sleep Med. 2007, 176, 2012. [Google Scholar]
- Massimini, M.; Ferrarelli, F.; Murphy, M.J.; Huber, R.; Riedner, B.A.; Casarotto, S.; Tononi, G. Cortical Reactivity and Effective Connectivity during REM Sleep in Humans. Cogn. Neurosci. 2010, 1, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical Oscillations in the Sleeping and Aroused Brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, E.; Laufs, H. Decoding Wakefulness Levels from Typical FMRI Resting-State Data Reveals Reliable Drifts between Wakefulness and Sleep. Neuron 2014, 82, 695–708. [Google Scholar] [CrossRef]
- Houldin, E.; Fang, Z.; Ray, L.B.; Stojanoski, B.; Owen, A.M.; Fogel, S.M. Reversed and Increased Functional Connectivity in Non-REM Sleep Suggests an Altered Rather than Reduced State of Consciousness Relative to Wake. Sci. Rep. 2021, 11, 11943. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Rechtschaffen, A. Auditory Awakening Thresholds and Dream Recall in NREM Sleep. Percept. Mot. Ski. 1969, 29, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.; Rosadini, G.; Rossi, G.F.; Zattoni, J. Relations between Threshold of Arousal and Electroencephalographic Patterns during Sleep in Man. Physiol. Behav. 1966, 1, 55–58. [Google Scholar] [CrossRef]
- Bekinschtein, T.A.; Dehaene, S.; Rohaut, B.; Tadel, F.; Cohen, L.; Naccache, L. Neural Signature of the Conscious Processing of Auditory Regularities. Proc. Natl. Acad. Sci. USA 2009, 106, 1672–1677. [Google Scholar] [CrossRef]
- Perez, P.; Valente, M.; Hermann, B.; Sitt, J.; Faugeras, F.; Demeret, S.; Rohaut, B.; Naccache, L. Auditory Event-Related “Global Effect” Predicts Recovery of Overt Consciousness. Front. Neurol. 2021, 11, 588233. [Google Scholar] [CrossRef]
- Friston, K. A Theory of Cortical Responses. Philos. Trans. R. Soc. B: Biol. Sci. 2005, 360, 815–836. [Google Scholar] [CrossRef]
- Marti, S.; Thibault, L.; Dehaene, S. How Does the Extraction of Local and Global Auditory Regularities Vary with Context? PLoS ONE 2014, 9, e107227. [Google Scholar] [CrossRef]
- Chennu, S.; Noreika, V.; Gueorguiev, D.; Blenkmann, A.; Kochen, S.; Ibanez, A.; Owen, A.M.; Bekinschtein, T.A. Expectation and Attention in Hierarchical Auditory Prediction. J. Neurosci. 2013, 33, 11194–11205. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.; Sitt, J.D.; King, J.-R.; Elbaz, M.; Azizi, L.; Buiatti, M.; Naccache, L.; van Wassenhove, V.; Dehaene, S. Disruption of Hierarchical Predictive Coding during Sleep. Proc. Natl. Acad. Sci. USA 2015, 112, E1353–E1362. [Google Scholar] [CrossRef] [PubMed]
- Ivanitsky, A.M. Brain Mechanisms of the Signal Evaluation; Medicina Publishing House: Moscow, Russia, 1976; p. 264. (In Russian) [Google Scholar]
- Hajcak, G.; Foti, D. Significance? … Significance! Empirical, Methodological, and Theoretical Connections between the Late Positive Potential and P300 as Neural Responses to Stimulus Significance: An Integrative Review. Psychophysiology 2020, 57, e13570. [Google Scholar] [CrossRef] [PubMed]
- Faugeras, F.; Rohaut, B.; Weiss, N.; Bekinschtein, T.A.; Galanaud, D.; Puybasset, L.; Bolgert, F.; Sergent, C.; Cohen, L.; Dehaene, S.; et al. Probing Consciousness with Event-Related Potentials in the Vegetative State. Neurology 2011, 77, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Atienza, M.; Cantero, J.L.; Dominguez-Marin, E. Mismatch Negativity (MMN): An Objective Measure of Sensory Memory and Long-Lasting Memories during Sleep. Int. J. Psychophysiol. 2002, 46, 215–225. [Google Scholar] [CrossRef]
- Tivadar, R.I.; Knight, R.T.; Tzovara, A. Automatic Sensory Predictions: A Review of Predictive Mechanisms in the Brain and Their Link to Conscious Processing. Front. Hum. Neurosci. 2021, 15, 438. [Google Scholar] [CrossRef]
- Hobson, J.A. REM Sleep and Dreaming: Towards a Theory of Protoconsciousness. Nat. Rev. Neurosci. 2009, 10, 803–813. [Google Scholar] [CrossRef]
- Sviderskaya, N.E.; Bykov, P.V. Spatial Organization of EEG Activity during Active Hyperventilation (Cyclic Breath) I. General Patterns of Changes in Brain Functional State and the Effect of Paroxysmal Activity. Hum. Physiol. 2006, 32, 140–149. [Google Scholar] [CrossRef]
- Peter-Derex, L.; Magnin, M.; Bastuji, H. Heterogeneity of Arousals in Human Sleep: A Stereo-Electroencephalographic Study. NeuroImage 2015, 123, 229–244. [Google Scholar] [CrossRef]
- Rosanova, M.; Fecchio, M.; Casarotto, S.; Sarasso, S.; Casali, A.G.; Pigorini, A.; Comanducci, A.; Seregni, F.; Devalle, G.; Citerio, G.; et al. Sleep-like Cortical OFF-Periods Disrupt Causality and Complexity in the Brain of Unresponsive Wakefulness Syndrome Patients. Nat. Commun. 2018, 9, 4427. [Google Scholar] [CrossRef]
- Modolo, J.; Hassan, M.; Wendling, F.; Benquet, P. Decoding the Circuitry of Consciousness: From Local Microcircuits to Brain-Scale Networks. Netw. Neurosci. 2020, 4, 315–337. [Google Scholar] [CrossRef]
- van Driel, J.; Knapen, T.; van Es, D.M.; Cohen, M.X. Interregional Alpha-Band Synchrony Supports Temporal Cross-Modal Integration. NeuroImage 2014, 101, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Kujala, T.; Winkler, I. Auditory Processing That Leads to Conscious Perception: A Unique Window to Central Auditory Processing Opened by the Mismatch Negativity and Related Responses. Psychophysiology 2011, 48, 4–22. [Google Scholar] [CrossRef] [PubMed]
- El Karoui, I.; King, J.R.; Sitt, J.; Meyniel, F.; Van Gaal, S.; Hasboun, D.; Adam, C.; Navarro, V.; Baulac, M.; Dehaene, S.; et al. Event-Related Potential, Time-Frequency, and Functional Connectivity Facets of Local and Global Auditory Novelty Processing: An Intracranial Study in Humans. Cereb. Cortex 2015, 25, 4203–4212. [Google Scholar] [CrossRef]
- Ivanitsky, A.M.; Ivanitsky, G.A.; Sysoeva, O.V. Brain Science: On the Way to Solving the Problem of Consciousness. Int. J. Psychophysiol. 2009, 73, 101–108. [Google Scholar] [CrossRef]
- Beer, C. In the Theater of Consciousness: The Workspace of the Mind. Bernard J. Baars. Q. Rev. Biol. 1998, 73, 106–107. [Google Scholar] [CrossRef]
- Dehaene, S. Towards a Cognitive Neuroscience of Consciousness: Basic Evidence and a Workspace Framework. Cognition 2001, 79, 1–37. [Google Scholar] [CrossRef]
- Bor, D.; Seth, A.K. Consciousness and the Prefrontal Parietal Network: Insights from Attention, Working Memory, and Chunking. Front. Psychol. 2012, 3, 63. [Google Scholar] [CrossRef]
- Tassi, P.; Muzet, A. Sleep Inertia. Sleep Med. Rev. 2000, 4, 341–353. [Google Scholar] [CrossRef]
- Trotti, L.M. Waking up Is the Hardest Thing I Do All Day: Sleep Inertia and Sleep Drunkenness. Sleep Med. Rev. 2017, 35, 76–84. [Google Scholar] [CrossRef]
- Balkin, T.J. The Process of Awakening: A PET Study of Regional Brain Activity Patterns Mediating the Re-Establishment of Alertness and Consciousness. Brain 2002, 125, 2308–2319. [Google Scholar] [CrossRef]
- Ferrara, M.; Curcio, G.; Fratello, F.; Moroni, F.; Marzano, C.; Pellicciari, M.; Gennaro, L. The Electroencephalographic Substratum of the Awakening. Behav. Brain Res. 2006, 167, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Ferrara, M.; Moroni, F.; De Gennaro, L. Electroencephalographic Sleep Inertia of the Awakening Brain. Neuroscience 2011, 176, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; De Gennaro, L.; Ferlazzo, F.; Curcio, G.; Barattucci, M.; Bertini, M. Auditory Evoked Responses upon Awakening from Sleep in Human Subjects. Neurosci. Lett. 2001, 310, 145–148. [Google Scholar] [CrossRef]
- Bastuji, H.; Perrin, F.; Garcia-Larrea, L. Event-Related Potentials during Forced Awakening: A Tool for the Study of Acute Sleep Inertia. J. Sleep Res. 2003, 12, 189–206. [Google Scholar] [CrossRef]
- Shilov, M.O.; Liaukovich, K.M.; Martynova, O.V.; Ukraintseva, Y.V. Effects of Daytime Sleep Inertia on the Recognition of Barely Distinguishable Sounds. Neurosci. Behav. Physiol. 2021, 51, 938–946. [Google Scholar] [CrossRef]
- Feltin, M.; Broughton, R. Differential Effects of Arousal from Slow Wave versus REM Sleep. Psychophysiology 1968, 5, 231. [Google Scholar]
- Chow, H.M.; Horovitz, S.G.; Carr, W.S.; Picchioni, D.; Coddington, N.; Fukunaga, M.; Xu, Y.; Balkin, T.J.; Duyn, J.H.; Braun, A.R. Rhythmic Alternating Patterns of Brain Activity Distinguish Rapid Eye Movement Sleep from Other States of Consciousness. Proc. Natl. Acad. Sci. USA 2013, 110, 10300–10305. [Google Scholar] [CrossRef]
- Feige, B.; Nanovska, S.; Baglioni, C.; Bier, B.; Cabrera, L.; Diemers, S.; Quellmalz, M.; Siegel, M.; Xeni, I.; Szentkiralyi, A.; et al. Insomnia—Perchance a Dream? Results from a NREM/REM Sleep Awakening Study in Good Sleepers and Patients with Insomnia. Sleep 2018, 41, zsy032. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Nissen, C.; Hirscher, V.; Baglioni, C.; Feige, B. REM Sleep Instability—A New Pathway for Insomnia? Pharmacopsychiatry 2012, 45, 167–176. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]


| Step 1 | Step 2nonr | Step 2resp | Step 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWS a (N = 39) | REM a (N = 55) | p | SWS a (N = 35) | REM a (N = 52) | p | SWS a (N = 33) | REM a (N = 49) | p | SWS a (N = 38) | REM a (N = 54) | p | |
| Latency, s | 0 | 0 | 1 | 15.24 ± 19.82 | 2.42 ± 2.35 | <0.001 | 21.52 ± 13.55 | 8.41 ± 7.05 | <0.001 | 40.22 ± 20.87 | 22.72 ± 14.99 | <0.001 |
| Awakening from SWS | Awakening from REM | Awakening SWS + REM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Local a | Global a | p | Local a | Global a | p | Local a | Global a | p | |
| Latency | |||||||||
| Step 2resp, sec | 21.98 ± 14.14 (N = 17) | 21.02 ± 13.34 (N = 16) | 0.871 | 6.9 ± 5.19 (N = 22) | 9.63 ± 8.16 (N = 27) | 0.131 | 13.47 ± 12.51 (N = 39) | 13.87 ± 11.66 (N = 43) | 0.417 |
| Step 3, sec | 37.56 ± 17.63 (N = 20) | 43.18 ± 24.15 (N = 18) | 0.693 | 19.43 ± 12.82 (N = 28) | 26.26 ± 16.54 (N = 26) | 0.206 | 26.98 ± 17.37 (N = 48) | 33.18 ± 21.46 (N = 44) | 0.282 |
| Reaction time | |||||||||
| Wakepre, ms | 380.07 ± 79.83 (n = 11) | 449.29 ± 62.23 (n = 12) | 0.029 | 378.19 ± 76.73 (n = 19) | 452.93 ± 86.79 (n = 19) | 0.007 | 378.88 ± 76.5 (n = 30) | 451.52 ± 77.09 (n = 31) | <0.001 |
| Step 2resp, ms | 768.78 ± 442.14 (N = 16) | 807.87 ± 3511.45 (N = 16) | 0.72 | 786.93 ± 182.27 (N = 17) | 941.54 ± 358.78 (N = 25) | 0.148 | 778.13 ± 329.13 (N = 33) | 889.38 ± 357.65 (N = 41) | 0.217 |
| Step 3, ms | 418.89 ± 64.48 (N = 20) | 475.29 ± 58.43 (N = 18) | 0.007 | 423.42 ± 61.33 (N = 28) | 458.58 ± 58.58 (N = 26) | 0.043 | 421.53 ± 62.02 (N = 48) | 465.41 ± 58.43 (N = 44) | <0.001 |
| Wakepost, ms | 392.34 ± 90.08 (n = 11) | 468.77 ± 58.95 (n = 9) | 0.04 | 394.57 ± 108.76 (n = 20) | 449.78 ± 65.44 (n = 19) | 0.02 | 393.78 ± 100.98 (n = 31) | 455.88 ± 62.98 (n = 28) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liaukovich, K.; Sazhin, S.; Bobrov, P.; Ukraintseva, Y. Event-Related Potential Study of Recovery of Consciousness during Forced Awakening from Slow-Wave Sleep and Rapid Eye Movement Sleep. Int. J. Mol. Sci. 2022, 23, 11785. https://doi.org/10.3390/ijms231911785
Liaukovich K, Sazhin S, Bobrov P, Ukraintseva Y. Event-Related Potential Study of Recovery of Consciousness during Forced Awakening from Slow-Wave Sleep and Rapid Eye Movement Sleep. International Journal of Molecular Sciences. 2022; 23(19):11785. https://doi.org/10.3390/ijms231911785
Chicago/Turabian StyleLiaukovich, Krystsina, Sergei Sazhin, Pavel Bobrov, and Yulia Ukraintseva. 2022. "Event-Related Potential Study of Recovery of Consciousness during Forced Awakening from Slow-Wave Sleep and Rapid Eye Movement Sleep" International Journal of Molecular Sciences 23, no. 19: 11785. https://doi.org/10.3390/ijms231911785
APA StyleLiaukovich, K., Sazhin, S., Bobrov, P., & Ukraintseva, Y. (2022). Event-Related Potential Study of Recovery of Consciousness during Forced Awakening from Slow-Wave Sleep and Rapid Eye Movement Sleep. International Journal of Molecular Sciences, 23(19), 11785. https://doi.org/10.3390/ijms231911785





