The Role of the Paraventricular-Coerulear Network on the Programming of Hypertension by Prenatal Undernutrition
Abstract
1. Introduction
2. Hypothalamic CRF Expression and Subsequent Development of Hypertension in Subjects Who Suffered from Prenatal Undernutrition: The Role of Central Noradrenaline
2.1. Dysregulation of Hypothalamic CRF Levels in Prenatally Undernourished Subjects: The Role of the Glucocorticoid Feedback and Placental Barrier
2.2. Increased Expression of CRF in the PVN and Subsequent Hypertension Shown by Prenatally Malnourished Animals Are Likely due to Central Noradrenergic Hyperactivity
3. Maladaptive Programming of Paraventricular-Coerulear Network and Development of Hypertension in Prenatally Malnourished Adult Animals
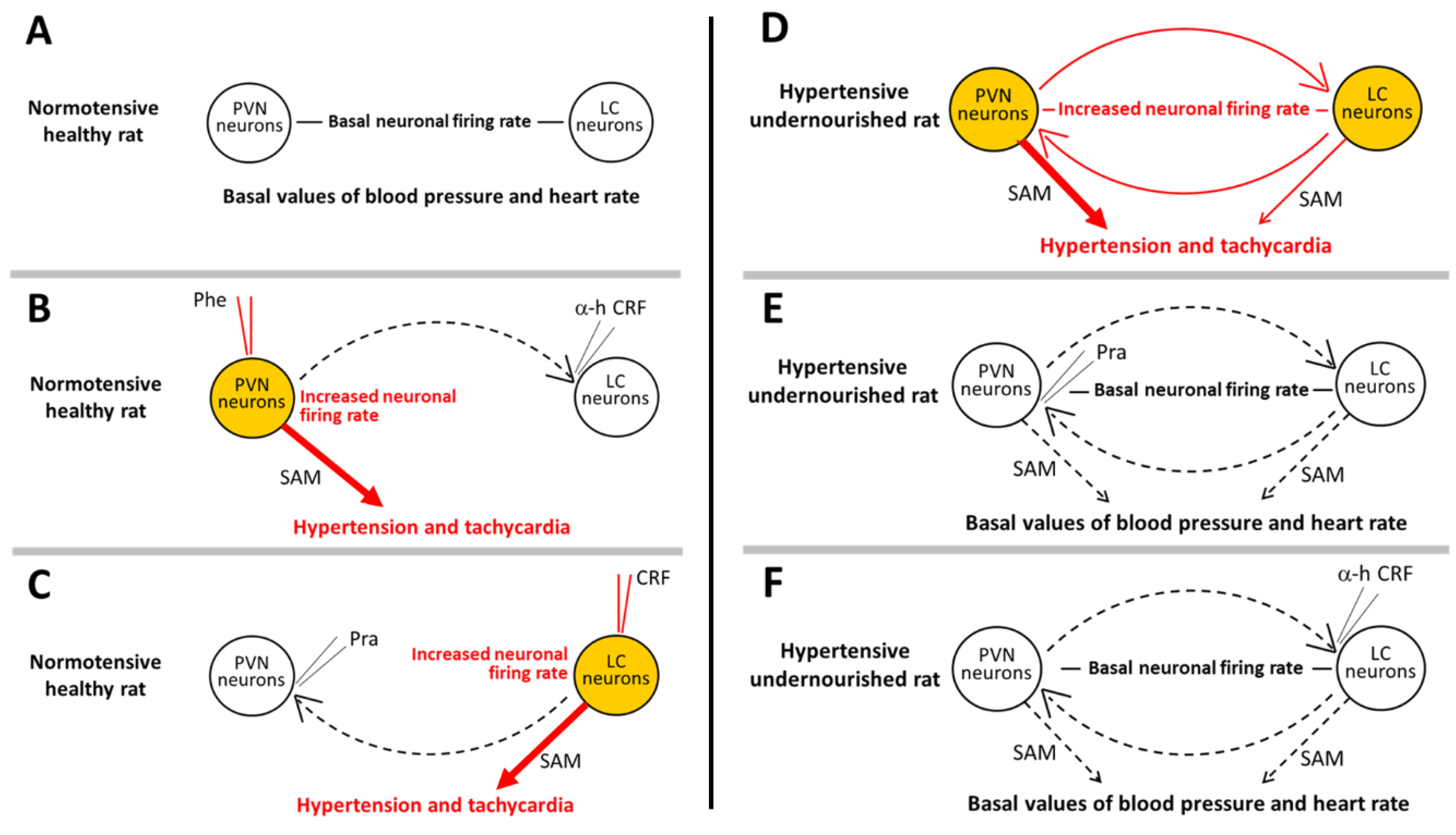
3.1. The Paraventricular-Coerulear Network: Differences between Eutrophic and Prenatally Undernourished States
3.2. Disruption of the PVN-LC Reciprocal Communication: Effects on Neuronal Activity and Cardiovascular Parameters in Undernourished and Eutrophic Animals
4. PVN as the Output System That Mediates Hypertension and Increased Heart Rate during Tonic Activation of the Paraventricular-Coerulear Network in Prenatally Malnourished Animals
- The PVN should not have a negative feedback mechanism associated with the down-regulation of PVN neurons (i.e., some inhibitory effect exerted backward on α1-adrenoceptor expression on PVN neurons). The absence of inhibition is required to sustain the continuous overactivity of the PVN in a pathological condition, such as in prenatal undernutrition. The evidence shows that prenatal undernutrition did not change the amounts of α1-adrenoceptor binding sites in the hypothalamus determined with [3H]-prazosin, but a lower expression of α1A-adrenoceptor mRNA was measured by in situ hybridization [93]. In that study [3H]-prazosin binding identified all three α1-adrenoceptor subtypes [126], while the deoxynucleotide probe used was specific for the α1A-adrenoceptor mRNA subtype [93]. It is also noteworthy that the binding assay was performed in the entire hypothalamus, while in-situ hybridization allowed specific recognition of mRNA in delimited regions of the PVN [93]. In this context, further experiments are needed to establish the net effect on the whole α1-adrenoceptor spectrum. This issue is of paramount importance because the three α1-adrenoceptor subtypes are expressed in the PVN [151,152,153], and they suffer different regulation processes depending on the primary condition. For example, α1D up-regulates while the α1A and α1B subtypes down-regulate in a concentration-dependent manner during an agonist challenge (i.e., endogenous noradrenaline), and down-regulation of the latter was accompanied by reductions of mRNA (for review see [154]). Interestingly, higher levels of α1A-adrenoceptor mRNA have reported in the PVN of rats suffering from chronic hypertension [155], but unchanged α1A, α1B, or α1D-adrenoceptor mRNA in the whole hypothalamus [156] and the VPN [157] has also been reported. Besides, chronic stress (which is often accompanied by hypertension) sensitizes the HPA axis to further acute stress (as measured by transient plasma ACTH increase) in rats, enhancing the response to α1-adrenergic receptor activation in the PVN [158]. Thus, despite the implicit importance of the above results, the specific regulation mechanisms of α1-adrenoceptor subtypes due to prenatal nutritional maladaptive programming remains still unknown.
- Permanent sensitization of the PVN should be required to maintain the integrity of the excitatory feed-forward loop. In this regard, it has been reported that noradrenaline induces an α1-adrenoceptor-mediated increase and an α2-adrenoceptor-mediated decrease in GABA-dependent spontaneous inhibitory postsynaptic current in a subset of parvocellular neurons of the PVN [159], which possibly represents a metaplastic regulation of GABAergic transmission in these neurons. Hippocampal long-term potentiation [160] and cerebral cortex long-term depression [161,162] have been reported also to be promoted by α1-adrenoceptors, but studies on neuroplasticity processes involved in long-lasting sensitization of neurons in the PVN, which can promote an enduring sympathetic activation thus favoring chronic hypertension, are still lacking.
5. Conclusions
- Both hypertension and tachycardia induced in healthy normotensive rats by either α1-adrenoceptor-mediated excitation of PVN neurons or CRF receptor-mediated excitation of LC neurons do not imply serial or reciprocal excitatory interactions between the two nuclei, as revealed by the fact that the cardiovascular effects observed were not prevented by disruption of the communication between the nuclei.
- Simultaneous concurrent tonic neuronal activity in the PVN and the LC is required to maintain elevated arterial blood pressure and heart rate scores in prenatally malnourished animals. In addition, reciprocal noradrenergic and CRFergic excitatory connections between the PVN and the LC give rise to a feedforward paraventricular-coerulear closed loop of neuronal activity, which is an essential part of the molecular etiological component of hypertension and tachycardia generated in animals submitted to prenatal undernutrition.
- The PVN may act as the exit point of the paraventricular-coerulear loop that downstream activates the sympathetic system, producing hypertension and tachycardia in malnourished animals. As such, it is essential that α1-adrenoceptor desensitization does not occur in the PVN of malnourished rats, allowing the PVN to function as the output locus in the paraventricular-coerulear network. More research is required to support this point.
- Whether noradrenergic hyperactivity in prenatally and perinatally undernourished animals is the primary factor involved in the triggering of neuronal hyperactivity in the PVN-LC communication, or on the contrary, it is a consequence of activity in such an interconnected set of neurons, is not entirely clear at present. Additionally, whether some epigenetic mechanisms may be underlying some of the remarkable characteristics that such an interactive neural system acquires under conditions of malnutrition remains unknown. Indeed, both early-life stress and early-life undernutrition similarly led to life-long alterations in the neuroendocrine stress system, partially by modifying epigenetic regulation of gene expression [163]. Increased CRF production via epigenetic mechanisms cannot be discarded since prenatal restraint stress is associated with the demethylation of CRF promoter, thereby enhancing CRF transcriptional responses to stress in adolescent rats [164]. However, no epigenetic modifications underlying altered CRF expression in prenatally undernourished animals have been reported so far.
- Other central nervous system programming factors that may underlie hypertension due to prenatal undernutrition, such as enhanced sympathetic-respiratory coupling at early life, inappropriate activation of the renin-angiotensin system, glucocorticoid neuronal remodeling, should not be neglected. Carefully designed experimental protocols should be arranged in order to study the specific contributions of those neural/endocrine components as well as the possibility of a relationship with the neuronal hyperactivity in the paraventricular-coerulear network.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langley-Evans, S.C. Developmental programming of health and disease. Proc. Nutr. Soc. 2006, 65, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 1997, 13, 807–813. [Google Scholar] [CrossRef]
- Bunker, S.; Pandey, J. Educational Case: Understanding Kwashiorkor and Marasmus: Disease Mechanisms and Pathologic Consequences. Acad. Pathol. 2021, 8, 23742895211037027. [Google Scholar] [CrossRef]
- Bergmann, R.L.; Bergmann, K.E.; Dudenhausen, J.W. Undernutrition and growth restriction in pregnancy. Nestle Nutr. Workshop Ser. Pediatr. Program 2008, 61, 103–121. [Google Scholar] [CrossRef]
- Morgane, P.J.; Austin-LaFrance, R.; Bronzino, J.; Tonkiss, J.; Díaz-Cintra, S.; Cintra, L.; Kemper, T.; Galler, J.R. Prenatal malnutrition and development of the brain. Neurosci. Biobehav. Rev. 1993, 17, 91–128. [Google Scholar] [CrossRef]
- Barra, R.; Morgan, C.; Sáez-Briones, P.; Reyes-Parada, M.; Burgos, H.; Morales, B.; Hernández, A. Facts and hypotheses about the programming of neuroplastic deficits by prenatal malnutrition. Nutr. Rev. 2019, 77, 65–80. [Google Scholar] [CrossRef]
- van Abeelen, A.F.; Veenendaal, M.V.; Painter, R.C.; de Rooij, S.R.; Thangaratinam, S.; van der Post, J.A.; Bossuyt, P.M.; Elias, S.G.; Uiterwaal, C.S.; Grobbee, D.E.; et al. The fetal origins of hypertension: A systematic review and meta-analysis of the evidence from animal experiments of maternal undernutrition. J. Hypertens. 2012, 30, 2255–2267. [Google Scholar] [CrossRef]
- Barker, D.J. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc. Biol. Sci. 1995, 262, 37–43. [Google Scholar] [CrossRef]
- Breslau, N.; Chilcoat, H.D.; Johnson, E.O.; Andreski, P.; Lucia, V.C. Neurologic soft signs and low birthweight: Their association and neuropsychiatric implications. Biol. Psychiatry 2000, 47, 71–79. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2017. Building Resilience for Peace and Food Security; FAO: Rome, Italy, 2017; Available online: https://www.fao.org/3/I7695e/I7695e.pdf (accessed on 30 September 2022).
- Goland, R.S.; Jozak, S.; Warren, W.B.; Conwell, I.M.; Stark, R.I.; Tropper, P.J. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J. Clin. Endocrinol. Metab. 1993, 77, 1174–1179. [Google Scholar] [CrossRef]
- Bertram, C.E.; Hanson, M.A. Animal models and programming of the metabolic syndrome. Br. Med. Bull. 2001, 60, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.D.; Zybert, P.A.; van der Bruin, K.P.; Lumey, L.H. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: Evidence from the Dutch Famine. Eur. J. Epidemiol. 2006, 21, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feng, X.; He, A.; Ding, Y.; Zhou, X.; Xu, Z. Prenatal exposure to the Great Chinese Famine and mid-age hypertension. PLoS ONE 2017, 12, e0176413. [Google Scholar] [CrossRef]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef]
- Liu, L.; Xu, X.; Zeng, H.; Zhang, Y.; Shi, Z.; Zhang, F.; Cao, X.; Xie, Y.J.; Reis, C.; Zhao, Y. Increase in the prevalence of hypertension among adults exposed to the Great Chinese Famine during early life. Environ. Health Prev. Med. 2017, 22, 64. [Google Scholar] [CrossRef][Green Version]
- Abate, K.H.; Arage, G.; Hassen, H.; Abafita, J.; Belachew, T. Differential effect of prenatal exposure to the Great Ethiopian Famine (1983-85) on the risk of adulthood hypertension based on sex: A historical cohort study. BMC Womens Health 2022, 22, 220. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Vonnahme, K.A.; Lemley, C.O.; Redmer, D.A.; Grazul-Bilska, A.T.; Borowicz, P.P.; Caton, J.S. Maternal stress and placental vascular function and remodeling. Curr. Vasc. Pharmacol. 2013, 11, 564–593. [Google Scholar] [CrossRef]
- Dissanayake, H.U.; Skilton, M.R.; Polson, J.W. Autonomic dysfunction in programmed hypertension. J. Hum. Hypertens. 2019, 33, 267–276. [Google Scholar] [CrossRef]
- Langley-Evans, S.C.; Gardner, D.S.; Jackson, A.A. Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J. Reprod. Fertil. 1996, 106, 307–312. [Google Scholar] [CrossRef]
- Woodall, S.M.; Johnston, B.M.; Breier, B.H.; Gluckman, P.D. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr. Res. 1996, 40, 438–443. [Google Scholar] [CrossRef]
- Pérez, H.; Ruiz, S.; Soto-Moyano, R. Prenatal malnutrition-induced hypertension in young rats is prevented by neonatal capsaicin treatment. Neurosci. Lett. 2002, 328, 253–256. [Google Scholar] [CrossRef]
- Pérez, H.; Ruiz, S.; Núñez, H.; White, A.; Gotteland, M. Coerulear activation by crh and its role in hypertension induced by prenatal malnutrition in the rat. Int. J. Neurosci. 2007, 117, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Wattez, J.S.; Delahaye, F.; Barella, L.F.; Dickes-Coopman, A.; Montel, V.; Breton, C.; Mathias, P.; Foligné, B.; Lesage, J.; Vieau, D. Short- and long-term effects of maternal perinatal undernutrition are lowered by cross-fostering during lactation in the male rat. J. Dev. Orig. Health Dis. 2014, 5, 109–120. [Google Scholar] [CrossRef]
- Barros, M.A.; de Alves, J.L.B.; Nogueira, V.O.; Wanderley, A.G.; Costa-Silva, J.H. Maternal low-protein diet induces changes in the cardiovascular autonomic modulation in male rat offspring. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Welberg, L.A.; Seckl, J.R.; Holmes, M.C. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: Possible implications for behaviour. Neuroscience 2001, 104, 71–79. [Google Scholar] [CrossRef]
- Pérez, H.; Soto-Moyano, R.; Ruiz, S.; Hernández, A.; Sierralta, W.; Olivares, R.; Núñez, H.; Flores, O.; Morgan, C.; Valladares, L.; et al. A putative role for hypothalamic glucocorticoid receptors in hypertension induced by prenatal undernutrition in the rat. Neurosci. Lett. 2010, 483, 41–46. [Google Scholar] [CrossRef]
- Clark, P.M. Programming of the hypothalamo-pituitary-adrenal axis and the fetal origins of adult disease hypothesis. Eur. J. Pediatr. 1998, 157, S7–S10. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Robinson, J.S. Conference report: Fetal origins of adult disease—Report of the First International Study Group, Sydney, 29–30 October 1994. Placenta 1995, 16, 317–320. [Google Scholar] [CrossRef]
- Zicha, J.; Kunes, J. Ontogenetic aspects of hypertension development: Analysis in the rat. Physiol. Rev. 1999, 79, 1227–1282. [Google Scholar] [CrossRef]
- Li, D.P.; Yang, Q.; Pan, H.M.; Pan, H.L. Plasticity of pre- and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H807–H815. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Yang, Q.; Pan, H.M.; Pan, H.L. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J. Physiol. 2008, 586, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.Y.; Li, D.P.; Byun, H.S.; Li, L.; Pan, H.L. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J. Neurosci. 2012, 32, 8560–8568. [Google Scholar] [CrossRef][Green Version]
- Jackson, K.L.; Head, G.A.; Gueguen, C.; Stevenson, E.R.; Lim, K.; Marques, F.Z. Mechanisms Responsible for Genetic Hypertension in Schlager BPH/2 Mice. Front. Physiol. 2019, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J.R. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol. Cell. Endocrinol. 2001, 185, 61–71. [Google Scholar] [CrossRef]
- Kawakami-Mori, F.; Nishimoto, M.; Reheman, L.; Kawarazaki, W.; Ayuzawa, N.; Ueda, K.; Hirohama, D.; Kohno, D.; Oba, S.; Shimosawa, T.; et al. Aberrant DNA methylation of hypothalamic angiotensin receptor in prenatal programmed hypertension. JCI Insight 2018, 3, e95625. [Google Scholar] [CrossRef] [PubMed]
- Paixão, A.D.; Alexander, B.T. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol. Reprod. 2013, 89, 144. [Google Scholar] [CrossRef]
- Moritz, K.M.; Dodic, M.; Wintour, E.M. Kidney development and the fetal programming of adult disease. Bioessays 2003, 25, 212–220. [Google Scholar] [CrossRef]
- Thornburg, K.L. The programming of cardiovascular disease. J. Dev. Orig. Health Dis. 2015, 6, 366–376. [Google Scholar] [CrossRef]
- Santos, M.S.; Joles, J.A. Early determinants of cardiovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 581–597. [Google Scholar] [CrossRef]
- Nuyt, A.M. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: Evidence from human studies and experimental animal models. Clin. Sci. 2008, 114, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, J.; Tang, K. The paraventricular nucleus of the hypothalamus: Development, function, and human diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef] [PubMed]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef]
- Savić, B.; Murphy, D.; Japundžić-Žigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef] [PubMed]
- Núñez, H.; Ruiz, S.; Soto-Moyano, R.; Navarrete, M.; Valladares, L.; White, A.; Pérez, H. Fetal undernutrition induces overexpression of CRH mRNA and CRH protein in hypothalamus and increases CRH and corticosterone in plasma during postnatal life in the rat. Neurosci. Lett. 2008, 448, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Sohlström, A.; Carlsson, C.; Uvnäs-Moberg, K. Effects of oxytocin treatment in early life on body weight and corticosterone in adult offspring from ad libitum-fed and food-restricted rats. Biol. Neonate 2000, 78, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rexhepaj, R.; Boini, K.M.; Huang, D.Y.; Amann, K.; Artunc, F.; Wang, K.; Brosens, J.J.; Kuhl, D.; Lang, F. Role of maternal glucocorticoid inducible kinase SGK1 in fetal programming of blood pressure in response to prenatal diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R2008–R2013. [Google Scholar] [CrossRef] [PubMed]
- Niwa, F.; Kawai, M.; Kanazawa, H.; Iwanaga, K.; Matsukura, T.; Shibata, M.; Hasegawa, T.; Heike, T. Limited response to CRH stimulation tests at 2 weeks of age in preterm infants born at less than 30 weeks of gestational age. Clin. Endocrinol. 2013, 78, 724–729. [Google Scholar] [CrossRef]
- Vallecillos, F.J.; Fernández, S.O. Histopathological features of post-mortem pituitaries: A retrospective analysis. Rev. Assoc. Med. Bras. 2016, 62, 399–406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez, H.; Ruiz, S.; Núñez, H.; White, A.; Gotteland, M.; Hernández, A. Paraventricular-coerulear interactions: Role in hypertension induced by prenatal undernutrition in the rat. Eur. J. Neurosci. 2006, 24, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, L. Glucocorticoids and vascular reactivity. Curr. Vasc. Pharmacol. 2004, 2, 1–12. [Google Scholar] [CrossRef]
- Pyner, S. Neurochemistry of the paraventricular nucleus of the hypothalamus: Implications for cardiovascular regulation. J. Chem. Neuroanat. 2009, 38, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lesage, J.; Blondeau, B.; Grino, M.; Bréant, B.; Dupouy, J.P. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 2001, 142, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Bertram, C.; Trowern, A.R.; Copin, N.; Jackson, A.A.; Whorwood, C.B. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: Potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology 2001, 142, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.; Hanson, M.A.; Matthews, S.G. Maternal undernutrition in early gestation alters molecular regulation of the hypothalamic-pituitary-adrenal axis in the ovine fetus. J. Neuroendocrinol. 2001, 13, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Núñez, H.; Ruiz, S.; Soto-Moyano, R.; Valladares, L.; White, A.; Pérez, H. Prenatal undernutrition decreases the sensitivity of the hypothalamo-pituitary-adrenal axis in rat, as revealed by subcutaneous and intra-paraventricular dexamethasone challenges. Neurosci. Lett. 2007, 419, 99–103. [Google Scholar] [CrossRef]
- Langley-Evans, S.; Jackson, A. Intrauterine programming of hypertension: Nutrient-hormone interactions. Nutr. Rev. 1996, 54, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Vasku, M.; Kleine-Eggebrecht, N.; Rath, W.; Mohaupt, M.G.; Escher, G.; Pecks, U. Apparent systemic 11ß-dehydroxysteroid dehydrogenase 2 activity is increased in preeclampsia but not in intrauterine growth restriction. Pregnancy Hypertens. 2018, 11, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Rosewicz, S.; McDonald, A.R.; Maddux, B.A.; Goldfine, I.D.; Miesfeld, R.L.; Logsdon, C.D. Mechanism of glucocorticoid receptor down-regulation by glucocorticoids. J. Biol. Chem. 1988, 263, 2581–2584. [Google Scholar] [CrossRef]
- Shimojo, M.; Hiroi, N.; Yakushiji, F.; Ueshiba, H.; Yamaguchi, N.; Miyachi, Y. Differences in down-regulation of glucocorticoid receptor mRNA by cortisol, prednisolone and dexamethasone in HeLa cells. Endocr. J. 1995, 42, 629–636. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, S.; Sarabdjitsingh, R.A.; van Batenburg, M.F.; Lachize, S.B.; Li, H.; Dijkmans, T.F.; Vreugdenhil, E.; de Kloet, E.R.; Meijer, O.C. Chromatin immunoprecipitation scanning identifies glucocorticoid receptor binding regions in the proximal promoter of a ubiquitously expressed glucocorticoid target gene in brain. J. Neurochem. 2008, 106, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C.; Gardner, D.S.; Jackson, A.A. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J. Nutr. 1996, 126, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, F.H.; Oliver, M.H.; Hawkins, P.; Holloway, A.C.; Campbell, M.; Gluckman, P.D.; Harding, J.E.; Challis, J.R. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology 2004, 145, 4278–4285. [Google Scholar] [CrossRef]
- Dutriez-Casteloot, I.; Breton, C.; Coupé, B.; Hawchar, O.; Enache, M.; Dickes-Coopman, A.; Keyzer, Y.; Deloof, S.; Lesage, J.; Vieau, D. Tissue-specific programming expression of glucocorticoid receptors and 11 beta-HSDs by maternal perinatal undernutrition in the HPA axis of adult male rats. Horm. Metab. Res. 2008, 40, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.; Begum, G.; Cook, A.; Connor, K.; Rumball, C.; Oliver, M.; Challis, J.; Bloomfield, F.; White, A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 2010, 151, 3652–3664. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ramahi, E.; Nijland, M.J.; Choi, J.; Myers, D.A.; Nathanielsz, P.W.; McDonald, T.J. Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: Coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation. Endocrinology 2013, 154, 2365–2373. [Google Scholar] [CrossRef]
- Shoemaker, W.J.; Wurtman, R.J. Perinatal undernutrition: Accumulation of catecholamines in rat brain. Science 1971, 171, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Marichich, E.S.; Molina, V.A.; Orsingher, O.A. Persistent changes in central catecholaminergic system after recovery of perinatally undernourished rats. J. Nutr. 1979, 109, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Resnick, O.; Morgane, P.J. Animal models for small-for-gestational-age (SGA) neonates and infants-at-risk (IAR). Brain Res. 1983, 312, 221–225. [Google Scholar] [CrossRef]
- Soto-Moyano, R.; Hernandez, A.; Perez, H.; Ruiz, S.; Diaz-Veliz, G.; Belmar, J. Early malnutrition and changes in the induced release of noradrenaline in the prefrontal cortex of adult rats. Int. J. Neurosci. 1987, 37, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Soto-Moyano, R.; Hernández, A.; Pérez, H.; Ruiz, S.; Carreño, P.; Alarcón, S.; Belmar, J. Clonidine treatment during gestation prevents functional deficits induced by prenatal malnutrition in the rat visual cortex. Int. J. Neurosci. 1994, 76, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Turiak, G.; Galler, J.; Volicer, L. Effect of prenatal malnutrition on release of monoamines from hippocampal slices. Life Sci. 1995, 57, 1467–1475. [Google Scholar] [CrossRef]
- Schlesinger, L.; Arevalo, M.; Simon, V.; Lopez, M.; Muñoz, C.; Hernandez, A.; Carreño, P.; Belmar, J.; White, A.; Häffner-Cavaillon, N. Immune depression induced by protein calorie malnutrition can be suppressed by lesioning central noradrenaline systems. J. Neuroimmunol. 1995, 57, 1–7. [Google Scholar] [CrossRef]
- Soto-Moyano, R.; Belmar, J.; Perez, H.; Ruiz, S.; Hernandez, A. Central noradrenergic hyperactivity early in life: A hypothesis on the origin of morpho-functional brain disorders induced by malnutrition. Biol. Res. 1995, 28, 105–111. [Google Scholar]
- Soto-Moyano, R.; Alarcón, S.; Belmar, J.; Kusch, C.; Pérez, H.; Ruiz, S.; Hernández, A. Prenatal protein restriction alters synaptic mechanisms of callosal connections in the rat visual cortex. Int. J. Dev. Neurosci. 1998, 16, 75–84. [Google Scholar] [CrossRef]
- Soto-Moyano, R.; Alarcon, S.; Hernández, A.; Pérez, H.; Ruiz, S.; Carreño, P.; Kusch, C.; Belmar, J. Prenatal malnutrition-induced functional alterations in callosal connections and in interhemispheric asymmetry in rats are prevented by reduction of noradrenaline synthesis during gestation. J. Nutr. 1998, 128, 1224–1231. [Google Scholar] [CrossRef][Green Version]
- Soto-Moyano, R.; Fernandez, V.; Sanhueza, M.; Belmar, J.; Kusch, C.; Perez, H.; Ruiz, S.; Hernandez, A. Effects of mild protein prenatal malnutrition and subsequent postnatal nutritional rehabilitation on noradrenaline release and neuronal density in the rat occipital cortex. Dev. Brain Res. 1999, 116, 51–58. [Google Scholar] [CrossRef]
- Church, N.T.; Weissner, W.; Galler, J.R.; Amaral, A.C.; Rosene, D.L.; McGaughy, J.A.; Rushmore, R.J.; Larrabee, E.; Mokler, D.J. In vivo microdialysis shows differential effects of prenatal protein malnutrition and stress on norepinephrine, dopamine, and serotonin levels in rat orbital frontal cortex. Behav. Neurosci. 2021, 135, 629–641. [Google Scholar] [CrossRef]
- Sodero, A.O.; Valdomero, A.; Cuadra, G.R.; Ramírez, O.A.; Orsingher, O.A. Locus coeruleus activity in perinatally protein-deprived rats: Effects of fluoxetine administration. Eur. J. Pharmacol. 2004, 503, 35–42. [Google Scholar] [CrossRef]
- Barra, R.; Soto-Moyano, R.; Valladares, L.; Morgan, C.; Pérez, H.; Burgos, H.; Olivares, R.; Sáez-Briones, P.; Laurido, C.; Hernández, A. Knockdown of α2C-adrenoceptors in the occipital cortex rescued long-term potentiation in hidden prenatally malnourished rats. Neurobiol. Learn. Mem. 2012, 98, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sawchenko, P.E.; Swanson, L.W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982, 257, 275–325. [Google Scholar] [CrossRef]
- Cunningham, E.T.; Sawchenko, P.E. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J. Comp. Neurol. 1988, 274, 60–76. [Google Scholar] [CrossRef]
- Nieuwenhuys, R. Chemoarchitecture of the Brain; Springer: Berlin/Heidelberg, Germany, 1985; Volume 33. [Google Scholar]
- Giorgi, F.S.; Galgani, A.; Puglisi-Allegra, S.; Busceti, C.L.; Fornai, F. The connections of Locus Coeruleus with hypothalamus: Potential involvement in Alzheimer’s disease. J. Neural. Transm. 2021, 128, 589–613. [Google Scholar] [CrossRef]
- Cole, R.L.; Sawchenko, P.E. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J. Neurosci. 2002, 22, 959–969. [Google Scholar] [CrossRef]
- Itoi, K.; Suda, T.; Tozawa, F.; Dobashi, I.; Ohmori, N.; Sakai, Y.; Abe, K.; Demura, H. Microinjection of norepinephrine into the paraventricular nucleus of the hypothalamus stimulates corticotropin-releasing factor gene expression in conscious rats. Endocrinology 1994, 135, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Milanick, W.J.; Polo-Parada, L.; Dantzler, H.A.; Kline, D.D. Activation of alpha-1 adrenergic receptors increases cytosolic calcium in neurones of the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2019, 31, e12791. [Google Scholar] [CrossRef]
- Calogero, A.E.; Bernardini, R.; Gold, P.W.; Chrousos, G.P. Regulation of rat hypothalamic corticotropin-releasing hormone secretion in vitro: Potential clinical implications. Adv. Exp. Med. Biol. 1988, 245, 167–181. [Google Scholar] [CrossRef]
- Yang, J.H.; Li, L.H.; Lee, S.; Jo, I.H.; Lee, S.Y.; Ryu, P.D. Effects of adrenalectomy on the excitability of neurosecretory parvocellular neurones in the hypothalamic paraventricular nucleus. J. Neuroendocrinol. 2007, 19, 293–301. [Google Scholar] [CrossRef]
- Mendonça, M.M.; Santana, J.S.; da Cruz, K.R.; Ianzer, D.; Ghedini, P.C.; Nalivaiko, E.; Fontes, M.A.P.; Ferreira, R.N.; Pedrino, G.R.; Colugnati, D.B.; et al. Involvement of GABAergic and Adrenergic Neurotransmissions on Paraventricular Nucleus of Hypothalamus in the Control of Cardiac Function. Front. Physiol. 2018, 9, 670. [Google Scholar] [CrossRef]
- Cayupe, B.; Morgan, C.; Puentes, G.; Valladares, L.; Burgos, H.; Castillo, A.; Hernández, A.; Constandil, L.; Ríos, M.; Sáez-Briones, P.; et al. Hypertension in Prenatally Undernourished Young-Adult Rats Is Maintained by Tonic Reciprocal Paraventricular-Coerulear Excitatory Interactions. Molecules 2021, 26, 3568. [Google Scholar] [CrossRef] [PubMed]
- Saphier, D.; Zhang, K. Inhibition by the serotonin1A agonist, 8-hydroxy-2- (di-n-propylamino)tetralin, of antidromically identified paraventricular nucleus neurons in the rat. Brain Res. 1993, 615, 7–12. [Google Scholar] [CrossRef]
- Saphier, D.; Feldman, S. Adrenoceptor specificity in the central regulation of adrenocortical secretion. Neuropharmacology 1989, 28, 1231–1237. [Google Scholar] [CrossRef]
- Saphier, D.; Feldman, S. Catecholaminergic projections to tuberoinfundibular neurones of the paraventricular nucleus: III. Effects of adrenoceptor agonists and antagonists. Brain Res. Bull. 1991, 26, 863–870. [Google Scholar] [CrossRef]
- Saphier, D. Electrophysiology and neuropharmacology of noradrenergic projections to rat PVN magnocellular neurons. Am. J. Physiol. 1993, 264, R891–R902. [Google Scholar] [CrossRef]
- Nasif, F.J.; Ramírez, O.A.; Cuadra, G.R.; Orsingher, O.A. Increased neuronal activity in locus coeruleus from adult rats undernourished at perinatal age: Its reversal by desipramine. Life Sci. 2001, 69, 2551–2559. [Google Scholar] [CrossRef]
- Dunn, A.J.; Berridge, C.W. Is corticotropin-releasing factor a mediator of stress responses? Ann. N. Y. Acad. Sci. 1990, 579, 183–191. [Google Scholar] [CrossRef]
- Dunn, A.J.; Berridge, C.W. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res. Rev. 1990, 15, 71–100. [Google Scholar] [CrossRef]
- Szabadi, E. Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol. 2013, 27, 659–693. [Google Scholar] [CrossRef]
- Dunn, A.J.; Swiergiel, A.H.; Palamarchouk, V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann. N. Y. Acad. Sci. 2004, 1018, 25–34. [Google Scholar] [CrossRef]
- Koob, G.F. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry 1999, 46, 1167–1180. [Google Scholar] [CrossRef]
- Valentino, R.J.; Page, M.; van Bockstaele, E.; Aston-Jones, G. Corticotropin-releasing factor innervation of the locus coeruleus region: Distribution of fibers and sources of input. Neuroscience 1992, 48, 689–705. [Google Scholar] [CrossRef]
- van Bockstaele, E.J.; Colago, E.E.; Aicher, S. Light and electron microscopic evidence for topographic and monosynaptic projections from neurons in the ventral medulla to noradrenergic dendrites in the rat locus coeruleus. Brain Res. 1998, 784, 123–138. [Google Scholar] [CrossRef]
- Reyes, B.A.; Valentino, R.J.; Xu, G.; van Bockstaele, E.J. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur. J. Neurosci. 2005, 22, 93–106. [Google Scholar] [CrossRef]
- Reyes, B.A.; Fox, K.; Valentino, R.J.; van Bockstaele, E.J. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur. J. Neurosci. 2006, 23, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Reyes, B.A.; Glaser, J.D.; van Bockstaele, E.J. Ultrastructural evidence for co-localization of corticotropin-releasing factor receptor and mu-opioid receptor in the rat nucleus locus coeruleus. Neurosci. Lett. 2007, 413, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.L.; Lechner, S.M.; Pavcovich, L.A.; Valentino, R.J. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: Effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J. Pharmacol. Exp. Ther. 1997, 281, 163–172. [Google Scholar] [PubMed]
- Swinny, J.D.; O’Farrell, E.; Bingham, B.C.; Piel, D.A.; Valentino, R.J.; Beck, S.G. Neonatal rearing conditions distinctly shape locus coeruleus neuronal activity, dendritic arborization, and sensitivity to corticotrophin-releasing factor. Int. J. Neuropsychopharmacol. 2010, 13, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Jedema, H.P.; Grace, A.A. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J. Neurosci. 2004, 24, 9703–9713. [Google Scholar] [CrossRef] [PubMed]
- Prouty, E.W.; Waterhouse, B.D.; Chandler, D.J. Corticotropin releasing factor dose-dependently modulates excitatory synaptic transmission in the noradrenergic nucleus locus coeruleus. Eur. J. Neurosci. 2017, 45, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.M.; Chen, X.Q.; Wang, X.; Hao, K.; Du, J.Z. Corticotropin-releasing factor receptor type 1 colocalizes with type 2 in corticotropin-releasing factor-containing cellular profiles in rat brain. Neuro. Endocrinol. Lett. 2014, 35, 417–426. [Google Scholar]
- Chen, C.L.; Tang, J.S.; Chiu, T.H.; Yang, Y.R. Influence of external and intracellular pH on propofol-induced responses in rat locus coeruleus neurons. Eur. J. Pharmacol. 2006, 545, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Tan, L.; Li, L.S.; Ding, X. Role of corticotropin-releasing factor and substance P in pressor responses of nuclei controlling emotion and stress. Peptides 1998, 19, 677–682. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Felder, R.B. Hypothalamic corticotrophin-releasing factor and norepinephrine mediate sympathetic and cardiovascular responses to acute intracarotid injection of tumour necrosis factor-alpha in the rat. J. Neuroendocrinol. 2008, 20, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, R.J.; Cabrera, C.L.; Baird, T.J.; Rice, K.C.; Woods, J.H. Antalarmin blockade of corticotropin releasing hormone-induced hypertension in rats. Brain Res. 2000, 881, 204–207. [Google Scholar] [CrossRef]
- Valentino, R.J.; Curtis, A.L. Antidepressant interactions with corticotropin-releasing factor in the noradrenergic nucleus locus coeruleus. Psychopharmacol. Bull. 1991, 27, 263–269. [Google Scholar]
- Morimoto, A.; Nakamori, T.; Morimoto, K.; Tan, N.; Murakami, N. The central role of corticotrophin-releasing factor (CRF-41) in psychological stress in rats. J. Physiol. 1993, 460, 221–229. [Google Scholar] [CrossRef]
- Ebihara, H.; Kawasaki, H.; Nakamura, S.; Takasaki, K.; Wada, A. Pressor response to microinjection of clonidine into the hypothalamic paraventricular nucleus in conscious rats. Brain Res. 1993, 624, 44–52. [Google Scholar] [CrossRef]
- Bealer, S.L.; Abell, S.O. Paraventricular nucleus histamine increases blood pressure by adrenoreceptor stimulation of vasopressin release. Am. J. Physiol. 1995, 269, H80–H85. [Google Scholar] [CrossRef] [PubMed]
- Domingos-Souza, G.; Martinez, D.; Sinkler, S.; Heesch, C.M.; Kline, D.D. Alpha adrenergic receptor signaling in the hypothalamic paraventricular nucleus is diminished by the chronic intermittent hypoxia model of sleep apnea. Exp. Neurol. 2021, 335, 113517. [Google Scholar] [CrossRef]
- Conti, L.H.; Youngblood, K.L.; Printz, M.P.; Foote, S.L. Locus coeruleus electrophysiological activity and responsivity to corticotropin-releasing factor in inbred hypertensive and normotensive rats. Brain Res. 1997, 774, 27–34. [Google Scholar] [CrossRef]
- Soto-Moyano, R.; Valladares, L.; Sierralta, W.; Pérez, H.; Mondaca, M.; Fernández, V.; Burgos, H.; Hernández, A. Mild prenatal protein malnutrition increases alpha2C-adrenoceptor density in the cerebral cortex during postnatal life and impairs neocortical long-term potentiation and visuo-spatial performance in rats. J. Neurochem. 2005, 93, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Taniguchi, T.; Takauji, R.; Suzuki, F.; Tanaka, T.; Muramatsu, I. Inverse agonism and neutral antagonism at a constitutively active alpha-1a adrenoceptor. Br. J. Pharmacol. 2000, 131, 546–552. [Google Scholar] [CrossRef]
- Docherty, J.R. The pharmacology of α. Eur. J. Pharmacol. 2019, 855, 305–320. [Google Scholar] [CrossRef]
- Hauger, R.L.; Grigoriadis, D.E.; Dallman, M.F.; Plotsky, P.M.; Vale, W.W.; Dautzenberg, F.M. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol. Rev. 2003, 55, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Arriza, J.L.; Simerly, R.B.; Swanson, L.W.; Evans, R.M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron 1988, 1, 887–900. [Google Scholar] [CrossRef]
- Herman, J.P. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell. Mol. Neurobiol. 1993, 13, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Pulsinelli, W.A. Glucocorticoids potentiate ischemic injury to neurons: Therapeutic implications. Science 1985, 229, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J. Neurosci. 1985, 5, 1222–1227. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Donnelly, T.M. Vulnerability to stress-induced tumor growth increases with age in rats: Role of glucocorticoids. Endocrinology 1985, 117, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Watkins, N.D.; Cork, S.C.; Pyner, S. An immunohistochemical investigation of the relationship between neuronal nitric oxide synthase, GABA and presympathetic paraventricular neurons in the hypothalamus. Neuroscience 2009, 159, 1079–1088. [Google Scholar] [CrossRef]
- Han, S.K.; Chong, W.; Li, L.H.; Lee, I.S.; Murase, K.; Ryu, P.D. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J. Neurophysiol. 2002, 87, 2287–2296. [Google Scholar] [CrossRef]
- Zhang, L.; Taniguchi, T.; Tanaka, T.; Shinozuka, K.; Kunitomo, M.; Nishiyama, M.; Kamata, K.; Muramatsu, I. Alpha-1 adrenoceptor up-regulation induced by prazosin but not KMD-3213 or reserpine in rats. Br. J. Pharmacol. 2002, 135, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Daftary, S.S.; Boudaba, C.; Tasker, J.G. Noradrenergic regulation of parvocellular neurons in the rat hypothalamic paraventricular nucleus. Neuroscience 2000, 96, 743–751. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Glucocorticoid toxicity in the hippocampus: Temporal aspects of neuronal vulnerability. Brain Res. 1985, 359, 300–305. [Google Scholar] [CrossRef]
- Sheline, Y.I. Hippocampal atrophy in major depression: A result of depression-induced neurotoxicity? Mol. Psychiatry 1996, 1, 298–299. [Google Scholar]
- Kathol, R.G.; Jaeckle, R.S.; Lopez, J.F.; Meller, W.H. Consistent reduction of ACTH responses to stimulation with CRH, vasopressin and hypoglycaemia in patients with major depression. Br. J. Psychiatry 1989, 155, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Breton-Provencher, V.; Sur, M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 2019, 22, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Tyagi, S.C. Nutriepigenetic regulation by folate-homocysteine-methionine axis: A review. Mol. Cell. Biochem. 2014, 387, 55–61. [Google Scholar] [CrossRef]
- Maekawa, M.; Watanabe, A.; Iwayama, Y.; Kimura, T.; Hamazaki, K.; Balan, S.; Ohba, H.; Hisano, Y.; Nozaki, Y.; Ohnishi, T.; et al. Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes. Transl. Psychiatry 2017, 7, e1229. [Google Scholar] [CrossRef]
- Dampney, R.A.; Michelini, L.C.; Li, D.P.; Pan, H.L. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1200–H1214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Ma, H.J.; Shao, J.Y.; Pan, H.L.; Li, D.P. Impaired Hypothalamic Regulation of Sympathetic Outflow in Primary Hypertension. Neurosci. Bull. 2019, 35, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Milner, T.A.; Reis, D.J.; Pickel, V.M.; Aicher, S.A.; Giuliano, R. Ultrastructural localization and afferent sources of corticotropin-releasing factor in the rat rostral ventrolateral medulla: Implications for central cardiovascular regulation. J. Comp. Neurol. 1993, 333, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Gurtu, S.; Pant, K.K.; Sinha, J.N.; Bhargava, K.P. An investigation into the mechanism of cardiovascular responses elicited by electrical stimulation of locus coeruleus and subcoeruleus in the cat. Brain Res. 1984, 301, 59–64. [Google Scholar] [CrossRef]
- van Bockstaele, E.J.; Pieribone, V.A.; Aston-Jones, G. Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: Retrograde and anterograde tracing studies. J. Comp. Neurol. 1989, 290, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Head, G.A.; Burke, S.L. Relative importance of medullary brain nuclei for the sympatho-inhibitory actions of rilmenidine in the anaesthetized rabbit. J. Hypertens. 1998, 16, 503–517. [Google Scholar] [CrossRef] [PubMed]
- de Alves, J.L.B.; Nogueira, V.O.; Neto, M.P.C.; Leopoldino, A.M.; Curti, C.; Colombari, D.S.; Colombari, E.; Wanderley, A.G.; Leandro, C.G.; Zoccal, D.B.; et al. Maternal protein restriction increases respiratory and sympathetic activities and sensitizes peripheral chemoreflex in male rat offspring. J. Nutr. 2015, 145, 907–914. [Google Scholar] [CrossRef]
- Domyancic, A.V.; Morilak, D.A. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J. Comp. Neurol. 1997, 386, 358–378. [Google Scholar] [CrossRef]
- Day, H.E.; Campeau, S.; Watson, S.J.; Akil, H. Expression of alpha(1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J. Neurosci. 1999, 19, 10098–10106. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Morilak, D.A. Expression of alpha1D adrenergic receptor messenger RNA in oxytocin- and corticotropin-releasing hormone-synthesizing neurons in the rat paraventricular nucleus. Neuroscience 1999, 91, 639–649. [Google Scholar] [CrossRef]
- Hein, P.; Michel, M.C. Signal transduction and regulation: Are all alpha1-adrenergic receptor subtypes created equal? Biochem. Pharmacol. 2007, 73, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Ploj, K.; Larsson, B.M.; Holmäng, A. Rats with steroid-induced polycystic ovaries develop hypertension and increased sympathetic nervous system activity. Reprod. Biol. Endocrinol. 2005, 3, 44. [Google Scholar] [CrossRef]
- Begg, D.P.; Puskás, L.G.; Kitajka, K.; Ménesi, D.; Allen, A.M.; Li, D.; Mathai, M.L.; Shi, J.R.; Sinclair, A.J.; Weisinger, R.S. Hypothalamic gene expression in ω-3 PUFA-deficient male rats before, and following, development of hypertension. Hypertens. Res. 2012, 35, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Thorsdottir, D.; Cruickshank, N.C.; Einwag, Z.; Hennig, G.W.; Erdos, B. BDNF downregulates β-adrenergic receptor-mediated hypotensive mechanisms in the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1258–H1271. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Morilak, D.A. Norepinephrine release in medial amygdala facilitates activation of the hypothalamic-pituitary-adrenal axis in response to acute immobilisation stress. J. Neuroendocrinol. 2005, 17, 22–28. [Google Scholar] [CrossRef]
- Yang, J.H.; Li, L.H.; Shin, S.Y.; Lee, S.; Lee, S.Y.; Han, S.K.; Ryu, P.D. Adrenalectomy potentiates noradrenergic suppression of GABAergic transmission in parvocellular neurosecretory neurons of hypothalamic paraventricular nucleus. J. Neurophysiol. 2008, 99, 514–523. [Google Scholar] [CrossRef][Green Version]
- Doze, V.A.; Papay, R.S.; Goldenstein, B.L.; Gupta, M.K.; Collette, K.M.; Nelson, B.W.; Lyons, M.J.; Davis, B.A.; Luger, E.J.; Wood, S.G.; et al. Long-term α1A-adrenergic receptor stimulation improves synaptic plasticity, cognitive function, mood, and longevity. Mol. Pharmacol. 2011, 80, 747–758. [Google Scholar] [CrossRef]
- Marzo, A.; Bai, J.; Caboche, J.; Vanhoutte, P.; Otani, S. Cellular mechanisms of long-term depression induced by noradrenaline in rat prefrontal neurons. Neuroscience 2010, 169, 74–86. [Google Scholar] [CrossRef]
- Treviño, M.; Frey, S.; Köhr, G. Alpha-1 adrenergic receptors gate rapid orientation-specific reduction in visual discrimination. Cereb. Cortex 2012, 22, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.Y.; Naninck, E.F.; Schmidt, M.V.; Lucassen, P.J.; Korosi, A. Early-life adversity programs emotional functions and the neuroendocrine stress system: The contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress 2015, 18, 328–342. [Google Scholar] [CrossRef]
- Xu, J.; He, G.; Zhu, J.; Zhou, X.; St Clair, D.; Wang, T.; Xiang, Y.; Zhao, Q.; Xing, Q.; Liu, Y.; et al. Prenatal nutritional deficiency reprogrammed postnatal gene expression in mammal brains: Implications for schizophrenia. Int. J. Neuropsychopharmacol. 2014, 18, pyu054. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cayupe, B.; Troncoso, B.; Morgan, C.; Sáez-Briones, P.; Sotomayor-Zárate, R.; Constandil, L.; Hernández, A.; Morselli, E.; Barra, R. The Role of the Paraventricular-Coerulear Network on the Programming of Hypertension by Prenatal Undernutrition. Int. J. Mol. Sci. 2022, 23, 11965. https://doi.org/10.3390/ijms231911965
Cayupe B, Troncoso B, Morgan C, Sáez-Briones P, Sotomayor-Zárate R, Constandil L, Hernández A, Morselli E, Barra R. The Role of the Paraventricular-Coerulear Network on the Programming of Hypertension by Prenatal Undernutrition. International Journal of Molecular Sciences. 2022; 23(19):11965. https://doi.org/10.3390/ijms231911965
Chicago/Turabian StyleCayupe, Bernardita, Blanca Troncoso, Carlos Morgan, Patricio Sáez-Briones, Ramón Sotomayor-Zárate, Luis Constandil, Alejandro Hernández, Eugenia Morselli, and Rafael Barra. 2022. "The Role of the Paraventricular-Coerulear Network on the Programming of Hypertension by Prenatal Undernutrition" International Journal of Molecular Sciences 23, no. 19: 11965. https://doi.org/10.3390/ijms231911965
APA StyleCayupe, B., Troncoso, B., Morgan, C., Sáez-Briones, P., Sotomayor-Zárate, R., Constandil, L., Hernández, A., Morselli, E., & Barra, R. (2022). The Role of the Paraventricular-Coerulear Network on the Programming of Hypertension by Prenatal Undernutrition. International Journal of Molecular Sciences, 23(19), 11965. https://doi.org/10.3390/ijms231911965






