Promyelocytic Leukemia Protein Potently Restricts Human Cytomegalovirus Infection in Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. PML-NB Protein Expression in Endothelial Cells and Fibroblasts
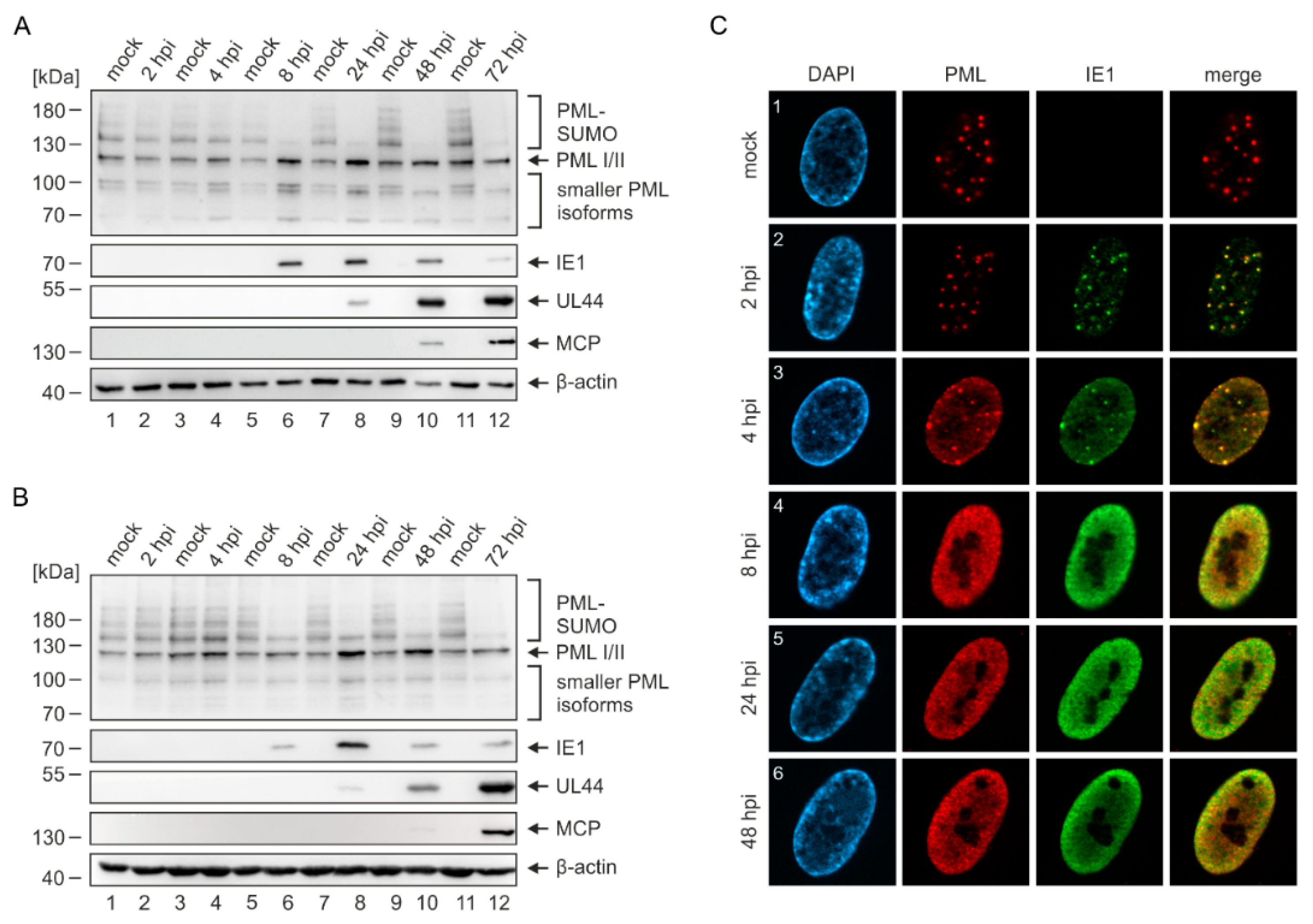
2.2. Disruption of PML-NBs in Endothelial Cells during HCMV Infection
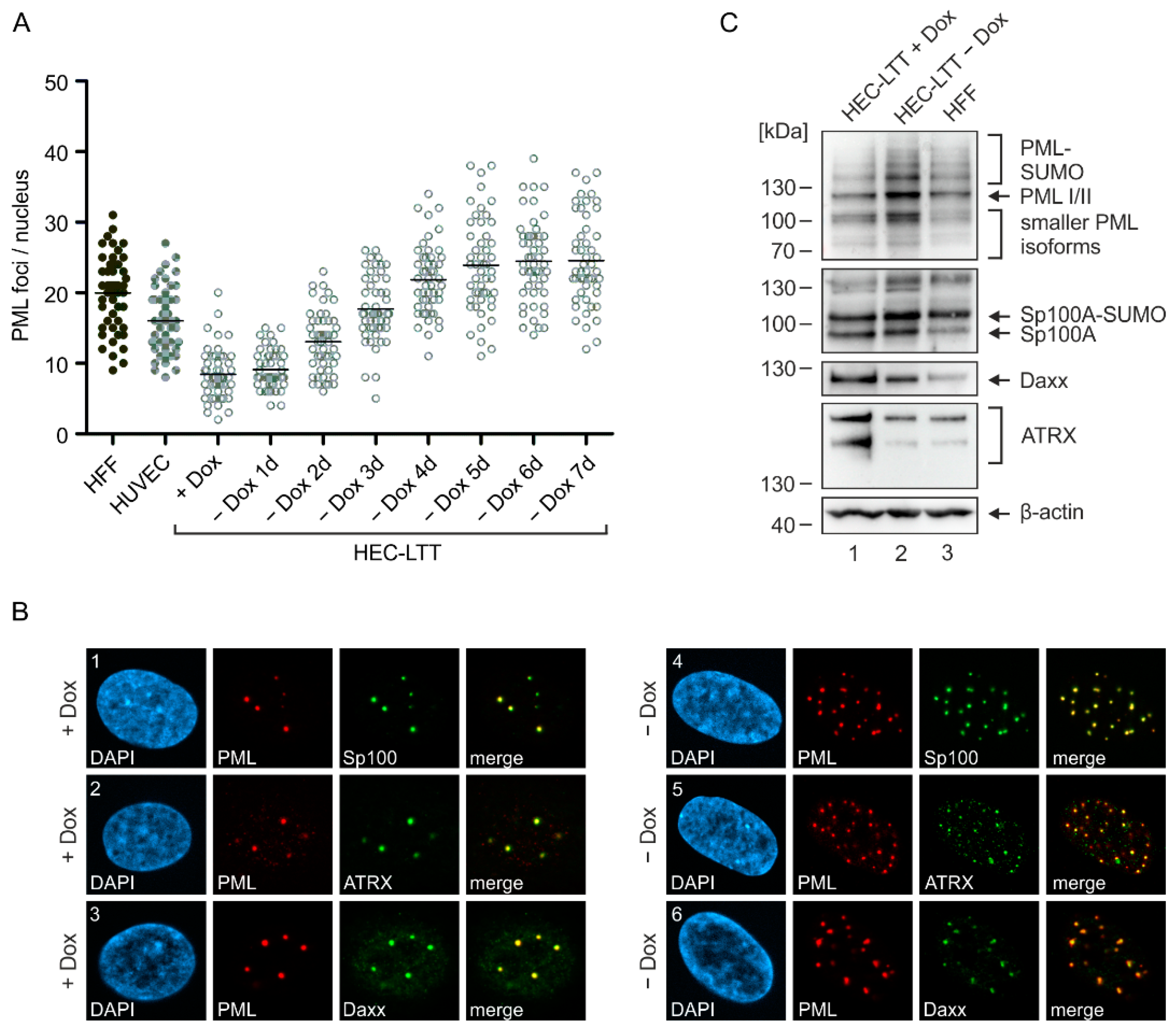
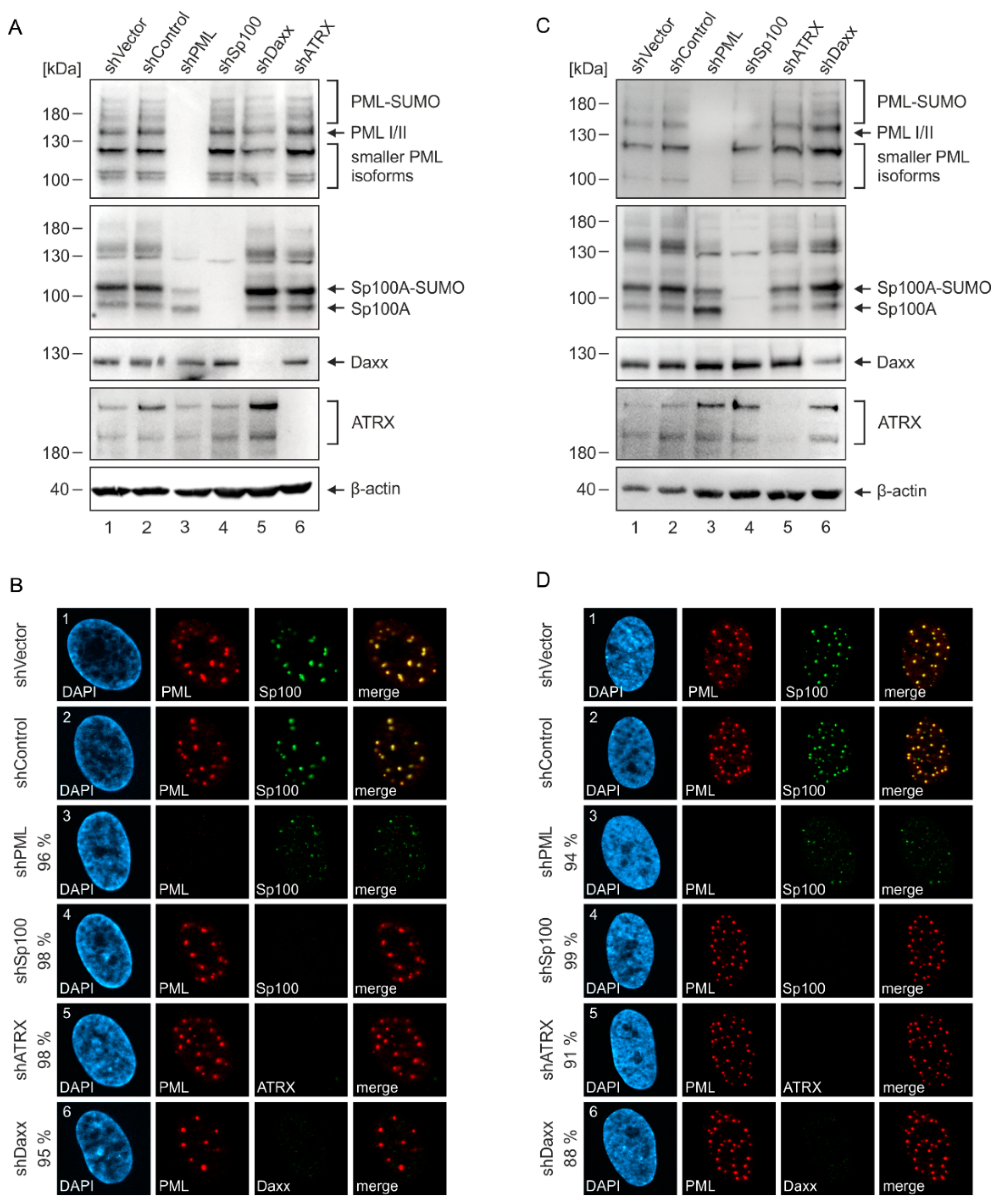
2.3. Generation of Endothelial Cells with a Stable Knockdown of PML-NB Proteins
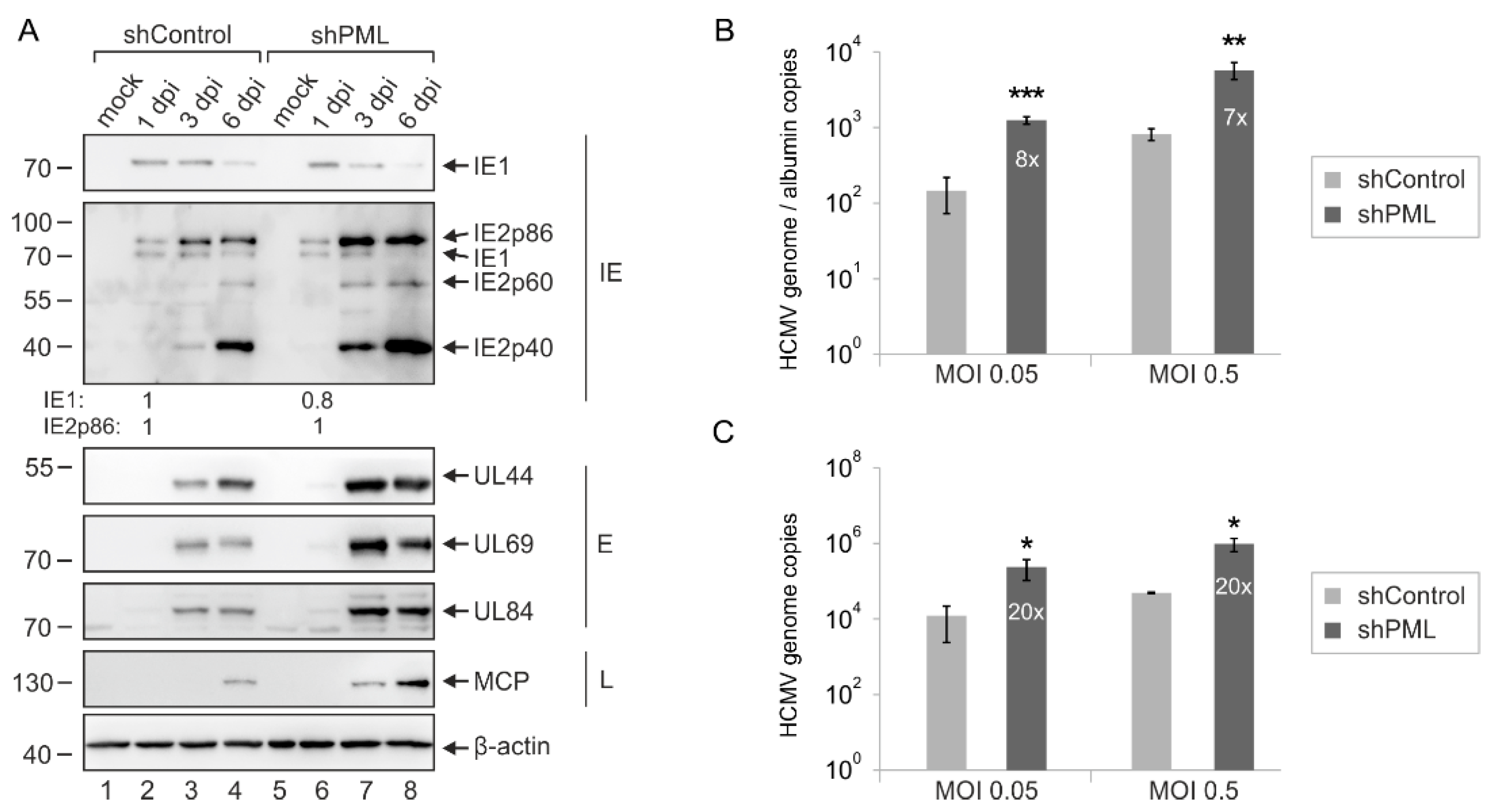
2.4. Enhanced Initiation of HCMV Gene Expression in PML- but Not Daxx-Depleted HEC-LTT
2.5. Multiplicity-Independent Inhibition of HCMV Infection by PML
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Lentiviral Gene Transfer
4.3. Western Blotting
4.4. Indirect Immunofluorescence
4.5. Nuclear Localization Assay
4.6. Antibodies
4.7. TaqMan Real-Time PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Kagan, K.O.; Hamprecht, K. Cytomegalovirus infection in pregnancy. Arch. Gynecol. Obstet. 2017, 296, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Sinzger, C.; Digel, M.; Jahn, G. Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 2008, 325, 63–83. [Google Scholar] [PubMed]
- Gerna, G.; Baldanti, F.; Revello, M.G. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum. Immunol. 2004, 65, 381–386. [Google Scholar] [CrossRef]
- Adler, B.; Sinzger, C. Endothelial cells in human cytomegalovirus infection: One host cell out of many or a crucial target for virus spread? Thromb. Haemost. 2009, 102, 1057–1063. [Google Scholar]
- Forte, E.; Zhang, Z.; Thorp, E.B.; Hummel, M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay with the Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 130. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, S. The role of human cytomegalovirus in atherosclerosis: A systematic review. Acta Biochim. Biophys. Sin. 2020, 52, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.D.; Rota, T.R.; Andrews, C.A.; Hirsch, M.S. Replication of human cytomegalovirus in endothelial cells. J. Infect. Dis. 1984, 150, 956–957. [Google Scholar] [CrossRef]
- Lieber, D.; Hochdorfer, D.; Stoehr, D.; Schubert, A.; Lotfi, R.; May, T.; Wirth, D.; Sinzger, C. A permanently growing human endothelial cell line supports productive infection with human cytomegalovirus under conditional cell growth arrest. BioTechniques 2015, 59, 127–136. [Google Scholar] [CrossRef]
- May, T.; Butueva, M.; Bantner, S.; Markusic, D.; Seppen, J.; MacLeod, R.A.; Weich, H.; Hauser, H.; Wirth, D. Synthetic gene regulation circuits for control of cell expansion. Tissue Eng. Part A 2010, 16, 441–452. [Google Scholar] [CrossRef]
- Bieniasz, P.D. Intrinsic immunity: A front-line defense against viral attack. Nat. Immunol. 2004, 5, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Tavalai, N.; Papior, P.; Rechter, S.; Stamminger, T. Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J. Virol. 2008, 82, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Adler, M.; Tavalai, N.; Muller, R.; Stamminger, T. Human cytomegalovirus immediate-early gene expression is restricted by the nuclear domain 10 component Sp100. J. Gen. Virol. 2011, 92, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Lukashchuk, V.; McFarlane, S.; Everett, R.D.; Preston, C.M. Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J. Virol. 2008, 82, 12543–12554. [Google Scholar] [CrossRef] [Green Version]
- Sloan, E.; Orr, A.; Everett, R.D. MORC3, a Component of PML Nuclear Bodies, Has a Role in Restricting Herpes Simplex virus 1 and Human Cytomegalovirus. J. Virol. 2016, 90, 8621–8633. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, R.; Pandolfi, P.P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 2007, 8, 1006–1016. [Google Scholar] [CrossRef]
- Lallemand-Breitenbach, V.; de Thé, H. PML nuclear bodies: From architecture to function. Curr. Opin. Cell Biol. 2018, 52, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tavalai, N.; Stamminger, T. Interplay between Herpesvirus Infection and Host Defense by PML Nuclear Bodies. Viruses 2009, 1, 1240–1264. [Google Scholar] [CrossRef]
- Scherer, M.; Read, C.; Neusser, G.; Kranz, C.; Kuderna, A.K.; Müller, R.; Full, F.; Wörz, S.; Reichel, A.; Schilling, E.M.; et al. Dual signaling via interferon and DNA damage response elicits entrapment by giant PML nuclear bodies. eLife 2022, 11, e73006. [Google Scholar] [CrossRef]
- Saffert, R.T.; Kalejta, R.F. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 2006, 80, 3863–3871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.R.; Kim, D.J.; Lee, J.M.; Choi, C.Y.; Ahn, B.Y.; Hayward, G.S.; Ahn, J.H. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 2004, 78, 6527–6542. [Google Scholar] [CrossRef]
- Schilling, E.M.; Scherer, M.; Reuter, N.; Schweininger, J.; Muller, Y.A.; Stamminger, T. The Human Cytomegalovirus IE1 Protein Antagonizes PML Nuclear Body-Mediated Intrinsic Immunity via the Inhibition of PML De Novo SUMOylation. J. Virol. 2017, 91, e02049-16. [Google Scholar] [CrossRef] [Green Version]
- Tavalai, N.; Adler, M.; Scherer, M.; Riedl, Y.; Stamminger, T. Evidence for a dual antiviral role of the major nuclear domain 10 component Sp100 during the immediate-early and late phases of the human cytomegalovirus replication cycle. J. Virol. 2011, 85, 9447–9458. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Gibbons, R.; Yan, Z.; Yang, D.; McDowell, T.L.; Sechi, S.; Qin, J.; Zhou, S.; Higgs, D.; Wang, W. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 2003, 100, 10635–10640. [Google Scholar] [CrossRef] [Green Version]
- Korioth, F.; Maul, G.G.; Plachter, B.; Stamminger, T.; Frey, J. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 1996, 229, 155–158. [Google Scholar] [CrossRef]
- Everett, R.D.; Rechter, S.; Papior, P.; Tavalai, N.; Stamminger, T.; Orr, A. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 2006, 80, 7995–8005. [Google Scholar] [CrossRef] [Green Version]
- Sinzger, C.; Hahn, G.; Digel, M.; Katona, R.; Sampaio, K.L.; Messerle, M.; Hengel, H.; Koszinowski, U.; Brune, W.; Adler, B. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J. Gen. Virol. 2008, 89, 359–368. [Google Scholar] [CrossRef]
- Tavalai, N.; Papior, P.; Rechter, S.; Leis, M.; Stamminger, T. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J. Virol. 2006, 80, 8006–8018. [Google Scholar] [CrossRef] [Green Version]
- Geoffroy, M.C.; Chelbi-Alix, M.K. Role of promyelocytic leukemia protein in host antiviral defense. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2011, 31, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Berscheminski, J.; Groitl, P.; Dobner, T.; Wimmer, P.; Schreiner, S. The adenoviral oncogene E1A-13S interacts with a specific isoform of the tumor suppressor PML to enhance viral transcription. J. Virol. 2013, 87, 965–977. [Google Scholar] [CrossRef] [Green Version]
- Everett, R.D.; Lomonte, P.; Sternsdorf, T.; van Driel, R.; Orr, A. Cell cycle regulation of PML modification and ND10 composition. J. Cell Sci. 1999, 112, 4581–4588. [Google Scholar] [CrossRef]
- Woodhall, D.L.; Groves, I.J.; Reeves, M.B.; Wilkinson, G.; Sinclair, J.H. Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J. Biol. Chem. 2006, 281, 37652–37660. [Google Scholar] [CrossRef] [Green Version]
- Ishov, A.M.; Maul, G.G. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J. Cell Biol. 1996, 134, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Ishov, A.M.; Stenberg, R.M.; Maul, G.G. Human cytomegalovirus immediate early interaction with host nuclear structures: Definition of an immediate transcript environment. J. Cell Biol. 1997, 138, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Everett, R.D.; Murray, J. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 2005, 79, 5078–5089. [Google Scholar] [CrossRef] [Green Version]
- Glass, M.; Everett, R.D. Components of promyelocytic leukemia nuclear bodies (ND10) act cooperatively to repress herpesvirus infection. J. Virol. 2013, 87, 2174–2185. [Google Scholar] [CrossRef] [Green Version]
- Maillet, S.; Nisole, S. Daxx, a broad-spectrum viral restriction factor. Virologie 2016, 20, 261–272. [Google Scholar]
- Mac Kain, A.; Maarifi, G.; Aicher, S.-M.; Arhel, N.; Baidaliuk, A.; Munier, S.; Donati, F.; Vallet, T.; Tran, Q.D.; Hardy, A.; et al. Identification of DAXX as a restriction factor of SARS-CoV-2 through a CRISPR/Cas9 screen. Nat. Commun. 2022, 13, 2442. [Google Scholar] [CrossRef]
- Kivipõld, P.; Võsa, L.; Ustav, M.; Kurg, R. DAXX modulates human papillomavirus early gene expression and genome replication in U2OS cells. Virol. J. 2015, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Agrawal, T.; Zhu, G.; Yu, S.; Tao, L.; Lin, J.; Marmorstein, R.; Shorter, J.; Yang, X. DAXX represents a new type of protein-folding enabler. Nature 2021, 597, 132–137. [Google Scholar] [CrossRef]
- Wagenknecht, N.; Reuter, N.; Scherer, M.; Reichel, A.; Muller, R.; Stamminger, T. Contribution of the Major ND10 Proteins PML, hDaxx and Sp100 to the Regulation of Human Cytomegalovirus Latency and Lytic Replication in the Monocytic Cell Line THP-1. Viruses 2015, 7, 2884–2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stilp, A.C.; Scherer, M.; König, P.; Fürstberger, A.; Kestler, H.A.; Stamminger, T. The chromatin remodeling protein ATRX positively regulates IRF3-dependent type I interferon production and interferon-induced gene expression. PLoS Pathog. 2022, 18, e1010748. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, M.; Faircloth, M.; Vugler, L.; Britt, W.J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 1989, 23, 157–167. [Google Scholar] [CrossRef]
- Hofmann, H.; Floss, S.; Stamminger, T. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J. Virol. 2000, 74, 2510–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebert, S.; Schmolke, S.; Sorg, G.; Floss, S.; Plachter, B.; Stamminger, T. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J. Virol. 1997, 71, 7048–7060. [Google Scholar] [CrossRef] [Green Version]
- Plachter, B.; Nordin, M.; Wirgart, B.Z.; Mach, M.; Stein, H.; Grillner, L.; Jahn, G. The DNA-binding protein P52 of human cytomegalovirus reacts with monoclonal antibody CCH2 and associates with the nuclear membrane at late times after infection. Virus Res. 1992, 24, 265–276. [Google Scholar] [CrossRef]
- Winkler, M.; Rice, S.A.; Stamminger, T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 1994, 68, 3943–3954. [Google Scholar] [CrossRef] [Green Version]
- Waldo, F.B.; Britt, W.J.; Tomana, M.; Julian, B.A.; Mestecky, J. Non-specific mesangial staining with antibodies against cytomegalovirus in immunoglobulin-A nephropathy. Lancet 1989, 1, 129–131. [Google Scholar] [CrossRef]

| Target Gene | siRNA Sequence |
|---|---|
| PML | AGATGCAGCTGTATCCAAG |
| Sp100 | GGAAGCACTGTTCAGCGATGT |
| ATRX | GAGGAAACCTTCAATTGTA |
| Daxx | GGAGTTGGATCTCTCAGAA |
| Control | GTGCGTTGCTAGTACCAAC |
| Name | Sequence |
|---|---|
| 5′CMV_gB | CTGCGTGATATGAACGTGAAGG |
| 3′CMV_gB | ACTGCACGTACGAGCTGTTGG |
| CMV_gB_FAM/TAMRA | CGCCAGGACGCTGCTACTCACGA |
| 5′Alb | GTGAACAGGCGACCATGCT |
| 3′Alb | GCATGGAAGGTGAATGTTTCAG |
| Alb_FAM/TAMRA | TCAGCTCTGGAAGTCGATGAAACATACGTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitz, S.; Heusel, A.T.; Stamminger, T.; Scherer, M. Promyelocytic Leukemia Protein Potently Restricts Human Cytomegalovirus Infection in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 11931. https://doi.org/10.3390/ijms231911931
Seitz S, Heusel AT, Stamminger T, Scherer M. Promyelocytic Leukemia Protein Potently Restricts Human Cytomegalovirus Infection in Endothelial Cells. International Journal of Molecular Sciences. 2022; 23(19):11931. https://doi.org/10.3390/ijms231911931
Chicago/Turabian StyleSeitz, Sven, Anna Theresa Heusel, Thomas Stamminger, and Myriam Scherer. 2022. "Promyelocytic Leukemia Protein Potently Restricts Human Cytomegalovirus Infection in Endothelial Cells" International Journal of Molecular Sciences 23, no. 19: 11931. https://doi.org/10.3390/ijms231911931





