Resveratrol Improves the Digestive Ability and the Intestinal Health of Siberian Sturgeon
Abstract
:1. Introduction
2. Results
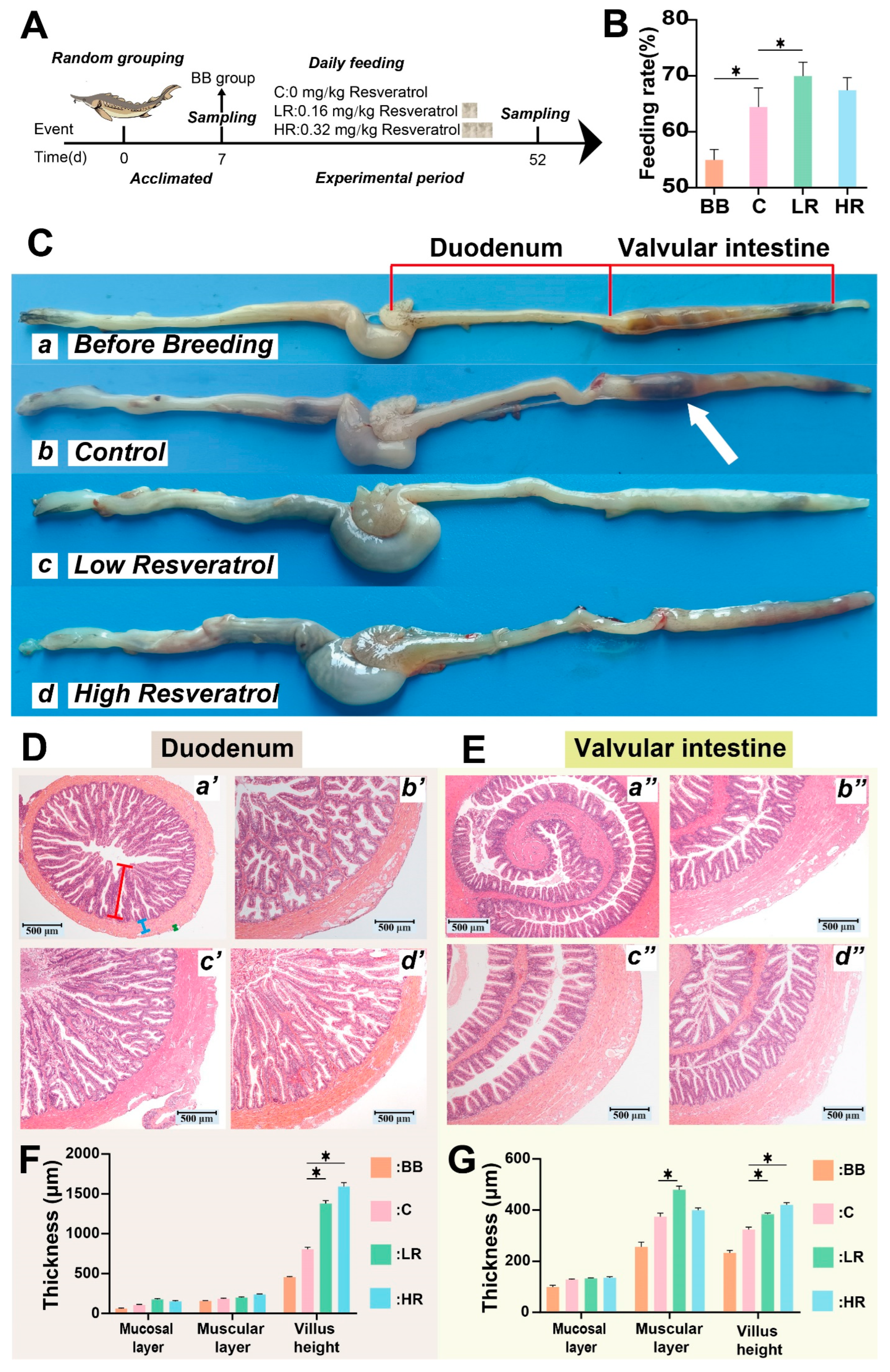
2.1. Resveratrol Improved Intestinal Digestion and Protected the Intestinal Structure in Siberian Sturgeon
2.2. Resveratrol Increased Digestive Enzyme Activity of Siberian Sturgeon
2.3. Global Analysis of OTUs
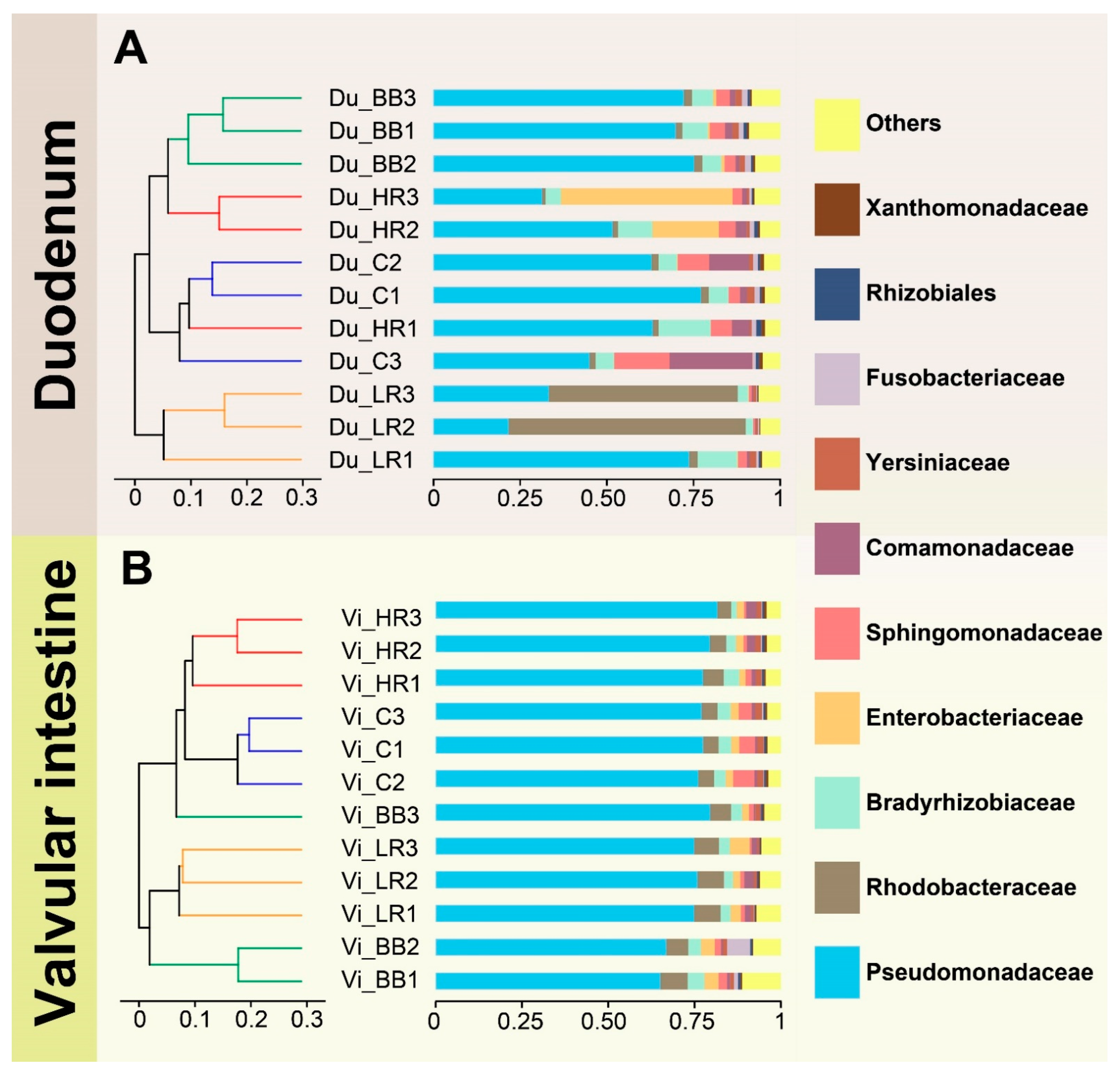
2.4. Resveratrol Alters the Community Composition in the Duodenum of Siberian Sturgeon
2.5. Resveratrol Influenced the Abundance of Duodenum Microbiota of Siberian Sturgeon
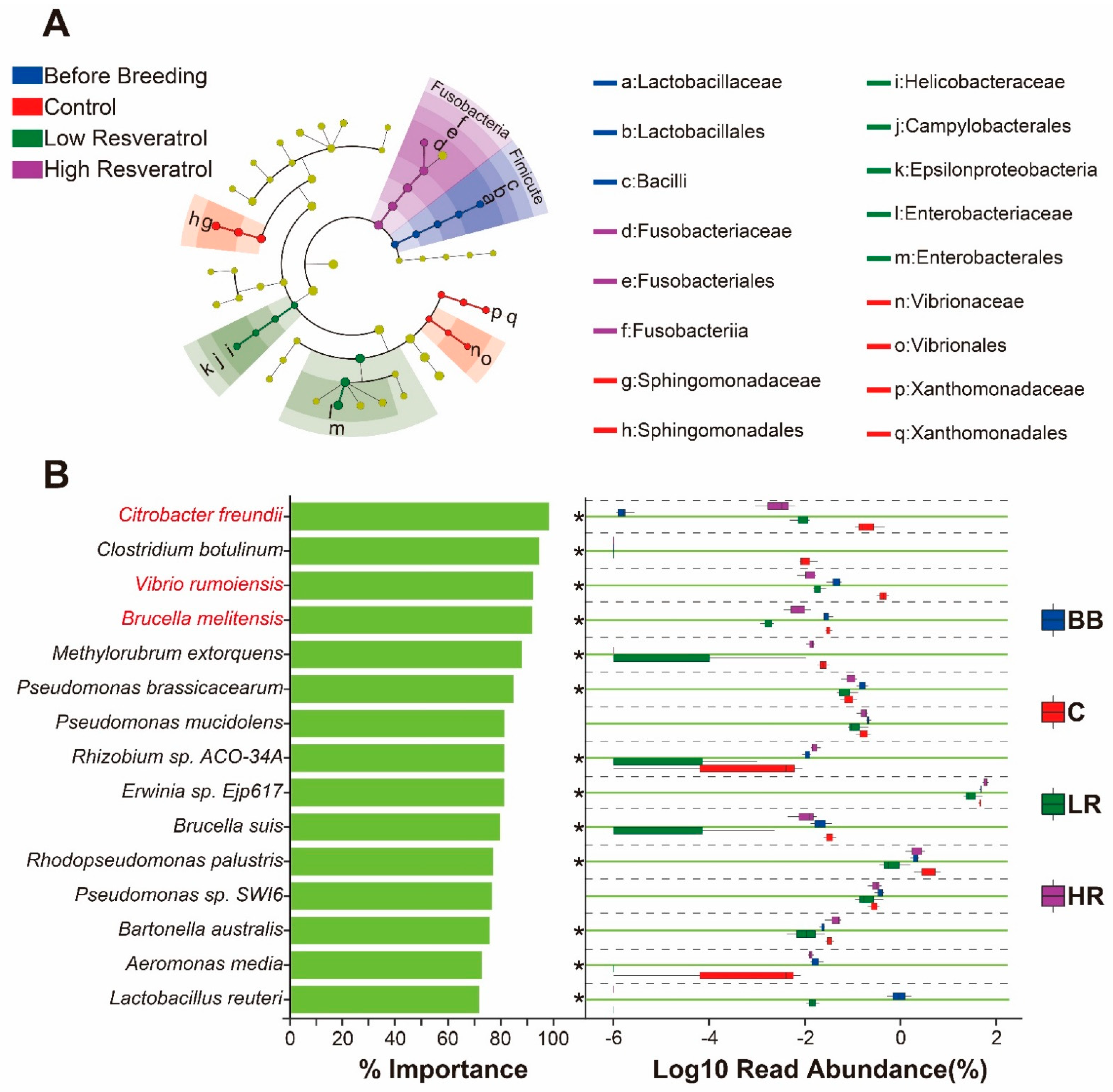
2.6. Resveratrol Reduced the Abundance of Potential Pathogens in the Duodenum of Siberian Sturgeon
3. Discussion
4. Materials and Methods
4.1. Fish and Experimental Design
4.2. Sample Collection, DNA Isolation, Library Construction, and Sequencing
4.3. Taxonomy Classification and Differential Abundance Analysis
4.4. Histological Observation
4.5. Digestive Enzyme Activity Assay of Intestine
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biltueva, L.S.; Prokopov, D.Y.; Romanenko, S.A.; Interesova, E.A.; Schartl, M.; Trifonov, V.A. Chromosome Distribution of Highly Conserved Tandemly Arranged Repetitive DNAs in the Siberian Sturgeon (Acipenser baerii). Genes 2020, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Bronzi, P.; Rosenthal, H.; Gessner, J. Global sturgeon aquaculture production: An overview. J. Appl. Ichthyol. 2011, 27, 169–175. [Google Scholar] [CrossRef]
- Abdolahnejad, Z.; Pourkazemi, M.; Khoshkholgh, M.R.; Yarmohammadi, M. Expression of growth hormone gene during early development of Siberian sturgeon (Acipenser baerii). Mol. Biol. Res. Commun. 2015, 4, 181–188. [Google Scholar] [PubMed]
- Pavasovic, M.; Richardson, N.A.; Anderson, A.J.; Mann, D.; Mather, P.B. Effect of pH, temperature and diet on digestive enzyme profiles in the mud crab, Scylla serrata. Aquaculture 2004, 242, 641–654. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and inflammation in the intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Saad, R.; Rizkallah, M.R.; Aziz, R.K. Gut Pharmacomicrobiomics: The tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 2012, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, C.; Xu, W.; Li, D.; Feng, Y.; Wu, J.; Luo, W.; Du, X.; Du, Z.; Huang, X. Heat Stress Decreases Intestinal Physiological Function and Facilitates the Proliferation of Harmful Intestinal Microbiota in Sturgeons. Front. Microbiol. 2022, 13, 755369. [Google Scholar] [CrossRef]
- Hieu, D.Q.; Hang, B.T.B.; Lokesh, J.; Garigliany, M.M.; Huong, D.T.T.; Yen, D.T.; Liem, P.T.; Tam, B.M.; Hai, D.M.; Son, V.N.; et al. Salinity significantly affects intestinal microbiota and gene expression in striped catfish juveniles. Appl. Microbiol. Biotechnol. 2022, 106, 3245–3264. [Google Scholar] [CrossRef]
- Deng, Y.; Mao, C.; Lin, Z.; Su, W.; Cheng, C.; Li, Y.; Gu, Q.; Gao, R.; Su, Y.; Feng, J. Nutrients, temperature, and oxygen mediate microbial antibiotic resistance in sea bass (Lateolabrax maculatus) ponds. Sci. Total Environ. 2022, 819, 153120. [Google Scholar] [CrossRef]
- Xia, J.H.; Lin, G.; Fu, G.H.; Wan, Z.Y.; Lee, M.; Wang, L.; Liu, X.J.; Yue, G.H. The intestinal microbiome of fish under starvation. BMC Genom. 2014, 15, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yúfera, M.; Nguyen, M.V.; Navarro-Guillén, C.; Moyano, F.J.; Jordal, A.O.; Espe, M.; Conceição, L.E.C.; Engrola, S.; Le, M.H.; Rønnestad, I. Effect of increased rearing temperature on digestive function in cobia early juvenile. Comp. Biochem. Physiology. Part A Mol. Integr. Physiol. 2019, 230, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hani, Y.M.I.; Marchand, A.; Turies, C.; Kerambrun, E.; Palluel, O.; Bado-Nilles, A.; Beaudouin, R.; Porcher, J.M.; Geffard, A.; Dedourge-Geffard, O. Digestive enzymes and gut morphometric parameters of threespine stickleback (Gasterosteus aculeatus): Influence of body size and temperature. PLoS ONE 2018, 13, e0194932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunier, P.; Peyron, D.; Trollat, P. Effect of Enological Practices on the Resveratrol Isomer Content of Wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Brockmueller, A.; Shayan, P.; Shakibaei, M. Evidence That β1-Integrin Is Required for the Anti-Viability and Anti-Proliferative Effect of Resveratrol in CRC Cells. Int. J. Mol. Sci. 2022, 23, 4714. [Google Scholar] [CrossRef]
- Moreira, H.; Szyjka, A.; Grzesik, J.; Pelc, K.; Żuk, M.; Kulma, A.; Emhemmed, F.; Muller, C.D.; Gąsiorowski, K.; Barg, E. Celastrol and Resveratrol Modulate SIRT Genes Expression and Exert Anticancer Activity in Colon Cancer Cells and Cancer Stem-like Cells. Cancers 2022, 14, 1372. [Google Scholar] [CrossRef]
- Cesmeli, S.; Goker Bagca, B.; Caglar, H.O.; Ozates, N.P.; Gunduz, C.; Biray Avci, C. Combination of resveratrol and BIBR1532 inhibits proliferation of colon cancer cells by repressing expression of LncRNAs. Med. Oncol. 2021, 39, 12. [Google Scholar] [CrossRef]
- Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. β1-Integrin plays a major role in resveratrol-mediated anti-invasion effects in the CRC microenvironment. Front. Pharmacol. 2022, 13, 978625. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Tan, Z.; Shi, L.; Xun, W. Resveratrol Attenuates Diquat-Induced Oxidative Stress by Regulating Gut Microbiota and Metabolome Characteristics in Piglets. Front. Microbiol. 2021, 12, 695155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, W.; Hu, G.; Qiu, L.; Meng, S.; Song, C.; Fan, L.; Zhao, Z.; Bing, X.; Chen, J. Gut microbiota analysis of juvenile genetically improved farmed tilapia (Oreochromis niloticus) by dietary supplementation of different resveratrol concentrations. Fish Shellfish Immunol. 2018, 77, 200–207. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Lv, H.; Pang, W.; Wang, J.; Ma, H.; Wang, S. Intestinal pharmacokinetics of resveratrol and regulatory effects of resveratrol metabolites on gut barrier and gut microbiota. Food Chem. 2021, 357, 129532. [Google Scholar] [CrossRef]
- Pan, H.H.; Zhou, X.X.; Ma, Y.Y.; Pan, W.S.; Zhao, F.; Yu, M.S.; Liu, J.Q. Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy. World J. Gastroenterol. 2020, 26, 4945–4959. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.T.; Ye, X.L.; Li, R.R.; Chen, H.; Wang, Y.Y.; Yong, H.J.; Pan, M.L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol Suppresses Cross-Talk between Colorectal Cancer Cells and Stromal Cells in Multicellular Tumor Microenvironment: A Bridge between In Vitro and In Vivo Tumor Microenvironment Study. Molecules 2020, 25, 4292. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, R.; Wei, W.; Wang, M.; Wang, S. Resveratrol reverses the cadmium-promoted migration, invasion, and epithelial-mesenchymal transition procession by regulating the expression of ZEB1. Hum. Exp. Toxicol. 2021, 40, S331–S338. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Chitrala, K.N.; Nagarkatti, M.; Nagarkatti, P. Alterations in the Gut Microbiome and Suppression of Histone Deacetylases by Resveratrol Are Associated with Attenuation of Colonic Inflammation and Protection Against Colorectal Cancer. J. Clin. Med. 2020, 9, 1796. [Google Scholar] [CrossRef]
- Faal, M.; Manouchehri, H.; Changizi, R.; Bootorabi, F.; Khorramizadeh, M.R. Assessment of resveratrol on diabetes of zebrafish (Danio rerio). J. Diabetes Metab. Disord. 2022, 21, 823–833. [Google Scholar] [CrossRef]
- Maruyama, K.; Sato, S.; Ohira, T.; Maeda, K.; Noda, H.; Kubota, Y.; Nishimura, S.; Kitamura, A.; Kiyama, M.; Okada, T.; et al. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: Cross sectional survey. BMJ 2008, 337, a2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Hou, W.; Wang, F.; Arcan, C. Factors Affecting Obesity and Waist Circumference among US Adults. Prev. Chronic Dis. 2019, 16, E02. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Yan, Y.; Tian, H.; Jiang, G.; Li, X.; Liu, W. Resveratrol supplementation improves lipid and glucose metabolism in high-fat diet-fed blunt snout bream. Fish Physiol. Biochem. 2018, 44, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Wierdsma, N.J.; Peters, J.H.; van Bokhorst-de van der Schueren, M.A.; Mulder, C.J.; Metgod, I.; van Bodegraven, A.A. Bomb calorimetry, the gold standard for assessment of intestinal absorption capacity: Normative values in healthy ambulant adults. J. Hum. Nutr. Diet. 2014, 27, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Effect of some phenolic compounds and beverages on pepsin activity during simulated gastric digestion. J. Agric. Food Chem. 2005, 53, 8706–8713. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Yan, Q.; Yu, Y.; Zhang, T. Factors influencing the grass carp gut microbiome and its effect on metabolism. FEMS Microbiol. Ecol. 2014, 87, 704–714. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Afrose, S.; Hossain, M.S.; Salma, U.; Miah, A.G.; Tsujii, H. Dietary Karaya Saponin and Rhodobacter capsulatus Exert Hypocholesterolemic Effects by Suppression of Hepatic Cholesterol Synthesis and Promotion of Bile Acid Synthesis in Laying Hens. Cholesterol 2010, 2010, 272731. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, Y.L.; Wang, W.K.; Zhang, Z.W.; Si, X.M.; Cao, Z.J.; Li, S.L.; Yang, H.J. Beneficial effect of Rhodopseudomonas palustris on in vitro rumen digestion and fermentation. Benef. Microbes 2020, 11, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, J.; Chertkov, O.; Lapidus, A.; Nolan, M.; Lucas, S.; Del Rio, T.G.; Tice, H.; Cheng, J.F.; Tapia, R.; Han, C.; et al. Complete genome sequence of Ilyobacter polytropus type strain (CuHbu1). Stand. Genom. Sci. 2010, 3, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Allen-Vercoe, E.; Strauss, J.; Chadee, K. Fusobacterium nucleatum: An emerging gut pathogen? Gut Microbes 2011, 2, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Heymans, R.; Vila, A.; van Heerwaarden, C.A.M.; Jansen, C.C.C.; Castelijn, G.A.A.; van der Voort, M.; Biesta-Peters, E.G. Rapid detection and differentiation of Salmonella species, Salmonella Typhimurium and Salmonella Enteritidis by multiplex quantitative PCR. PLoS ONE 2018, 13, e0206316. [Google Scholar] [CrossRef] [Green Version]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Tian, T.; Xiao, K.; Zeng, Q.; Tan, C.; Du, H. Pathogenic infection and immune-related gene expression of Chinese sturgeon (Acipenser sinensis) challenged by Citrobacter freundii. Dev. Comp. Immunol. 2021, 114, 103872. [Google Scholar] [CrossRef]
- Ji, Q.; Wang, S.; Ma, J.; Liu, Q. A review: Progress in the development of fish Vibrio spp. vaccines. Immunol. Lett. 2020, 226, 46–54. [Google Scholar] [CrossRef]
- El-Tras, W.F.; Tayel, A.A.; Eltholth, M.M.; Guitian, J. Brucella infection in fresh water fish: Evidence for natural infection of Nile catfish, Clarias gariepinus, with Brucella melitensis. Vet. Microbiol. 2010, 141, 321–325. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Okamoto-Shibayama, K.; Yoshida, A.; Ishihara, K. Inhibitory Effect of Resveratrol on Candida albicans Biofilm Formation. Bull. Tokyo Dent. Coll. 2021, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Peng, S.; Chen, D.; Yang, F.; Liu, J.; Wang, J.; Liu, Q.; Huang, X.; Ouyang, P.; Wang, K.; et al. Low lethal doses of Streptococcus iniae caused enteritis in Siberian sturgeon (Acipenser baerii). Fish Shellfish Immunol. 2020, 104, 654–662. [Google Scholar] [CrossRef] [PubMed]



| Sample Category | Replicate | IBW (g/fish) | FBW (g/fish) | PWG (%) |
|---|---|---|---|---|
| Before breeding | BB1 | 248.3 ± 2.6 a | / | / |
| BB2 | 249.8 ± 5.3 a | / | / | |
| BB3 | 251.2 ± 4.3 a | / | / | |
| Control | C1 | 247.9 ± 5.1 a | 357.6 ± 8.6 b | 44.53 c |
| C2 | 250.5 ± 2.9 a | 364.8 ± 7.0 b | 45.63 c | |
| C3 | 249.9 ± 6.5 a | 371.0 ± 15.6 b | 48.46 c | |
| Low resveratrol | LR1 | 253.2 ± 3.8 a | 372.4 ± 13.5 b | 47.08 c |
| LR2 | 252.3 ± 4.3 a | 369.1 ± 9.5 b | 46.29 c | |
| LR3 | 249.4 ± 1.6 a | 360.5 ± 6.3 b | 44.55 c | |
| High resveratrol | HR1 | 255.1 ± 5.6 a | 378.5 ± 10.1 b | 48.37 c |
| HR2 | 249.7 ± 5.8 a | 374.8 ± 13.0 b | 50.01 c | |
| HR3 | 251.9 ± 6.5 a | 366.1 ± 6.5 b | 45.34 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Xu, W.; Feng, L.; Zhang, C.; Yan, C.; Zhang, J.; Lai, J.; Yan, T.; He, Z.; Du, X.; et al. Resveratrol Improves the Digestive Ability and the Intestinal Health of Siberian Sturgeon. Int. J. Mol. Sci. 2022, 23, 11977. https://doi.org/10.3390/ijms231911977
Yang S, Xu W, Feng L, Zhang C, Yan C, Zhang J, Lai J, Yan T, He Z, Du X, et al. Resveratrol Improves the Digestive Ability and the Intestinal Health of Siberian Sturgeon. International Journal of Molecular Sciences. 2022; 23(19):11977. https://doi.org/10.3390/ijms231911977
Chicago/Turabian StyleYang, Shiyong, Wenqiang Xu, Langkun Feng, Chaoyang Zhang, Chaozhan Yan, Jiajin Zhang, Jiansheng Lai, Taiming Yan, Zhi He, Xiaogang Du, and et al. 2022. "Resveratrol Improves the Digestive Ability and the Intestinal Health of Siberian Sturgeon" International Journal of Molecular Sciences 23, no. 19: 11977. https://doi.org/10.3390/ijms231911977






