Physiological and Transcriptional Analysis Reveals the Response Mechanism of Camellia vietnamensis Huang to Drought Stress
Abstract
1. Introduction
2. Results
2.1. Comparative Analysis of Morphological and Physiological Characteristics of HD1 and WH1 C. vietnamensis Seedlings under PEG Stress
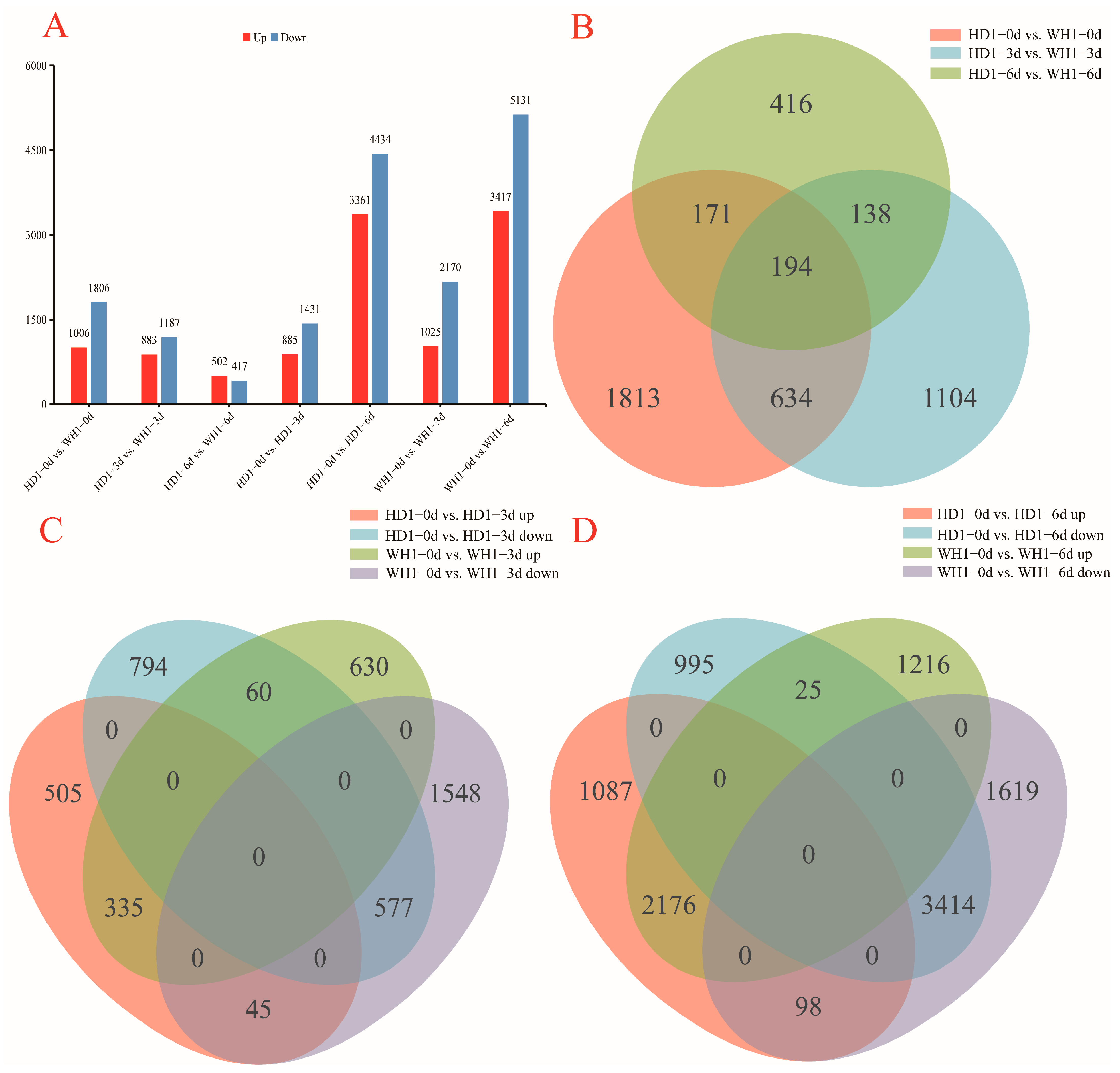
2.2. Overview of Leaf Transcriptome Sequencing Results of C. vietnamensis under Stress by PEG
2.3. Analysis of DEGs between HD1 and WH1 Cultivars
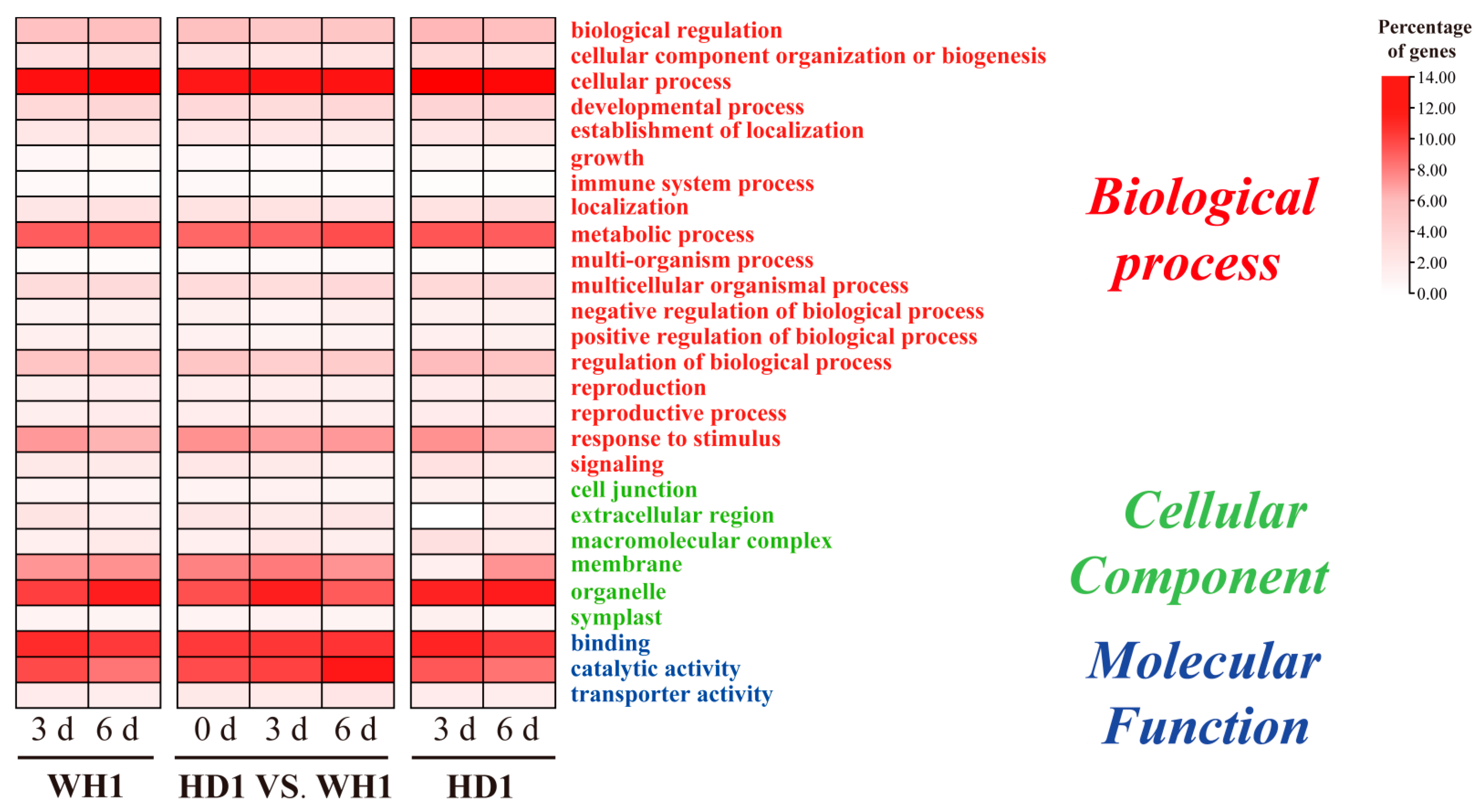
2.4. GO- and KEGG-Enrichment Analyses
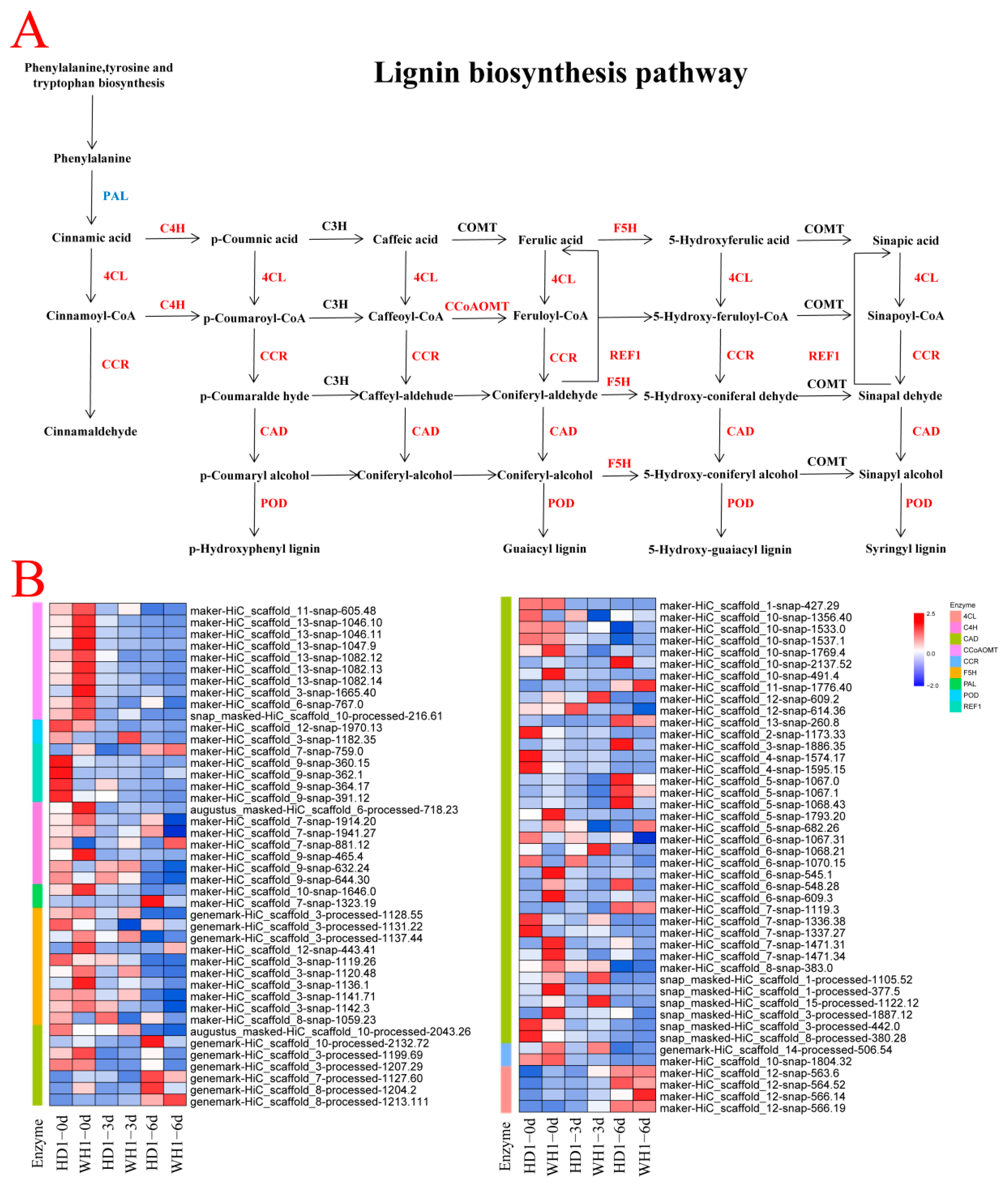
2.5. Different Expression Patterns of Genes Related to Phenylpropanoid Biosynthesis in Two C. vietnamensis Cultivars under PEG Stress
2.6. Different Expression Patterns of Genes Related to Flavonoid Biosynthesis in Two C. vietnamensis Cultivars under PEG Stress
2.7. Different Expression Patterns of Hormone Signal Transduction-Related Genes in Two C. vietnamensis Cultivars under PEG Stress
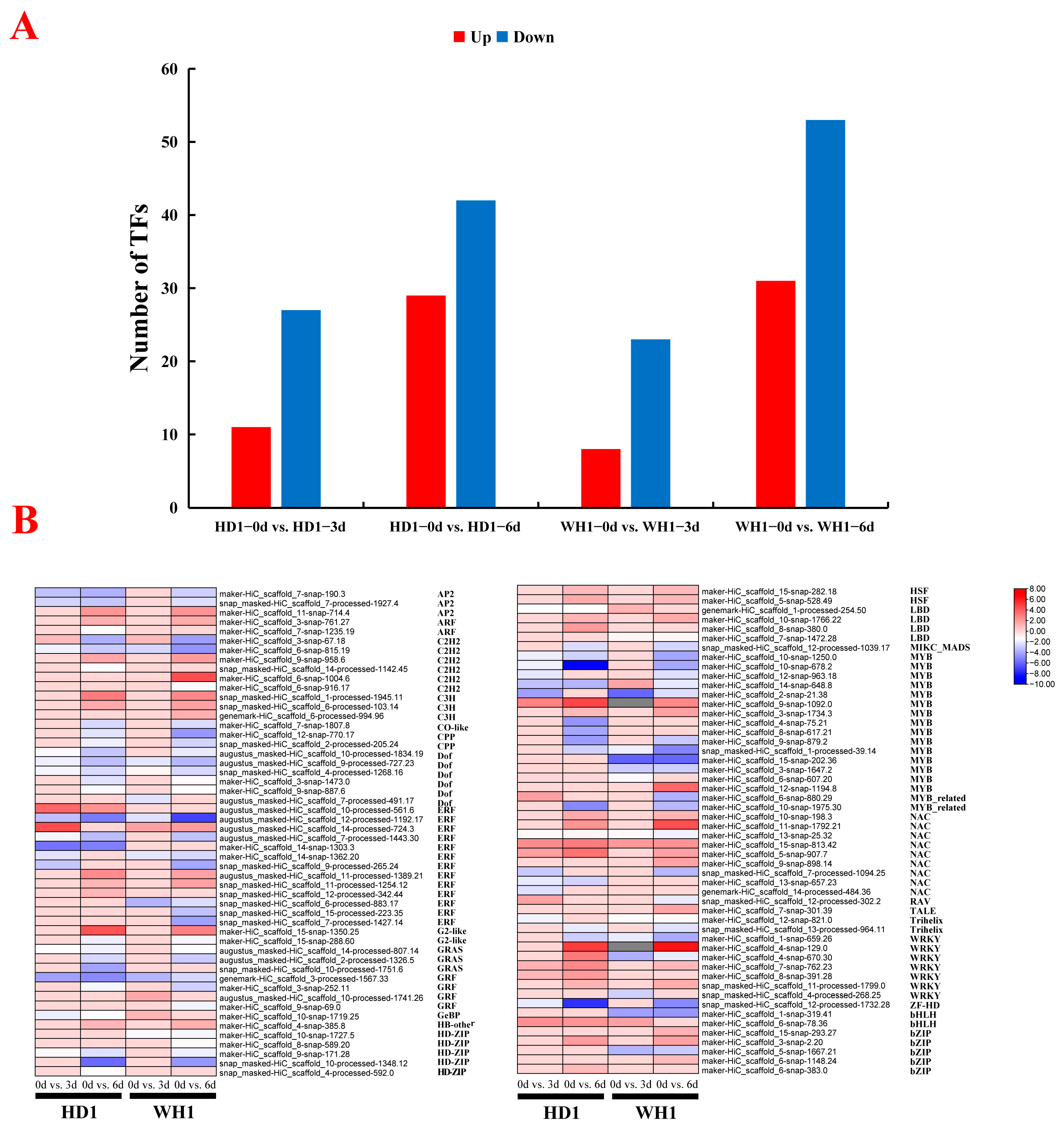
2.8. TF Prediction
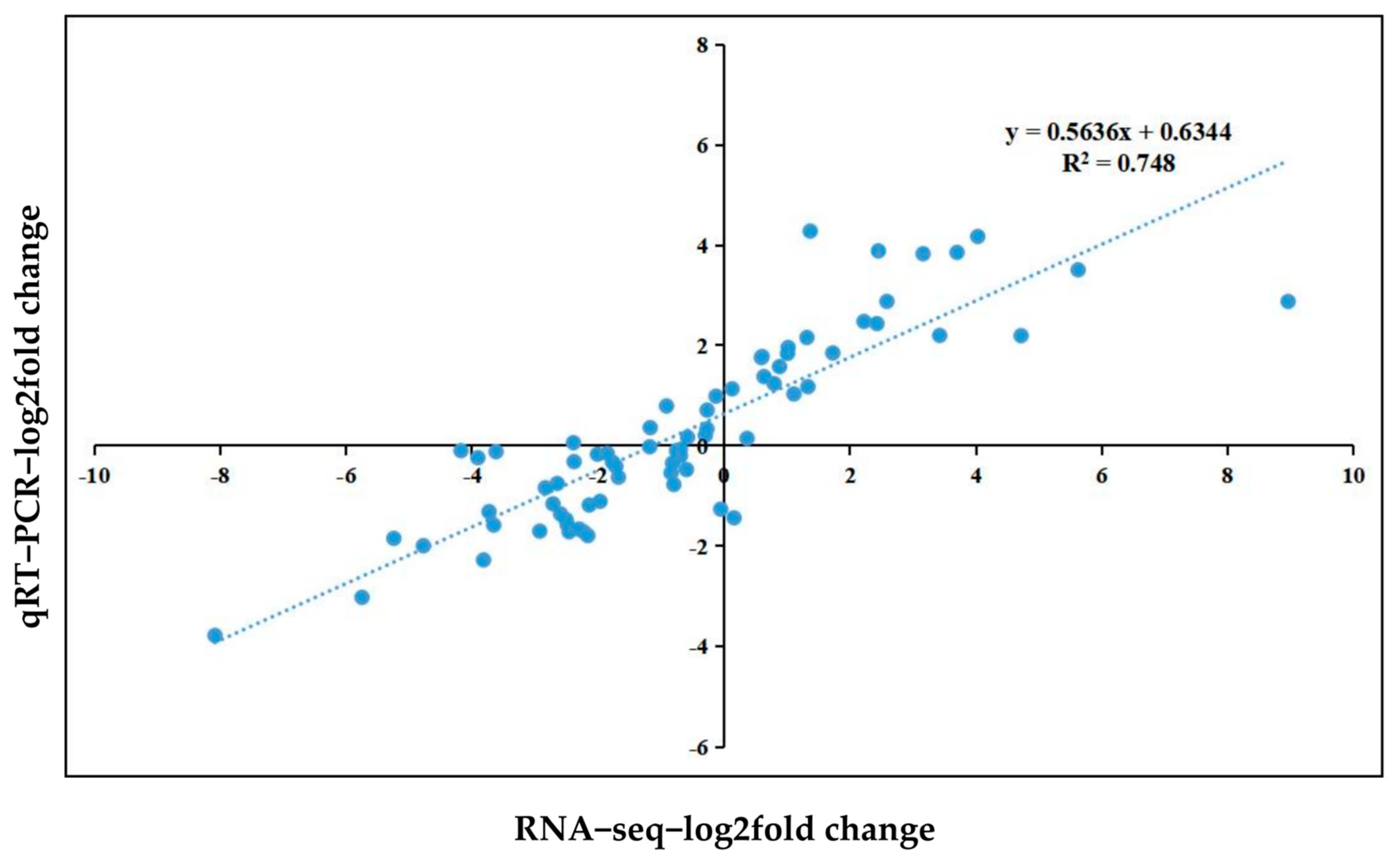
2.9. RNA-seq Expression Level Was Verified by qRT-PCR
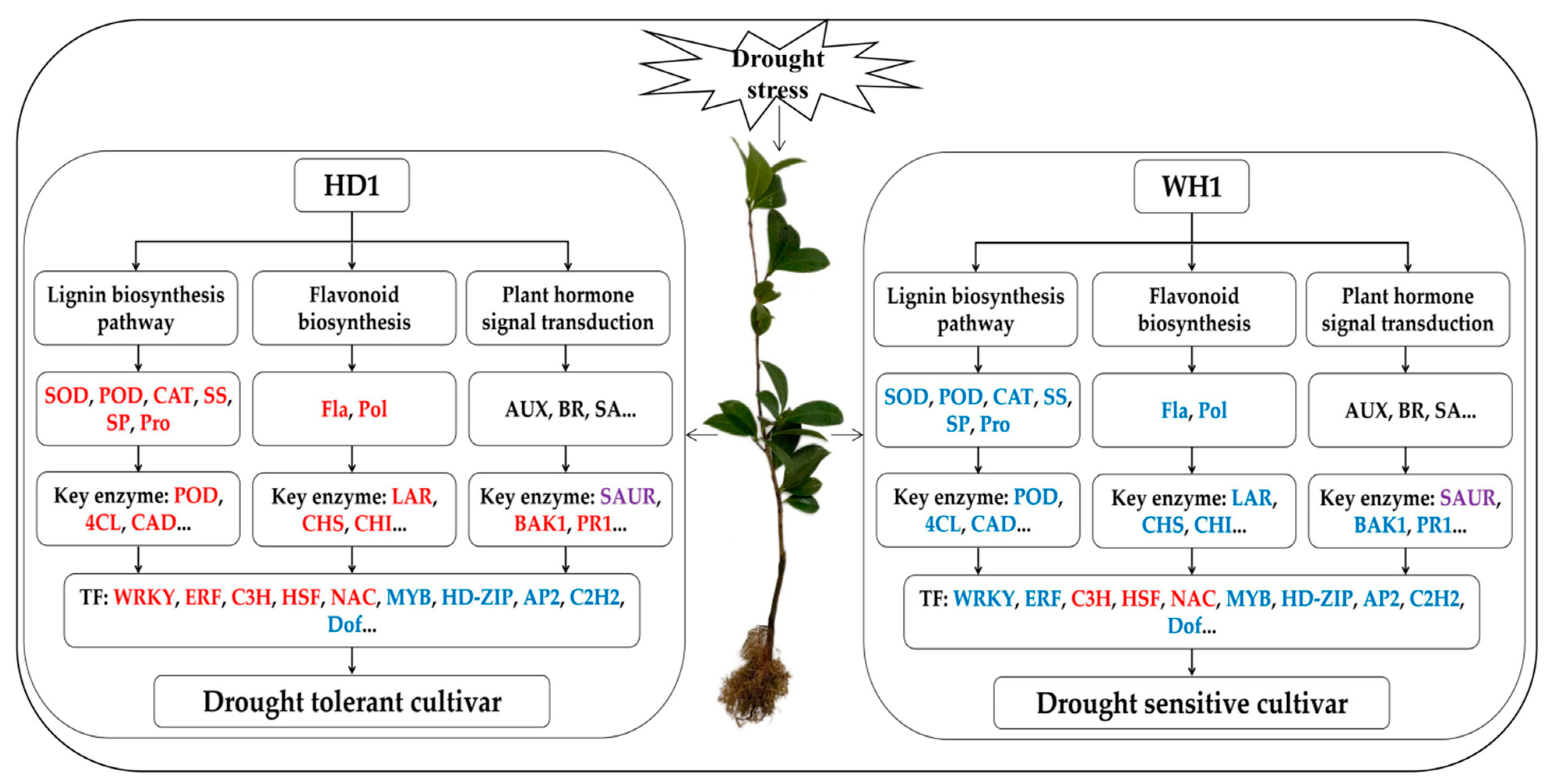
3. Discussion
3.1. Physiological Responses of Two C. vietnamensis Cultivars to Drought Stress
3.2. Increasing the Antioxidant Activity of C. vietnamensis Seedlings Plays a Key Role in Resisting Drought Stress
3.3. The Flavonoid Biosynthesis Pathway Plays an Important Role in Drought Tolerance of C. vietnamensis Seedlings
3.4. Plant-Hormone Signal Transduction Plays a Crucial Role in Drought Tolerance of C. vietnamensis Seedlings, Especially AUX and BR
4. Materials and Methods
4.1. Plant Materials and Methods
4.2. Measurement of Physiological, Biochemical and Secondary Metabolite Indexes
4.3. Transcriptome Sequencing and Analysis
4.4. Validation of DEG Expression by qRT-PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Zhou, H.Y.; Yang, X.N.; Zhan, J.J.; Zhou, H.; Wang, C.; Yu, Y.; Lu, X.Y.; Chen, Y.Z.; Tian, Y. Transcriptomic analysis of Camellia oleifera in response to drought stress using high throughput RNA-seq. Russ. J. Plant Physiol. 2017, 64, 728–737. [Google Scholar] [CrossRef]
- Gao, S.S.; Wang, Y.L.; Yu, S.; Huang, Y.Q.; Liu, H.C.; Chen, W.; He, X.Y. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Hou, W.; Yan, P.C.; Feng, G.L.; Zuo, D.D. A 3D Copula Method for the Impact and Risk Assessment of Drought Disaster and an Example Application. Front. Phys. 2021, 9, 656253. [Google Scholar] [CrossRef]
- Du, C.; Chen, J.S.; Nie, T.; Dai, C.L. Spatial–temporal changes in meteorological and agricultural droughts in Northeast China: Change patterns, response relationships and causes. Nat. Hazards. 2022, 110, 155–173. [Google Scholar] [CrossRef]
- Hu, X.; Yang, M.; Gong, S.F.; Li, H.B.; Zhang, J.; Sajjad, M.; Ma, X.L.; Yuan, D.Y. Ethylene-regulated immature fruit abscission is associated with higher expression of CoACO genes in Camellia oleifera. R. Soc. Open Sci. 2021, 8, 202340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qiu, F.C.; Chen, L.; Liu, R.J.; Chang, M.; Wang, X.G. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. 2021, 344, 128660. [Google Scholar] [CrossRef]
- Ye, Z.C.; Yu, J.; Yan, W.P.; Zhang, J.F.; Yang, D.M.; Yao, G.L.; Liu, Z.J.; Wu, Y.G.; Hou, X.L. Integrative iTRAQ-based proteomic and transcriptomic analysis reveals the accumulation patterns of key metabolites associated with oil quality during seed ripening of Camellia oleifera. Hortic. Res. 2021, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Wu, Y.G.; Ul Haq Muhammad, Z.; Yan, W.P.; Yu, J.; Zhang, J.F.; Yao, G.L.; Hu, X.W. Complementary transcriptome and proteome profiling in the mature seeds of Camellia oleifera from Hainan Island. PLoS ONE 2020, 15, e0226888. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.M.; He, L.M.; Chen, Y.Y.; Wu, L.H.; Wang, L.; Liu, Z.P. Anti-inflammatory and antioxidative effects of Camellia oleifera Abel components. Futur. Med. Chem. 2017, 9, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wu, B.; Hong, W.H.; Li, X.Q.; Li, Z.; Xue, L.; Huang, Y.F. Transcriptome analysis of the tea oil camellia (Camellia oleifera) reveals candidate drought stress genes. PLoS ONE 2017, 12, e0181835. [Google Scholar] [CrossRef] [PubMed]
- Elagib, N.A. Development and application of a drought risk index for food crop yield in Eastern Sahel. Ecol. Indic. 2014, 43, 114–125. [Google Scholar] [CrossRef]
- Price, A.H.; Cairns, J.E.; Horton, P.; Jones, H.G.; Griffiths, H. Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: Progress and new opportunities to integrate stomatal and mesophyll responses. J. Exp. Bot. 2002, 53, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Sarwat, M.; Tuteja, N. Hormonal signaling to control stomatal movement during drought stress. Plant Gene 2017, 11, 143–153. [Google Scholar] [CrossRef]
- Wan, J.X.; Griffiths, R.; Ying, J.F.; McCourt, P.; Huang, Y.F. Development of drought-tolerant canola (Brassica napus L.) through genetic modulation of ABA-mediated stomatal responses. Crop Sci. 2009, 49, 1539–1554. [Google Scholar] [CrossRef]
- Sperlich, D.; Barbeta, A.; Ogaya, R.; Sabaté, S.; Peñuelas, J. Balance between carbon gain and loss under long-term drought: Impacts on foliar respiration and photosynthesis in Quercus ilex L. J. Exp. Bot. 2016, 67, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Ogbaga, C.C.; Stepien, P.; Dyson, B.C.; Rattray, N.J.; Ellis, D.I.; Goodacre, R.; Johnson, G.N. Biochemical analyses of sorghum varietiescultivars reveal differential responses to drought. PLoS ONE 2016, 11, e0154423. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Casaretto, J.A.; El-Kereamy, A.; Zeng, B.; Stiegelmeyer, S.M.; Chen, X.; Bi, Y.M.; Rothstein, S.J. Expression of OsMYB55 in maize activates stress-responsive genes and enhances heat and drought tolerance. BMC Genom. 2016, 17, 312. [Google Scholar] [CrossRef]
- Wen, X.J.; Wang, J.X.; Zhang, D.Y.; Wang, Y.C. A gene regulatory network controlled by BpERF2 and BpMYB102 in Birch under drought conditions. Int. J. Mol. Sci. 2019, 20, 3071. [Google Scholar] [CrossRef]
- Chang, Y.; Nguyen, B.H.; Xie, Y.J.; Xiao, B.Z.; Tang, N.; Zhu, W.L.; Mou, T.M.; Xiong, L.Z. Co-overexpression of the constitutively active form of OsbZIP46 and ABA-activated protein kinase SAPK6 improves drought and temperature stress resistance in rice. Front Plant Sci. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Li, W.M.; Wang, Y.J.; Ren, H.; Guo, Z.H.; Li, N.; Zhao, C.Z.; Xie, Z.K. Transcriptomic and physiological analyses identifying Lanzhou lily (Lilium davidii var. unicolor) drought adaptation strategies. Hortic. Plant J. 2022. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Gao, X.L.; Li, J.; Gong, X.W.; Yang, P.; Gao, J.F.; Wang, P.K.; Feng, B.L. Comparative analysis of proso millet (Panicum miliaceum L.) leaf transcriptomes for insight into drought tolerance mechanisms. BMC Plant Biol. 2019, 19, 397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.L.; Ren, Y.J.; Li, Z. Transcriptome profiling of Jerusalem artichoke seedlings (Helianthus tuberosus L.) under polyethylene glycol-simulated drought stress. Ind. Crop. Prod. 2021, 170, 113696. [Google Scholar] [CrossRef]
- Clark, M.B.; Mercer, T.R.; Bussotti, G.; Leonardi, T.; Haynes, K.R.; Crawford, J.; Brunck, M.E.; Cao, K.L.; Thomas, G.P.; Chen, W.Y.; et al. Quantitative gene profiling of long noncoding RNAs with targeted RNA sequencing. Nat. Methods 2015, 12, 339–342. [Google Scholar] [CrossRef]
- Xu, K.J.; Sun, F.L.; Chai, G.Q.; Wang, Y.F.; Shi, L.L.; Liu, S.D.; Xi, Y.J. De novo assembly and transcriptome analysis of two contrary tillering mutants to learn the mechanisms of tillers outgrowth in switchgrass (Panicum virgatum L.). Front Plant Sci. 2015, 6, 749. [Google Scholar] [CrossRef]
- He, X.S.; Xu, L.C.; Pan, C.; Gong, C.; Wang, Y.J.; Liu, X.L.; Yu, Y.C. Drought resistance of Camellia oleifera under drought stress: Changes in physiology and growth characteristics. PLoS ONE 2020, 15, e0235795. [Google Scholar] [CrossRef]
- Lei, Y.T.; Xu, Y.X.; Hettenhausen, C.; Lu, C.K.; Shen, G.J.; Zhang, C.P.; Li, J.; Song, J.; Lin, H.H.; Wu, J.Q. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar] [CrossRef]
- Hayano-Kanashiro, C.; Calderón-Vázquez, C.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Simpson, J. Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS ONE 2009, 4, e7531. [Google Scholar] [CrossRef]
- Liu, G.; Zenda, T.; Liu, S.T.; Wang, X.; Jin, H.Y.; Dong, A.Y.; Yang, Y.T.; Duan, H.J. Comparative transcriptomic and physiological analyses of contrasting hybrid cultivars ND476 and ZX978 identify important differentially expressed genes and pathways regulating drought stress tolerance in maize. Genes Genom. 2020, 42, 937–955. [Google Scholar] [CrossRef]
- Waititu, J.K.; Zhang, X.G.; Chen, T.C.; Zhang, C.Y.; Zhao, Y.; Wang, H. Transcriptome analysis of tolerant and susceptible maize genotypes reveals novel insights about the molecular mechanisms underlying drought responses in leaves. Int. J. Mol. Sci. 2021, 22, 6980. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mo, Y.L.; Cui, Q.; Yang, X.Z.; Guo, Y.L.; Wei, C.H.; Yang, J.Q.; Zhang, Y.; Ma, J.X.; Zhang, X. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and-susceptible watermelon genotypes. Plant Sci. 2019, 278, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Man, D.; Bao, Y.X.; Han, L.B.; Zhang, X.Z. Drought tolerance associated with proline and hormone metabolism in two tall fescue cultivars. HortScience 2011, 46, 1027–1032. [Google Scholar] [CrossRef]
- Jespersen, D.; Leclerc, M.; Zhang, G.S.; Raymer, P. Drought performance and physiological responses of bermudagrass and seashore paspalum. Crop Sci. 2019, 59, 778–786. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.T.; Wang, X.; Liu, G.; Jin, H.Y.; Dong, A.Y.; Yang, Y.T.; Duan, H.J. Key maize drought-responsive genes and pathways revealed by comparative transcriptome and physiological analyses of contrasting inbred lines. Int. J. Mol. Sci. 2019, 20, 1268. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, H.; Wang, L.; Liu, H.J.; Huo, H.Q.; Zhang, C.J.; Liu, A.Z.; Zhu, A.D.; Hu, J.Y.; Lin, Y.J.; et al. Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front. Genet. 2019, 10, 55. [Google Scholar] [CrossRef]
- Fracasso, A.; Trindade, L.M.; Amaducci, S. Drought stress tolerance strategies revealed by RNA-Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol. 2016, 16, 115. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Geng, D.L.; Chen, P.X.; Shen, X.X.; Zhang, Y.; Li, X.W.; Jiang, L.J.; Xie, Y.P.; Niu, C.D.; Zhang, J.; Huang, X.H.; et al. MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox-and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Rangani, J.; Panda, A.; Patel, M.; Parida, A.K. Regulation of ROS through proficient modulations of antioxidative defense system maintains the structural and functional integrity of photosynthetic apparatus and confers drought tolerance in the facultative halophyte Salvadora persica L. J. Photochem. Photobiol. B. Biol. 2018, 189, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Van Nood, E.L.S.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Rani, A.; Singh, K.; Ahuja, P.S.; Kumar, S. Molecular regulation of catechins biosynthesis in tea [Camellia sinensis (L.) O. Kuntze]. Gene 2012, 495, 205–210. [Google Scholar] [CrossRef]
- Wang, F.B.; Ren, G.L.; Li, F.S.; Qi, S.T.; Xu, Y.; Wang, B.W.; Yang, Y.L.; Ye, Y.X.; Zhou, Q.; Chen, X.H. A chalcone synthase gene AeCHS from Abelmoschus esculentus regulates flavonoid accumulation and abiotic stress tolerance in transgenic Arabidopsis. Acta Physiol Plant. 2018, 40, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Wei, K.; Cheng, H.; Wang, L.Y.; Zhang, C.C. Accumulation of catechins and expression of catechin synthetic genes in Camellia sinensis at different developmental stages. Bot. Stud. 2016, 57, 1–8. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Wang, W.D.; Xin, H.H.; Wang, M.L.; Ma, Q.P.; Wang, L.; Kaleri, N.A.; Wang, Y.H.; Li, X.H. Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Front. Plant Sci. 2016, 7, 385. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Singh, V.P.; Prasad, S.M.; Munné-Bosch, S.; Müller, M. Phytohormones and the regulation of stress tolerance in plants: Current status and future directions. Front. Plant Sci. 2017, 8, 1871. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.D.; Huang, Y.Z.; Zhou, L.; Deng, S.S.; Wang, C.C.; Xu, J.Y.; Wang, H.T.; Zhao, J.M.; Guo, N.; Xing, H. Key Soybean Seedlings Drought-Responsive Genes and Pathways Revealed by Comparative Transcriptome Analyses of Two Cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, D.K.; Do Choi, Y.; Kim, J.K. OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Bai, Y.H.; Shen, C.J.; Wu, Y.R.; Zhang, S.N.; Jiang, D.A.; Guilfoyle, T.J.; Chen, M.; Qi, Y.H. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct. Integr. Genom. 2010, 10, 533–546. [Google Scholar] [CrossRef]
- Kant, S.; Rothstein, S. Auxin-responsive SAUR39 gene modulates auxin level in rice. Plant Signal. Behav. 2009, 4, 1174–1175. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.P.; Pareek, S.; Mudgal, G.; Kumar, D. Auxin response factors in plant adaptation to drought and salinity stress. Physiol Plantarum. 2022, 174, e13714. [Google Scholar] [CrossRef]
- Yang, T.X.; Wang, Y.Y.; Teotia, S.; Wang, Z.H.; Shi, C.N.; Sun, H.W.; Gu, Y.Y.; Zhang, Z.H.; Tang, G.L. The interaction between miR160 and miR165/166 in the control of leaf development and drought tolerance in Arabidopsis. Sci. Rep. 2019, 9, 2832. [Google Scholar] [CrossRef]
- Chen, J.N.; Nolan, T.M.; Ye, H.X.; Zhang, M.C.; Tong, H.N.; Xin, P.Y.; Chu, J.F.; Chu, C.C.; Li, Z.H.; Yin, Y.H. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Hothorn, M.; Belkhadir, Y.; Dreux, M.; Dabi, T.; Noel, J.; Wilson, I.A.; Chory, J. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 2011, 474, 467–471. [Google Scholar] [CrossRef]
- Tang, W.Q.; Kim, T.W.; Oses-Prieto, J.A.; Sun, Y.; Deng, Z.P.; Zhu, S.W.; Wang, R.J.; Burlingame, A.L.; Wang, Z.Y. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 2008, 321, 557–560. [Google Scholar] [CrossRef]
- Kim, T.W.; Guan, S.; Burlingame, A.L.; Wang, Z.Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell 2011, 43, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tang, B.Y.; Xie, Z.L.; Nolan, T.; Ye, H.X.; Song, G.Y.; Walley, J.; Yin, Y.H. GSK 3-like kinase BIN2 phosphorylates RD26 to potentiate drought signaling in Arabidopsis. Plant J. 2019, 100, 923–937. [Google Scholar] [CrossRef]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamashino, T.; Yokoyama, A.; Mizuno, T. Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 47–57. [Google Scholar] [CrossRef]
- Mason, M.G.; Mathews, D.E.; Argyros, D.A.; Maxwell, B.B.; Kieber, J.J.; Alonso, J.M.; Ecker, J.R.; Schaller, G.E. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 2005, 17, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Zhang, T.Z. Expansion and stress responses of the AP2/EREBP superfamily in cotton. BMC Genom. 2017, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.Y.; Lin, Y.C.; Zu, Y.G.; Efferth, T.; Li, D.W.; Tang, Z.H. Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. J. Plant Biol. 2015, 58, 193–201. [Google Scholar] [CrossRef]
- Scarpeci, T.E.; Frea, V.S.; Zanor, M.I.; Valle, E.M. Overexpression of AtERF019 delays plant growth and senescence, and improves drought tolerance in Arabidopsis. J. Exp. Bot. 2017, 68, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.J.; Mandaokar, A.; Liu, G.H.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Chini, A.; Boter, M.; Solano, R. Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module. FEBS J. 2009, 276, 4682–4692. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H.; Ma, S.Q.; Xiang, D.H.; Liu, R.Y.; Xiong, L.Z. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Chico, J.M.; Saénchez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. R. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Liu, P.; Xu, Z.S.; Pan-Pan, L.; Hu, D.; Chen, M.; Li, L.C.; Ma, Y.Z. A wheat PI4K gene whose product possesses threonine autophophorylation activity confers tolerance to drought and salt in Arabidopsis. J. Exp. Bot. 2013, 64, 2915–2927. [Google Scholar] [CrossRef]
- Dong, B. Comprehensive Evaluation of Drought Resistance and Transcriptome Analysis under Drought Stress in Camellia oleifera; South China Agricultural University: Guangzhou, China, 2018. [Google Scholar]
- Galmés, J.; Flexas, J.; Savé, R.; Medrano, H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: Responses to water stress and recovery. Plant Soil 2007, 290, 139–155. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur. Food Res. Technol. 2007, 225, 321–328. [Google Scholar] [CrossRef]
- Ye, Z.C. The Comparative Study on Chemical Constituents and Bioactivities of Camellia oleifera Oils and Cakes from Different Locations; Hainan University: Haikou, China, 2017. [Google Scholar]
- He, Y.; Deng, C.; Xiong, L.; Qin, S.S.; Peng, C. Transcriptome sequencing provides insights into the metabolic pathways of patchouli alcohol and pogostone in Pogostemon cablin (Blanco) Benth. Genes Genom. 2016, 38, 1031–1039. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Wang, K.L.; Wang, Y.P.; Hu, Z.K.; Yan, C.; Huang, H.; Ma, X.J.; Cao, Y.Q.; Long, W.; Liu, W.X.; et al. The genome of oil-Camellia and population genomics analysis provide insights into seed oil domestication. Genome Biol. 2022, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and abundance estimation from RNA-Seq reveals thousands of new transcripts and switching among isoforms. Nat. Biotechnol. 2010, 28, 511. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression of RNA-Seq data at the gene level–the DESeq package; European Molecular Biology Laboratory (EMBL): Heidelberg, Germany, 2012. [Google Scholar]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; Liu, Y.J.; Li, W.W.; Zhao, L.; Meng, F.; Wang, Y.S.; Tan, H.R.; Yang, H.; Wei, C.L.; Wan, X.C.; et al. Tissue-specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant [Camellia sinensis]. PLoS ONE 2013, 8, e62315. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Cultivars | ‘Wanhai 1’ | ‘Wanhai 3’ | ‘Wanhai 4’ | ‘Haida 1’ | ‘Haida 4’ |
|---|---|---|---|---|---|
| RWC | 0.0000 | 0.2796 | 0.5581 | 1.0000 | 0.7181 |
| REC | 0.0000 | 0.5174 | 0.3795 | 1.0000 | 0.9255 |
| MDA | 0.2997 | 0.0000 | 0.4412 | 1.0000 | 0.5235 |
| SOD | 0.0000 | 1.0000 | 0.1059 | 0.4973 | 0.5317 |
| POD | 0.0000 | 0.4896 | 0.2886 | 1.0000 | 0.4465 |
| CAT | 0.1656 | 0.7576 | 0.9696 | 1.0000 | 0.0000 |
| SP | 0.5955 | 0.8538 | 0.8462 | 1.0000 | 0.0000 |
| SS | 0.4839 | 0.8015 | 0.0000 | 1.0000 | 0.6483 |
| Pro | 0.3798 | 0.0000 | 0.3063 | 1.0000 | 0.6258 |
| Fla | 0.0000 | 1.0000 | 0.7868 | 0.6880 | 0.5426 |
| Pol | 0.2237 | 0.7244 | 1.0000 | 0.7664 | 0.0000 |
| Subordinate function Mean value | 0.1953 | 0.5840 | 0.5166 | 0.9047 | 0.4511 |
| Rank | 5 | 2 | 3 | 1 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Yan, W.; Xie, S.; Yu, J.; Yao, G.; Xia, P.; Wu, Y.; Yang, H. Physiological and Transcriptional Analysis Reveals the Response Mechanism of Camellia vietnamensis Huang to Drought Stress. Int. J. Mol. Sci. 2022, 23, 11801. https://doi.org/10.3390/ijms231911801
Shen S, Yan W, Xie S, Yu J, Yao G, Xia P, Wu Y, Yang H. Physiological and Transcriptional Analysis Reveals the Response Mechanism of Camellia vietnamensis Huang to Drought Stress. International Journal of Molecular Sciences. 2022; 23(19):11801. https://doi.org/10.3390/ijms231911801
Chicago/Turabian StyleShen, Shuaishuai, Wuping Yan, Shuao Xie, Jing Yu, Guanglong Yao, Pengguo Xia, Yougen Wu, and Huageng Yang. 2022. "Physiological and Transcriptional Analysis Reveals the Response Mechanism of Camellia vietnamensis Huang to Drought Stress" International Journal of Molecular Sciences 23, no. 19: 11801. https://doi.org/10.3390/ijms231911801
APA StyleShen, S., Yan, W., Xie, S., Yu, J., Yao, G., Xia, P., Wu, Y., & Yang, H. (2022). Physiological and Transcriptional Analysis Reveals the Response Mechanism of Camellia vietnamensis Huang to Drought Stress. International Journal of Molecular Sciences, 23(19), 11801. https://doi.org/10.3390/ijms231911801






