Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia
Abstract
:1. Introduction
2. Soy Isoflavones and Conversion of Daidzein into S-equol
3. Bioavailability of S-equol as Compared to Daidzein and Genistein
3.1. Bioavailability in the Circulation
3.2. Permeability to the Gastrointestinal Tract and Blood–Brain Barrier
4. Evidence from Epidemiological and Clinical Studies Evaluating the Cognitive Benefits of S-equol
5. Potential Protective Mechanisms of S-equol for Cognitive Decline and ADRD
5.1. Potential Effect of S-equol on Arterial Stiffness
5.2. Inverse Association of S-equol with White Matter Lesions (WMLs)
5.3. Other Neuroprotective Properties of S-equol
5.3.1. Antioxidant Properties
5.3.2. Anti-Inflammatory Properties
5.3.3. Reduction in Amyloid-β Induced Neurotoxicity and Tau Phosphorylation
5.3.4. Other Reported Mechanisms
6. ERβ and Cognition
7. Neuroprotection by ERβ Agonists
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimers Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solfrizzi, V.; . Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C. Nutrition and risk of dementia: Overview and methodological issues. Ann. N. Y. Acad. Sci. 2016, 1367, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Tzagarakis-Foster, C.; Scharschmidt, T.C.; Lomri, N.; Leitman, D.C. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J. Biol. Chem. 2001, 276, 17808–17814. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Larrea, M.B.; Mohan, A.R.; Paganga, G.; Miller, N.J.; Bolwell, G.P.; Rice-Evans, C.A. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997, 26, 63–70. [Google Scholar] [CrossRef]
- Chacko, B.K.; Chandler, R.T.; Mundhekar, A.; Khoo, N.; Pruitt, H.M.; Kucik, D.F.; Parks, D.A.; Kevil, C.G.; Barnes, S.; Patel, R.P. Revealing anti-inflammatory mechanisms of soy isoflavones by flow: Modulation of leukocyte-endothelial cell interactions. Am. J. Physiol. —Heart Circ. Physiol. 2005, 289, H908–H915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, M.S.; Clarkson, T.B.; Bullock, B.C.; Wagner, J.D. Soy Protein Versus Soy Phytoestrogens in the Prevention of Diet-Induced Coronary Artery Atherosclerosis of Male Cynomolgus Monkeys. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharm. 2019, 74, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [Green Version]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Bai, W.; Wang, C.; Ren, C. Intakes of total and individual flavonoids by US adults. Int. J. Food Sci. Nutr. 2014, 65, 9–20. [Google Scholar] [CrossRef]
- Nakamoto, M.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Kato, Y.; Imai, T.; Sakai, T.; Ando, F.; Shimokata, H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018, 72, 1458–1462. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Henderson, V.W.; St John, J.A.; Hodis, H.N.; Kono, N.; McCleary, C.A.; Franke, A.A.; Mack, W.J. Long-term soy isoflavone supplementation and cognition in women: A randomized, controlled trial. Neurology 2012, 78, 1841–1848. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite sequel-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Kono, N.; Azen, S.P.; Shoupe, D.; Hwang-Levine, J.; Petitti, D.; Whitfield-Maxwell, L.; Yan, M.; Franke, A.A.; et al. Isoflavone Soy Protein Supplementation and Atherosclerosis Progression in Healthy Postmenopausal Women. Stroke 2011, 42, 3168–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Greany, K.; A Nettleton, J.; E Wangen, K.; Thomas, W.; Kurzer, M.S. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur. J. Clin. Nutr. 2008, 62, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Chiriboga, D.; Olendzki, B.C.; Nicolosi, R.; Merriam, P.A.; Ockene, I.S. Effect of soy protein containing isoflavones on blood lipids in moderately hypercholesterolemic adults: A randomized controlled trial. J. Am. Coll. Nutr. 2005, 24, 275–285. [Google Scholar] [CrossRef]
- West, S.G.; Hilpert, K.F.; Juturu, V.; Bordi, P.L.; Lampe, J.W.; Mousa, S.A.; Kris-Etherton, P.M. Effects of including soy protein in a blood cholesterol-lowering diet on markers of cardiac risk in men and in postmenopausal women with and without hormone replacement therapy. J. Womens Health 2005, 14, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Pop, E.A.; Fischer, L.M.; Coan, A.D.; Gitzinger, M.; Nakamura, J.; Zeisel, S.H. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: A phase I clinical trial. Menopause 2008, 15 Pt 1, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, C.D.; Messina, M.; Kiazand, A.; Morris, J.L.; Franke, A.A. Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: A randomized trial. J. Am. Coll. Nutr. 2007, 26, 669–677. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Cassidy, A. Dietary Isoflavones: Biological Effects and Relevance to Human Health. J. Nutr. 1999, 129, 758. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr. Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.-F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Ueno, T.; Uchiyama, S.; Nagao, Y.; Yamamoto, S.; Shibuya, C.; Kashiki, Y.; Shimizu, H. Dietary and lifestyle correlates of urinary excretion status of equol in Japanese women. Nutr. Cancer 2008, 60, 49–54. [Google Scholar] [CrossRef]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef]
- Maskarinec, G.; Yamakawa, R.; Hebshi, S.; A Franke, A. Urinary isoflavonoid excretion and soy consumption in three generations of Japanese women in Hawaii. Eur. J. Clin. Nutr. 2007, 61, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Igase, M.; Igase, K.; Tabara, Y.; Ohyagi, Y.; Kohara, K. Cross-sectional study of equol producer status and cognitive impairment in older adults. Geriatr. Gerontol. Int. 2017, 17, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.-P.; Wang, J.-H.; Liu, X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef]
- Newton, K.M.; Reed, S.D.; Uchiyama, S.; Qu, C.; Ueno, T.; Iwashita, S.; Gunderson, G.; Fuller, S.; Lampe, J.W. A cross-sectional study of equol producer status and self-reported vasomotor symptoms. Menopause 2015, 22, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Gardner, P.T.; McPhail, D.B.; Morrice, P.C.; Collins, A.R.; Duthie, G.G. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch. Biochem. Biophys. 1998, 360, 142–148. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; A Katzenellenbogen, J. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Zhao, X.; Shoaf, S.E.; Ragland, K. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [PubMed] [Green Version]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anti-Cancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Bicker, J.; Alves, G.; Fortuna, A.; Soares-Da-Silva, P.; Falcão, A. A new PAMPA model using an in-house brain lipid extract for screening the blood-brain barrier permeability of drug candidates. Int. J. Pharm. 2016, 501, 102–111. [Google Scholar] [CrossRef]
- Talaei, M.; Feng, L.; Yuan, J.-M.; Pan, A.; Koh, W.-P. Dairy, soy, and calcium consumption and risk of cognitive impairment: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1541–1552. [Google Scholar] [CrossRef]
- Greendale, G.A.; Huang, M.H.; Leung, K.; Crawford, S.L.; Gold, E.B.; Wight, R.; Waetjen, E.; Karlamangla, A.S. Dietary phytoestrogen intakes and cognitive function during the menopausal transition: Results from the Study of Women’s Health Across the Nation Phytoestrogen Study. Menopause 2012, 19, 894–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Birru, R.L.; E Snitz, B.; Ihara, M.; Kakuta, C.; Lopresti, B.J.; Aizenstein, H.J.; Lopez, O.L.; A Mathis, C.; Miyamoto, Y.; et al. Effects of soy isoflavones on cognitive function: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 78, 134–144. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Ferrucci, L.; Kapogiannis, D. Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res. Rev. 2021, 68, 101339. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement. 2021, 7, e12179. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Gottesman, R.F.; Bernstein, K.E.; Seshadri, S.; McKee, A.; Snyder, H.; Greenberg, S.M.; Yaffe, K.; Schaffer, C.B.; Yuan, C.; et al. Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020, 16, 1714–1733. [Google Scholar] [CrossRef]
- The SPRINT MIND Investigators for the SPRINT Research Group; Nasrallah, I.M.; Pajewski, N.M.; Auchus, A.P.; Chelune, G.; Cheung, A.K.; Cleveland, M.L.; Coker, L.H.; Crowe, M.G.; Cushman, W.C.; et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral White Matter Lesions. JAMA 2019, 322, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.D.; Pajewski, N.M.; Auchus, A.P.; Bryan, R.N.; Chelune, G.; Cheung, A.K.; Cleveland, M.L.; Coker, L.H.; Crowe, M.G.; Cushman, W.C.; et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019, 321, 553–561. [Google Scholar] [PubMed] [Green Version]
- Zeki Al Hazzouri, A.; Newman, A.B.; Simonsick, E.; Sink, K.M.; Sutton Tyrrell, K.; Watson, N.; Satterfield, S.; Harris, T.; Yaffe, K. Pulse wave velocity and cognitive decline in elders: The Health, Aging, and Body Composition study. Stroke 2013, 44, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, I.; Goldstein, F.C.; Martin, G.S.; Quyyumi, A.A. Roles of Arterial Stiffness and Blood Pressure in Hypertension-Associated Cognitive Decline in Healthy Adults. Hypertension 2016, 67, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Waldstein, S.R.; Rice, S.C.; Thayer, J.F.; Najjar, S.S.; Scuteri, A.; Zonderman, A.B. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008, 51, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araghi, M.; Shipley, M.J.; Wilkinson, I.B.; McEniery, C.M.; Valencia-Hernández, C.A.; Kivimaki, M.; Sabia, S.; Singh-Manoux, A.; Brunner, E.J. Association of aortic stiffness with cognitive decline: Whitehall II longitudinal cohort study. Eur. J. Epidemiol. 2019, 35, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Bueno, C.; Cunha, P.G.; Martinez-Vizcaino, V.; Pozuelo-Carrascosa, D.P.; Visier-Alfonso, M.E.; Jimenez-Lopez, E.; Cavero-Redondo, I. Arterial Stiffness and Cognition Among Adults: A Systematic Review and Meta-Analysis of Observational and Longitudinal Studies. J. Am. Heart Assoc. 2020, 9, e014621. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Sekikawa, A.; Kuller, L.H.; Lopez, O.L.; Newman, A.B.; Kuipers, A.L.; Mackey, R.H. Aortic Stiffness is Associated with Increased Risk of Incident Dementia in Older Adults. J. Alzheimers Dis. 2018, 66, 297–306. [Google Scholar] [CrossRef]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.; Mackey, R.H.; McDade, E.M.; Klunk, W.E.; Aizenstein, H.J.; Cohen, A.D.; Snitz, B.E.; Mathis, C.A.; et al. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology 2013, 81, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.; McDade, E.M.; Klunk, W.E.; Cohen, A.D.; Mathis, C.A.; Dekosky, S.T.; Price, J.C.; Lopez, O.L. Arterial Stiffness and beta-Amyloid Progression in Nondemented Elderly Adults. JAMA Neurol. 2014, 71, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, T.M.; Wagenknecht, L.E.; Craft, S.; Mintz, A.; Heiss, G.; Palta, P.; Wong, D.; Zhou, Y.; Knopman, D.; Mosley, T.H.; et al. Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology 2018, 90, e1248–e1256. [Google Scholar] [CrossRef]
- Moore, E.E.; Liu, D.; Li, J.; Schimmel, S.J.; Cambronero, F.E.; Terry, J.G.; Nair, S.; Pechman, K.R.; Moore, M.E.; Bell, S.P.; et al. Association of Aortic Stiffness With Biomarkers of Neuroinflammation, Synaptic Dysfunction, and Neurodegeneration. Neurology 2021, 97, e329. [Google Scholar] [CrossRef]
- Man, B.; Cui, C.; Zhang, X.; Sugiyama, D.; Barinas-Mitchell, E.; Sekikawa, A. The effect of soy isoflavones on arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2020, 60, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H. Effects of Equol Supplement on Bone and Cardiovascular Parameters in Middle-Aged Japanese Women: A Prospective Observational Study. J. Altern. Complement Med. 2018, 24, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H.; Ishigaki, Y. Effects of an equol-containing supplement on advanced glycation end products, visceral fat and climacteric symptoms in postmenopausal women: A randomized controlled trial. PLoS ONE 2021, 16, e0257332. [Google Scholar] [CrossRef]
- AlGhatrif, M.; Strait, J.B.; Morrell, C.H.; Canepa, M.; Wright, J.; Elango, P.; Scuteri, A.; Najjar, S.S.; Ferrucci, L.; Lakatta, E.G. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore Longitudinal Study of Aging. Hypertension 2013, 62, 934–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, R.K.; Gillespie, D.G.; Imthurn, B.; Rosselli, M.; Jackson, E.K.; Keller, P.J. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension 1999, 33 Pt 2, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Gopaul, R.; Knaggs, H.E.; Lephart, E.D. Biochemical investigation and gene analysis of equol: A plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications. Biofactors 2012, 38, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Veliky, C.V.; Birru, R.L.; Barinas-Mitchell, E.; Magnani, J.W.; Sekikawa, A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases-From Molecular Mechanisms to Studies in Humans. Nutrients 2021, 13, 3739. [Google Scholar] [CrossRef]
- Iulita, M.F.; Noriega de la Colina, A.; Girouard, H. Arterial stiffness, cognitive impairment and dementia: Confounding factor or real risk? J. Neurochem. 2018, 144, 527–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pase, M.P.; Beiser, A.; Himali, J.J.; Tsao, C.; Satizabal, C.L.; Vasan, R.S.; Seshadri, S.; Mitchell, G.F. Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke 2016, 47, 2256–2261. [Google Scholar] [CrossRef] [Green Version]
- Poels, M.M.; van Oijen, M.; Mattace-Raso, F.U.; Hofman, A.; Koudstaal, P.J.; Witteman, J.C.; Breteler, M.M. Arterial stiffness, cognitive decline, and risk of dementia: The Rotterdam study. Stroke 2007, 38, 888–892. [Google Scholar] [CrossRef] [Green Version]
- Joy, S.; Siow, R.C.; Rowlands, D.J.; Becker, M.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J. Biol. Chem. 2006, 281, 27335–27345. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, D.J.; Chapple, S.; Siow, R.C.; Mann, G.E. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: Roles for F-actin and GPR30. Hypertension 2011, 57, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, X.; Shi, L.; Wang, L.; Chen, J.; Kang, C.; Zhu, J.; Mi, M. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE 2013, 8, e79075. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Hu, Q.; Shi, L.; Qin, L.; Zhang, Q.; Mi, M. Equol Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice by Inhibiting Endoplasmic Reticulum Stress via Activation of Nrf2 in Endothelial Cells. PLoS ONE 2016, 11, e0167020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, O.L.; Becker, J.T.; Chang, Y.; Klunk, W.E.; Mathis, C.; Price, J.; Aizenstein, H.J.; Snitz, B.; Cohen, A.D.; DeKosky, S.T.; et al. Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology 2018, 90, e1920–e1928. [Google Scholar] [CrossRef]
- Alber, J.; Alladi, S.; Bae, H.; Barton, D.A.; Beckett, L.A.; Bell, J.M.; Berman, S.E.; Biessels, G.J.; Black, S.E.; Bos, I.; et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement. 2019, 5, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Higashiyama, A.; Lopresti, B.J.; Ihara, M.; Aizenstein, H.; Watanabe, M.; Chang, Y.; Kakuta, C.; Yu, Z.; Mathis, C.; et al. Associations of equol-producing status with white matter lesion and amyloid-β deposition in cognitively normal elderly Japanese. Alzheimers Dement. 2020, 6, e12089. [Google Scholar] [CrossRef]
- Okamura, T.; Kokubo, Y.; Watanabe, M.; Higashiyama, A.; Miyamoto, Y.; Yoshimasa, Y.; Okayama, A. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: The Suita study. Atherosclerosis 2009, 203, 587–592. [Google Scholar] [CrossRef]
- Zhai, F.-F.; Ye, Y.-C.; Chen, S.-Y.; Ding, F.-M.; Han, F.; Yang, X.-L.; Wang, Q.; Zhou, L.-X.; Ni, J.; Yao, M.; et al. Arterial Stiffness and Cerebral Small Vessel Disease. Front. Neurol. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Stringer, M.S.; Shi, Y.; Thrippleton, M.J.; Wardlaw, J.M. Associations Between White Matter Hyperintensity Burden, Cerebral Blood Flow and Transit Time in Small Vessel Disease: An Updated Meta-Analysis. Front. Neurol. 2021, 12, 647848. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Song, Z.; Zhao, L.-M.; Li, G.-R.; Deng, X.-L. Equol increases cerebral blood flow in rats via activation of large-conductance Ca(2+)-activated K(+) channels in vascular smooth muscle cells. Pharmacol. Res. 2016, 107, 186–194. [Google Scholar] [CrossRef]
- Jackman, K.A.; Woodman, O.L.; Chrissobolis, S.; Sobey, C.G. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007, 1141, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, X.; Weakley, S.M.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J. Nutr. 2010, 140, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S. Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Scuderi, S.A.; Ardizzone, A.; Paterniti, I.; Esposito, E.; Campolo, M. Antioxidant and Anti-inflammatory Effect of Nrf2 Inducer Dimethyl Fumarate in Neurodegenerative Diseases. Antioxidants 2020, 9, 630. [Google Scholar] [CrossRef]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, F.; Westfall, S.; Brathwaite, J.; Pasinetti, G.M. Suppression of Presymptomatic Oxidative Stress and Inflammation in Neurodegeneration by Grape-Derived Polyphenols. Front. Pharmacol. 2018, 9, 867. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [PubMed]
- Grimm, A.; Friedland, K.; Eckert, A. Mitochondrial dysfunction: The missing link between aging and sporadic Alzheimer’s disease. Biogerontology 2016, 17, 281–296. [Google Scholar] [CrossRef]
- Lim, J.-H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, K.; Ohta, Y.; Inufusa, H.; Loon, A.F.N.; Abe, K. Prevention of Cognitive Decline in Alzheimer’s Disease by Novel Antioxidative Supplements. Int. J. Mol. Sci. 2020, 21, 1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem. Biophys. 1998, 356, 133–141. [Google Scholar] [CrossRef]
- Liu, T.-H.; Tsai, T.-Y. Effects of equol on deoxycorticosterone acetate salt-induced hypertension and associated vascular dementia in rats. Food Funct. 2016, 7, 3444–3457. [Google Scholar] [CrossRef]
- Ma, Y.; Sullivan, J.C.; Schreihofer, D.A. Dietary genistein and equol (4’, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R871–R877. [Google Scholar] [CrossRef]
- Yu, W.; Deng, X.; Ma, Z.; Wang, Y. Equol protects PC12 neuronal cells against hypoxia/reoxygenation injury in vitro by reducing reactive oxygen species production. Nan Fang Yi Ke Da Xue Xue Bao 2016, 36, 1–7. [Google Scholar]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, M.; Hashimoto, A.; Satoh, H.; Kawabe, K.; Ogawa, M.; Takano, K.; Nakamura, Y. S-Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway. Int. J. Inflam. 2018, 2018, 8496973. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Gao, R.; Zhang, Y.; Jiang, N.; Chen, Y.; Sun, J.; Wang, Q.; Fan, B.; Liu, X.-M.; Wang, Z.F. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021, 12, 5770–5778. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Okumura, N.; Yoshida, H.; Nishimura, Y.; Murakami, M.; Kitagishi, Y. Genistein downregulates presenilin 1 and ubiquilin 1 expression. Mol. Med. Rep. 2012, 5, 559–561. [Google Scholar] [CrossRef]
- Petry, F.D.S.; Coelho, B.P.; Gaelzer, M.M.; Kreutz, F.; Guma, F.T.C.R.; Salbego, C.G.; Trindade, V.M.T. Genistein protects against amyloid-beta-induced toxicity in SH-SY5Y cells by regulation of Akt and Tau phosphorylation. Phytother. Res. 2020, 34, 796–807. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D. Węgrzyn G.; Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef]
- Petry, F.D.S.; Hoppe, J.B.; Klein, C.P.; dos Santos, B.G.; Hözer, R.M.; Bifi, F.; Matté, C.; Salbego, C.G.; Trindade, V.M.T. Genistein attenuates amyloid-beta-induced cognitive impairment in rats by modulation of hippocampal synaptotoxicity and hyperphosphorylation of Tau. J. Nutr. Biochem. 2021, 87, 108525. [Google Scholar] [CrossRef]
- Shentu, Y.-P.; Hu, W.-T.; Liang, J.-W.; Liuyang, Z.-Y.; Wei, H.; Qun, W.; Wang, X.-C.; Wang, J.-Z.; Westermarck, J.; Liu, R. Genistein Decreases APP/tau Phosphorylation and Ameliorates Aβ Overproduction Through Inhibiting CIP2A. Curr. Alzheimer Res. 2019, 16, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Z.; Ding, X.-W.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Beneficial Effect of Genistein on Diabetes-Induced Brain Damage in the ob/ob Mouse Model. Drug Des. Devel. Ther. 2020, 14, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Lin, S.-H.; Hidayah, K.; Lin, C.-I. Equol Pretreatment Protection of SH-SY5Y Cells against Aβ (25-35)-Induced Cytotoxicity and Cell-Cycle Reentry via Sustaining Estrogen Receptor Alpha Expression. Nutrients 2019, 11, 2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Chen, Q.; Brinton, R.D. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp. Biol. Med. 2002, 227, 509–519. [Google Scholar] [CrossRef]
- Hirohata, M.; Ono, K.; Takasaki, J.-I.; Takahashi, R.; Ikeda, T.; Morinaga, A.; Yamada, M. Anti-amyloidogenic effects of soybean isoflavones in vitro: Fluorescence spectroscopy demonstrating direct binding to Aβ monomers, oligomers and fibrils. Biochim. Et Biophys. Acta 2012, 1822, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, I.F.; Purup, S.; Ehrich, M. Modulation of neurotoxicant-induced increases in intracellular calcium by phytoestrogens differ for amyloid beta peptide (Abeta) and 1-methyl-4-phenyl-pyridine (MPP(+)). J. Appl. Toxicol. 2009, 29, 84–89. [Google Scholar] [CrossRef]
- Ariyani, W.; Miyazaki, W.; Koibuchi, N. A Novel Mechanism of S-equol Action in Neurons and Astrocytes: The Possible Involvement of GPR30/GPER1. Int. J. Mol. Sci. 2019, 20, 5178. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.L.; Park, H.Y.; Vattem, D.A.; Grammas, P.; Ma, H.; Seeram, N.P. Equol, a Blood-Brain Barrier Permeable Gut Microbial Metabolite of Dietary Isoflavone Daidzein, Exhibits Neuroprotective Effects against Neurotoxins Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells and Caenorhabditis elegans. Plant Foods Hum. Nutr. 2020, 75, 512–517. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, N.; Barros, R.P.; Warner, M.; Gustafsson, J.A. ERbeta: Recent understanding of estrogen signaling. Trends Endocrinol. Metab. 2010, 21, 545–552. [Google Scholar] [CrossRef]
- Kelly, J.F.; Bienias, J.L.; Shah, A.; Meeke, K.A.; Schneider, J.A.; Soriano, E.; Bennett, D.A. Levels of estrogen receptors alpha and beta in frontal cortex of patients with Alzheimer’s disease: Relationship to Mini-Mental State Examination scores. Curr. Alzheimer Res. 2008, 5, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 2015, 74, 125–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Day, M.; Muñiz, L.C.; Bitran, D.; Arias, R.; Revilla-Sanchez, R.; Grauer, S.; Zhang, G.; Kelley, C.; Pulito, V.; et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 2008, 11, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.J.; Lee, T.Y.; Kim, N.S.; Kwon, J.S. The Role of Estrogen Receptors and Their Signaling across Psychiatric Disorders. Int. J. Mol. Sci. 2020, 22, 373. [Google Scholar] [CrossRef]
- Kim, H.J.; Casadesus, G. Estrogen-mediated effects on cognition and synaptic plasticity: What do estrogen receptor knockout models tell us? Biochim. Biophys. Acta 2010, 1800, 1090–1093. [Google Scholar] [CrossRef] [Green Version]
- Fehsel, K.; Schikowski, T.; Jänner, M.; Hüls, A.; Voussoughi, M.; Schulte, T.; Vierkötter, A.; Teichert, T.; Herder, C.; Sugiri, D.; et al. Estrogen receptor beta polymorphisms and cognitive performance in women: Associations and modifications by genetic and environmental influences. J. Neural Transm. 2016, 123, 1369–1379. [Google Scholar] [CrossRef]
- Janicki, S.C.; Park, N.; Cheng, R.; Lee, J.H.; Schupf, N.; Clark, L.N. Estrogen receptor β variants modify risk for Alzheimer’s disease in a multiethnic female cohort. J. Alzheimers Dis. 2014, 40, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Vargas, K.G.; Milic, J.; Zaciragic, A.; Wen, K.-X.; Jaspers, L.; Nano, J.; Dhana, K.; Bramer, W.M.; Kraja, B.; van Beeck, E.; et al. The functions of estrogen receptor beta in the female brain: A systematic review. Maturitas 2016, 93, 41–57. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Aenlle, K.K.; Bean, L.A.; Rani, A.; Semple-Rowland, S.L.; Kumar, A.; Foster, T.C. Role of estrogen receptor α and β in preserving hippocampal function during aging. J. Neurosci. 2013, 33, 2671–2683. [Google Scholar] [CrossRef] [Green Version]
- Spencer-Segal, J.; Tsuda, M.; Mattei, L.; Waters, E.; Romeo, R.; Milner, T.; McEwen, B.; Ogawa, S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 2012, 202, 131–146. [Google Scholar] [CrossRef]
- Rissman, E.F.; Heck, A.L.; Leonard, J.E.; Shupnik, M.A.; Gustafsson, J.A. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc. Natl. Acad. Sci. USA 2002, 99, 3996–4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourtchuladze, R.; Frenguelli, B.; Blendy, J.; Cioffi, D.; Schutz, G.; Silva, A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 1994, 79, 59–68. [Google Scholar] [CrossRef]
- Khan, M.M.; Wakade, C.; de Sevilla, L.; Brann, D.W. Selective estrogen receptor modulators (SERMs) enhance neurogenesis and spine density following focal cerebral ischemia. J. Steroid Biochem. Mol. Biol. 2015, 146, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, S.; Koehler, K.F.; Gustafsson, J. Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discov. 2011, 10, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fonseca, K.; Massieu, L.; de la Cadena, S.G.; Guzmán, C.; Camacho-Arroyo, I. Neuroprotective role of estradiol against neuronal death induced by glucose deprivation in cultured rat hippocampal neurons. Neuroendocrinology 2012, 96, 41–50. [Google Scholar] [CrossRef]
- Crawford, D.; Mangiardi, M.; Song, B.; Patel, R.; Du, S.; Sofroniew, M.V.; Voskuhl, R.R.; Tiwari-Woodruff, S.K. Oestrogen receptor β ligand: A novel treatment to enhance endogenous functional remyelination. Brain 2010, 133, 2999–3016. [Google Scholar] [CrossRef]
- Suwanna, N.; Thangnipon, W.; Soi-Ampornkul, R. Neuroprotective effects of diarylpropionitrile against β-amyloid peptide-induced neurotoxicity in rat cultured cortical neurons. Neurosci. Lett. 2014, 578, 44–49. [Google Scholar] [CrossRef]
- Suwanna, N.; Thangnipon, W.; Kumar, S.; De Vellis, J. Neuroprotection by diarylpropionitrile in mice with spinal cord injury. Excli J. 2014, 13, 1097–1103. [Google Scholar]
- Sárvári, M.; Kalló, I.; Hrabovszky, E.; Solymosi, N.; Liposits, Z. Ovariectomy and subsequent treatment with estrogen receptor agonists tune the innate immune system of the hippocampus in middle-aged female rats. PLoS ONE 2014, 9, e88540. [Google Scholar]
- Sárvári, M.; Kalló, I.; Hrabovszky, E.; Solymosi, N.; Rodolosse, A.; Liposits, Z. Long-Term Estrogen Receptor Beta Agonist Treatment Modifies the Hippocampal Transcriptome in Middle-Aged Ovariectomized Rats. Front. Cell Neurosci. 2016, 10, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, L.S.; Hernandez, G.; Zhao, L.; Franke, A.A.; Chen, Y.L.; Pawluczyk, S.; Mack, W.J.; Brinton, R.D. Safety and feasibility of estrogen receptor-β targeted phytoSERM formulation for menopausal symptoms: Phase 1b/2a randomized clinical trial. Menopause 2019, 26, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hernandez, G.; Mack, W.J.; Schneider, L.S.; Yin, F.; Brinton, R.D. Retrospective analysis of phytoSERM for management of menopause-associated vasomotor symptoms and cognitive decline: A pilot study on pharmacogenomic effects of mitochondrial haplogroup and APOE genotype on therapeutic efficacy. Menopause 2020, 27, 57–65. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, L.; Mao, Z.; Chen, S.; Wong, K.C.; To, J.; Brinton, R.D. Potentiation of brain mitochondrial function by S-equol and R/S-equol estrogen receptor beta-selective phytoSERM treatments. Brain Res. 2013, 1514, 128–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Mao, Z.; Brinton, R.D. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology 2009, 150, 770–783. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Mao, Z.; Chen, S.; Schneider, L.S.; Brinton, R.D. Early intervention with an estrogen receptor β-selective phytoestrogenic formulation prolongs survival, improves spatial recognition memory, and slows progression of amyloid pathology in a female mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2013, 37, 403–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Liu, B.; Cui, L.; Zhou, B.; Liu, W.; Xu, F.; Hayashi, T.; Hattori, S.; Ushiki-Kaku, Y.; Tashiro, S.-I.; et al. Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol. Behav. 2017, 179, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, B.; Cui, L.; Lei, D.; Zhang, P.; Yao, G.; Xia, M.; Hayashi, T.; Hattori, S.; Ushiki-Kaku, Y.; et al. Silibinin ameliorates Aβ(25-35)-induced memory deficits in rats by modulating autophagy and attenuating neuroinflammation as well as oxidative stress. Neurochem Res. 2017, 42, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, V.; Sharma, D.; Kalia, K.; Tiwari, V. Neuroprotective effects of silibinin: An in silico and in vitro study. Int. J. Neurosci. 2018, 128, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.M.; Perera, K.L.I.S.; Kim, J.; Pandey, R.K.; Sweeney, N.; Lu, X.; Imhoff, A.; Mackinnon, A.; Wargolet, A.J.; Van Hart, R.M.; et al. A-C Estrogens as Potent and Selective Estrogen Receptor-Beta Agonists (SERBAs) to Enhance Memory Consolidation under Low-Estrogen Conditions. J. Med. Chem. 2018, 61, 4720–4738. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.W.; Schalk, J.C.; Wetzel, E.A.; Hanson, A.M.; Sem, D.S.; Donaldson, W.A.; Frick, K.M. Long-term oral administration of a novel estrogen receptor beta agonist enhances memory and alleviates drug-induced vasodilation in young ovariectomized mice. Horm. Behav. 2021, 130, 104948. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Chow, J.; Fritzemeier, K.; Simpson, E.R.; Boon, W.C. Fas/FasL-mediated apoptosis in the arcuate nucleus and medial preoptic area of male ArKO mice is ameliorated by selective estrogen receptor alpha and estrogen receptor beta agonist treatment, respectively. Mol. Cell Neurosci. 2007, 36, 146–157. [Google Scholar] [CrossRef]
- Clark, J.; Alves, S.; Gundlah, C.; Rocha, B.; Birzin, E.; Cai, S.-J.; Flick, R.; Hayes, E.; Ho, K.; Warrier, S.; et al. Selective estrogen receptor-beta (SERM-beta) compounds modulate raphe nuclei tryptophan hydroxylase-1 (TPH-1) mRNA expression and cause antidepressant-like effects in the forced swim test. Neuropharmacology 2012, 63, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [Green Version]
- Hillisch, A.; Peters, O.; Kosemund, D.; Müller, G.; Walter, A.; Schneider, B.; Reddersen, G.; Elger, W.; Fritzemeier, K.H. Dissecting physiological roles of estrogen receptor alpha and beta with potent selective ligands from structure-based design. Mol. Endocrinol. 2004, 18, 1599–1609. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.A.; Lai, J.F.; Pagano, I.; Morimoto, Y.; Maskarinec, G. Equol production changes over time in pre-menopausal women. Br. J. Nutr. 2012, 107, 1201–1206. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Halm, B.M.; Pagano, I.; Kono, N.; Mack, W.J.; Hodis, H.N. Equol production changes over time in postmenopausal women. J. Nutr. Biochem. 2012, 23, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiwata, N.; Melby, M.; Mizuno, S.; Watanabe, S. New equol supplement for relieving menopausal symptoms: Randomized, placebo-controlled trial of Japanese women. Menopause 2009, 16, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause 2011, 18, 563–574. [Google Scholar] [CrossRef]
- Aso, T.; Uchiyama, S.; Matsumura, Y.; Taguchi, M.; Nozaki, M.; Takamatsu, K.; Ishizuka, B.; Kubota, T.; Mizunuma, H.; Ohta, H. A natural S-equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women. J. Womens Health 2012, 21, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Jenks, B.H.; Iwashita, S.; Nakagawa, Y.; Ragland, K.; Lee, J.; Carson, W.H.; Ueno, T.; Uchiyama, S. A pilot study on the effects of S-equol compared to soy isoflavones on menopausal hot flash frequency. J. Womens Health 2012, 21, 674–682. [Google Scholar] [CrossRef]
- Oyama, A.; Ueno, T.; Uchiyama, S.; Aihara, T.; Miyake, A.; Kondo, S.; Matsunaga, K. The effects of natural S-equol supplementation on skin aging in postmenopausal women: A pilot randomized placebo-controlled trial. Menopause 2012, 19, 202–210. [Google Scholar] [CrossRef] [PubMed]

| Arterial stiffness is an emerging target for preventing cognitive decline and dementia |
| • Emerging as a potential therapeutic target for preventing vascular contribution of cognitive impairment and dementia (VCID) and Alzheimer’s disease and related dementias (ADRD) [58,71]. • Significantly associated with cognitive decline [53,54,55,56,57,58]. • Signifincatly associated with multiple forms of dementia-related pathology, including white matter lesion volume and Amyloid-β deposition in the brain [53,54,55,56,57,58]. • Significantly associated with incident dementia in some [59] but not all [72,73] prospective cohort studies.  |
| Pathophysiology of arterial stiffness |
Major underlying mechanisms of arterial stiffness include pathological collagen production and elastin degradation in the arterial wall, endothelial dysfunction, oxidative stress, and inflammation [58].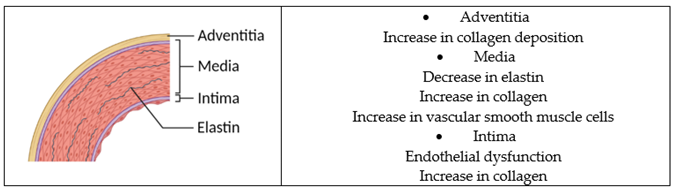 |
| Potential mechanisms of S-equol that slow the progression of arterial stiffness |
| • S-equol inhibits abnormal collagen synthesis, proliferation, and migration of smooth muscle cells in the human aorta [68]. • S-equol may inhibit the degradation of elastin in the arterial wall by decreasing the expression of the elastase enzyme [69]. • S-equol improves endothelial function in the human aorta and umbilical vein endothelial cells [74,75,76,77]. • S-equol possesses anti-inflammatory and antioxidant properties [70]. |
| Name | Findings |
|---|---|
| Diarylproprionitrile (DPN) | Treatment with DPN-protected rat cultured hippocampal neurons from glucose deprivation-induced cell death [136]. Treatment with DPN of chronic experimental autoimmune encephalomyelitis mice (transgenic proteolipid protein-enhanced green fluorescent protein transgenic mice in the C57BL/6J background) prevented histopathological (fewer demyelinated, damaged axons and more myelinated axons) and increased mature oligodendrocyte numbers [137]. Pretreatment with DPN decreased neuronal cell death by reducing reactive oxygen species in Aβ1-42 induced oxidative stress and inflammation in primary rat cortical cell culture [138]. In addition, DPN pretreatment decreased pro-inflammatory cytokines (IL-1β and IL-6) In C57/B1/6 female mice performed with spinal cord injury, the administration of DPN reduced neural apoptosis and inflammation compared to untreated groups [139].In middle-aged female Harlan-Wistar rats with ovariectomy followed by no replacement or replacement with estradiol, DPN, or LE2 (ERα agonist), gene expression related to the innate immune system, specifically macrophage-associated and complement genes, in the hippocampus was investigated. Ovariectomy caused the upregulation of these gene expressions, and estradiol, DPN, and LE2 attenuated the increase in these gene expressions [140]. In middle-aged female Harlan-Wistar rats implanted subcutaneously in the neck with DPN or vehicle only for 29 days, the effect of DPN on hippocampal transcriptome was investigated. DPN contributed to regulating transcription, translation, neurogenesis, neuromodulation, and neuroprotection in the hippocampal formation [141]. |
| PhytoSERM | A first human study of PhytoSERM-Phase 1b/2a randomized, double-blind placebo-controlled study of 50 or 100 mg/day PhytoSERM for 12 weeks, recruiting 71 peri-menopausal women aged 45–60, showed that both 50 and 100 mg/day were well tolerated but based on safety outcomes, 50 mg/day was considered preferable [142]. A retrospective responder analysis of the trial mentioned above showed that 50 mg/day of PhytoSERM significantly reduced hot flashes and preserved cognitive function in verbal learning and executive function [143]. In mitochondrial markers in rat hippocampal neuronal cultures and a female mouse ovariectomy model, both S-equol phytoSERM and R/S-equol phytoSERM treatments potentiated mitochondrial bioenergetics [144]. In rat primary hippocampal neuronal cultures challenged with neurotoxic glutamate or amyloid-β1–42, phytoSERM showed a greater effect on neuronal survival than genistein, daidzein, equol, or IBSO03569. In ovariectomized adult female rats, treatment with phytoSERV significantly enhanced brain mitochondrial bioenergetics. Furthermore, in western blot analyses of hippocampal protein, phytoSERM increased expression of brain mitochondrial anti-apoptotic protein Bcl-2 and Bcl-xL as well as insulin-degrading enzyme and neprilysin, which are amyloid-β degrading enzymes [145]. A 9-month supplementation of phytoSERM in a female triple transgenic mouse model of Alzheimer’s disease promoted physical health, prolonged survival, improved spatial recognition memory, and attenuated amyloid-β deposition and plaque formation in the brain [146]. |
| Silibinin | Oral gavage of silibinin protected amyloid-β1–42 induced anxiety/depression-like behaviors in male Sprague-Dawley rats. Silibinin significantly suppressed neuronal damage induced by amyloid-β1–42 in hippocampus regions, increased brain-derived neurotrophic factor, and attenuated the increase of autophagy levels in the hippocampus [147]. Silibinin-protected amyloid-β25–35 induced memory deficits in male Sprague-Dawley rats. Silibinin increased the expression of anti-inflammatory cytokine (interleukin 4) and decreased the expression of pro-inflammatory cytokines (interleukin 1β) in the hippocampus. Silibinin also increased glutathione levels and decreased malondialdehyde levels, indicating antioxidant properties of silibinin [148]. Treatment with silibinin of astrocyte induced with lipopolysaccharide decreased the reactive form of astrocyte, attenuated nitrite release, increased glutathione levels, reduced malonaldehyde, and reduced glial fibrillary activated protein [149]. |
| EGX358 or ISP358-2 | Intrahippocampal infusion or intraperitoneal injection of ISP358-2 enhanced memory consolidation in C57BL/6 ovariectomized female mice [150]. EGX358 reduced drug-induced vasodilation and enhanced spatial and object recognition memory without adversely affecting anxiety- or depression-like behaviors in C57BL/6 ovariectomized female mice gavaged with EGX358 for 9 weeks [151]. |
| 8β-VE2 | Injecting 8β-VE2 for 6 weeks in aromatase knockout male mice prevented dopaminergic cell death in the medial preoptic area but not in the rostral and medial arcuate nucleus [152]. |
| SERM-beta1 and SERM-beta2 | Treatment with SERM-beta 1 or SERM-beta 2 for 4 days in ovariectomized Sprague-Dawley rats stimulated an increase in the total number of cells in the dentate gyrus of the hippocampus [153]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekikawa, A.; Wharton, W.; Butts, B.; Veliky, C.V.; Garfein, J.; Li, J.; Goon, S.; Fort, A.; Li, M.; Hughes, T.M. Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. Int. J. Mol. Sci. 2022, 23, 11921. https://doi.org/10.3390/ijms231911921
Sekikawa A, Wharton W, Butts B, Veliky CV, Garfein J, Li J, Goon S, Fort A, Li M, Hughes TM. Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. International Journal of Molecular Sciences. 2022; 23(19):11921. https://doi.org/10.3390/ijms231911921
Chicago/Turabian StyleSekikawa, Akira, Whitney Wharton, Brittany Butts, Cole V. Veliky, Joshua Garfein, Jiatong Li, Shatabdi Goon, Annamaria Fort, Mengyi Li, and Timothy M. Hughes. 2022. "Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia" International Journal of Molecular Sciences 23, no. 19: 11921. https://doi.org/10.3390/ijms231911921
APA StyleSekikawa, A., Wharton, W., Butts, B., Veliky, C. V., Garfein, J., Li, J., Goon, S., Fort, A., Li, M., & Hughes, T. M. (2022). Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. International Journal of Molecular Sciences, 23(19), 11921. https://doi.org/10.3390/ijms231911921








