Microbiome of Nodules and Roots of Soybean and Common Bean: Searching for Differences Associated with Contrasting Performances in Symbiotic Nitrogen Fixation
Abstract
1. Introduction
2. Results
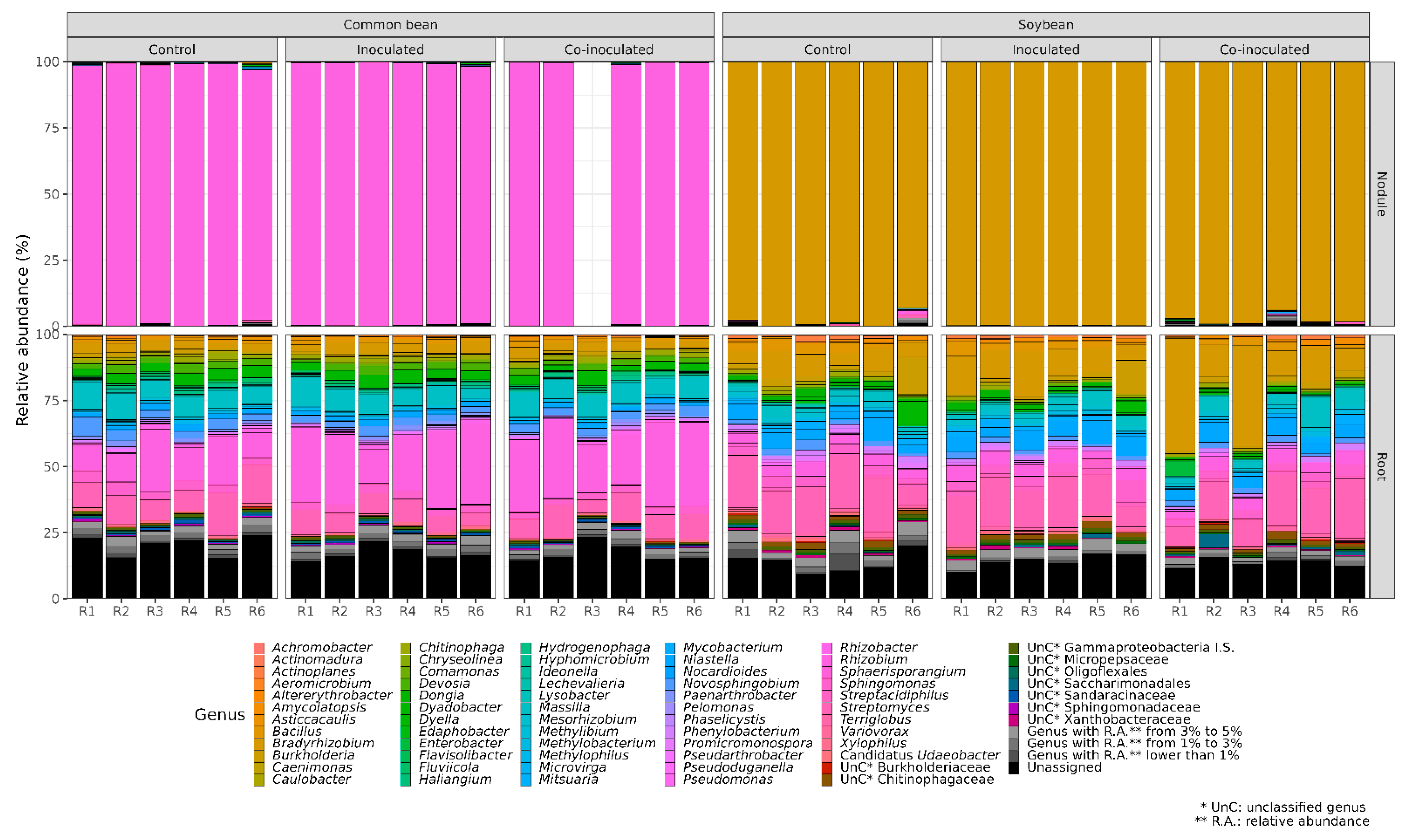
2.1. Relative Abundance of Bradyrhizobium on Soybean Nodules and Roots
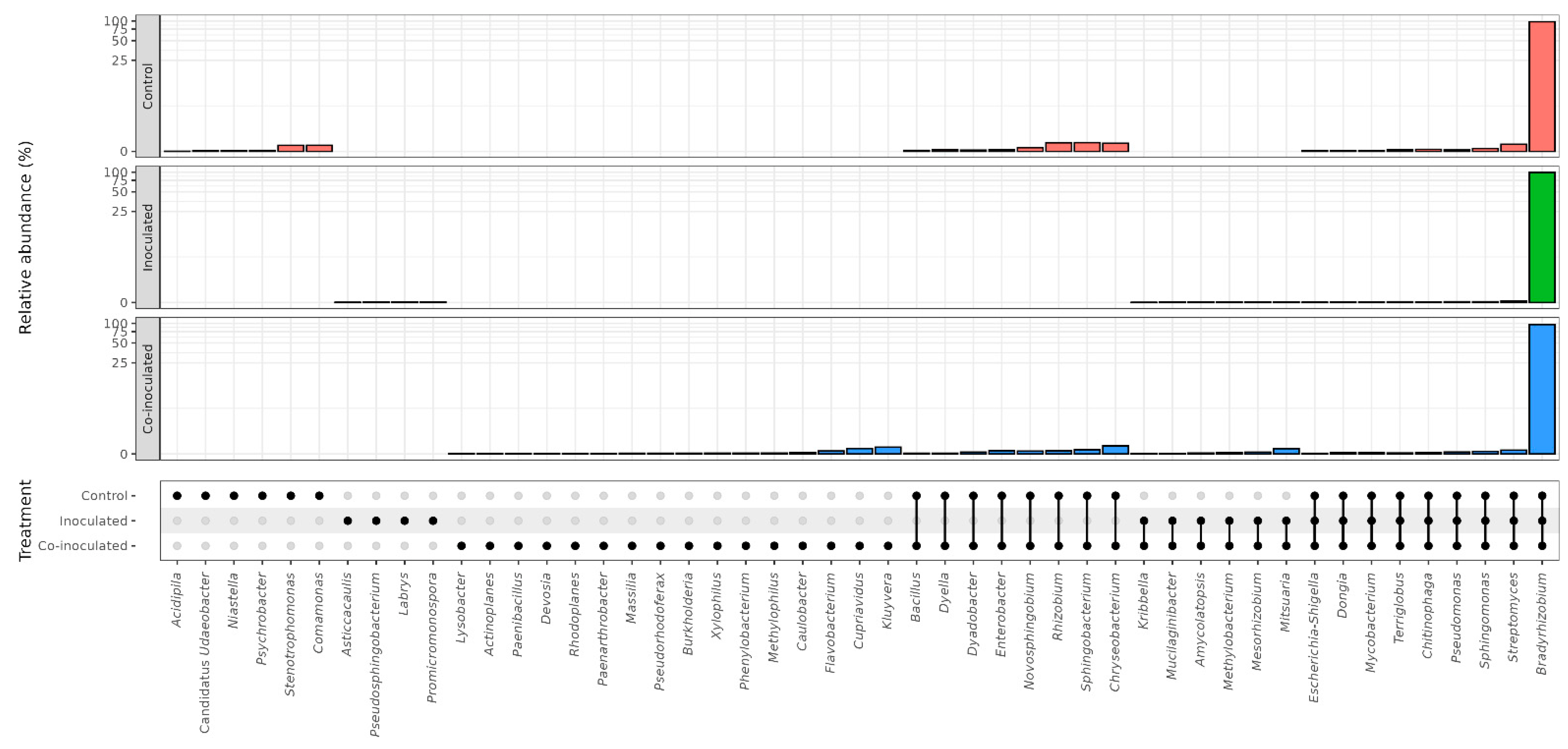
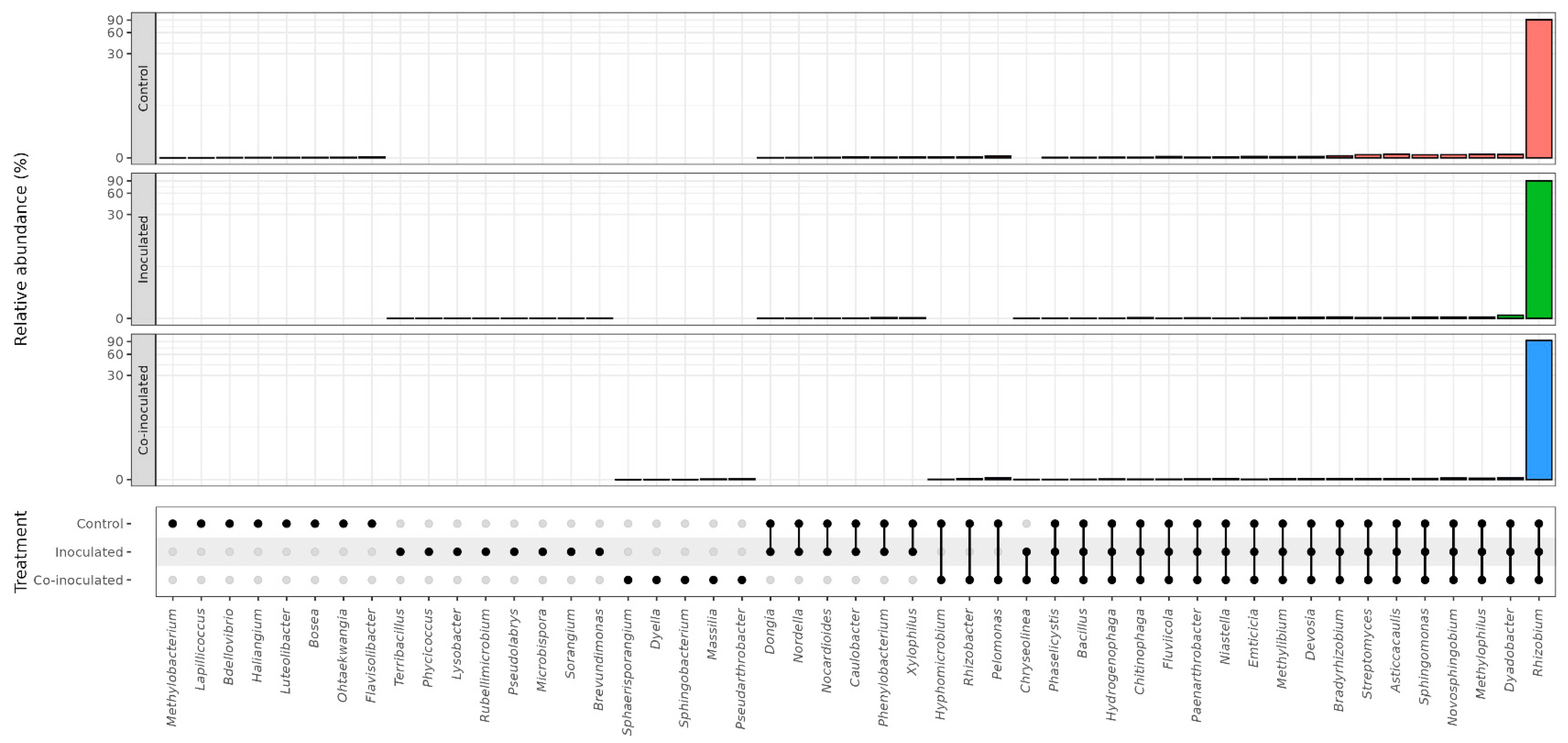
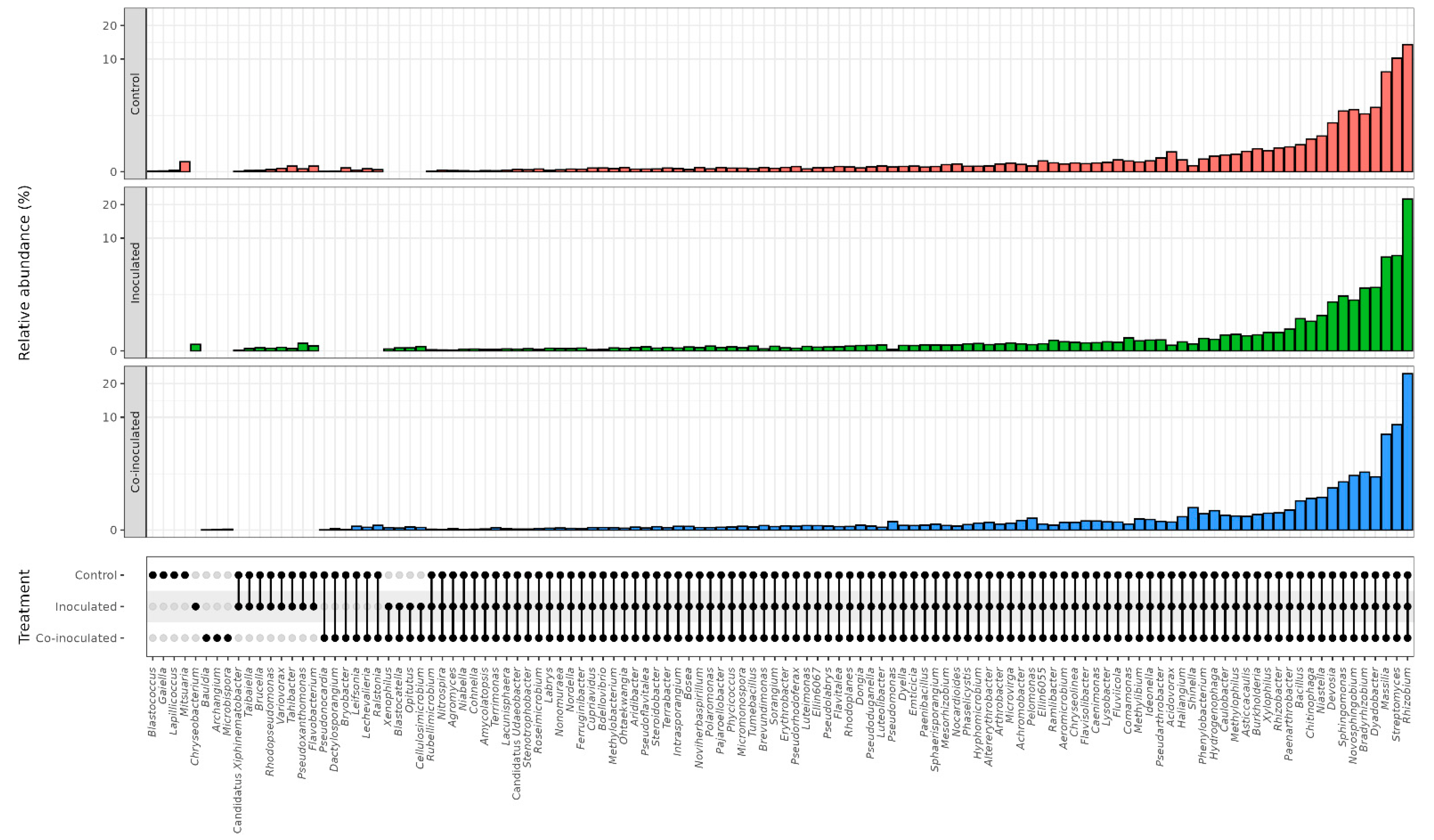
2.2. Microbial Genera Identified in the Microbiomes of Soybean Nodules
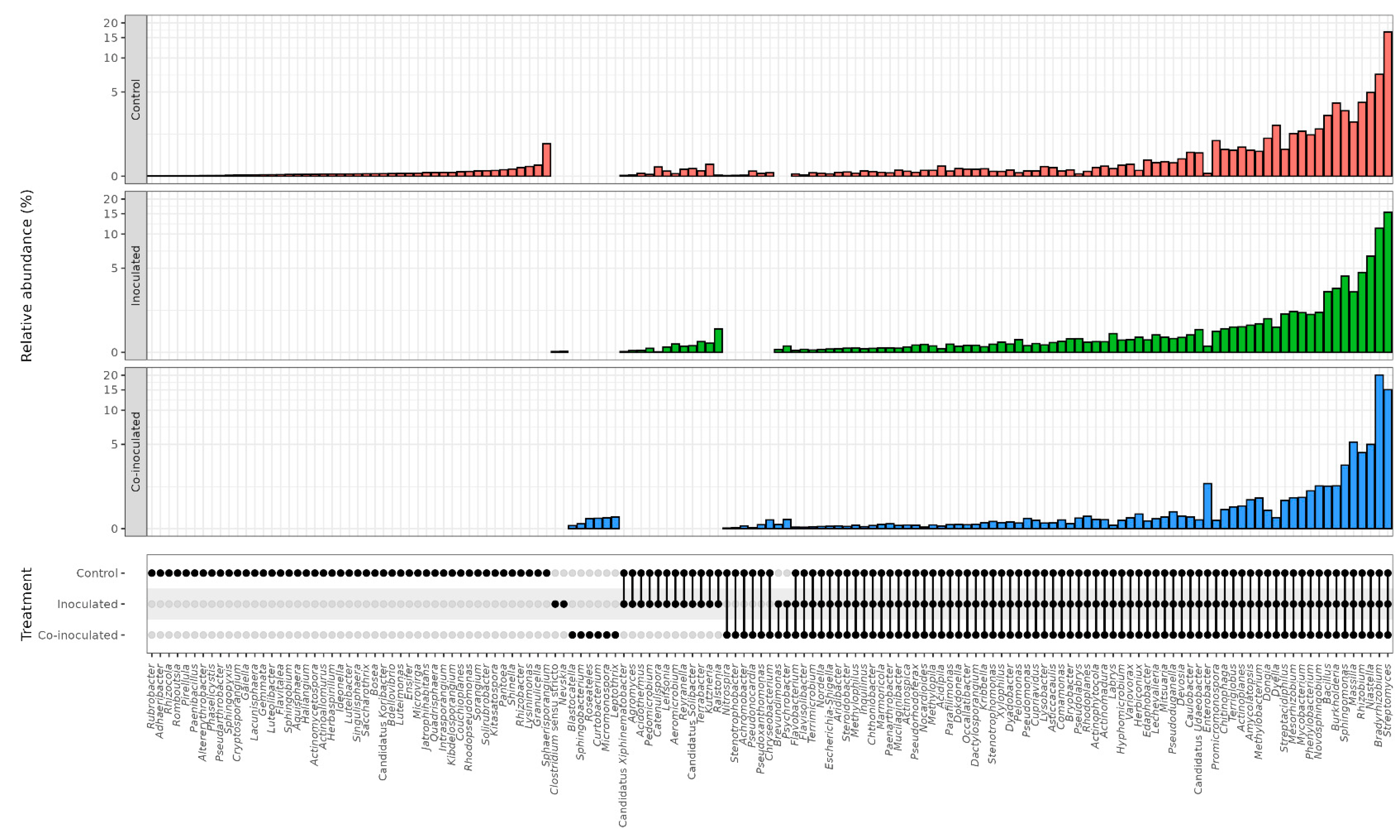
2.3. Microbial Genera Identified in the Microbiomes of Soybean Roots
2.4. Relative Abundance of Rhizobium on Common Bean Nodules and Roots
2.5. Microbial Genera Identified in the Microbiomes of Common Bean Nodules
2.6. Microbial Genera Identified in the Microbiomes of Common Bean Roots
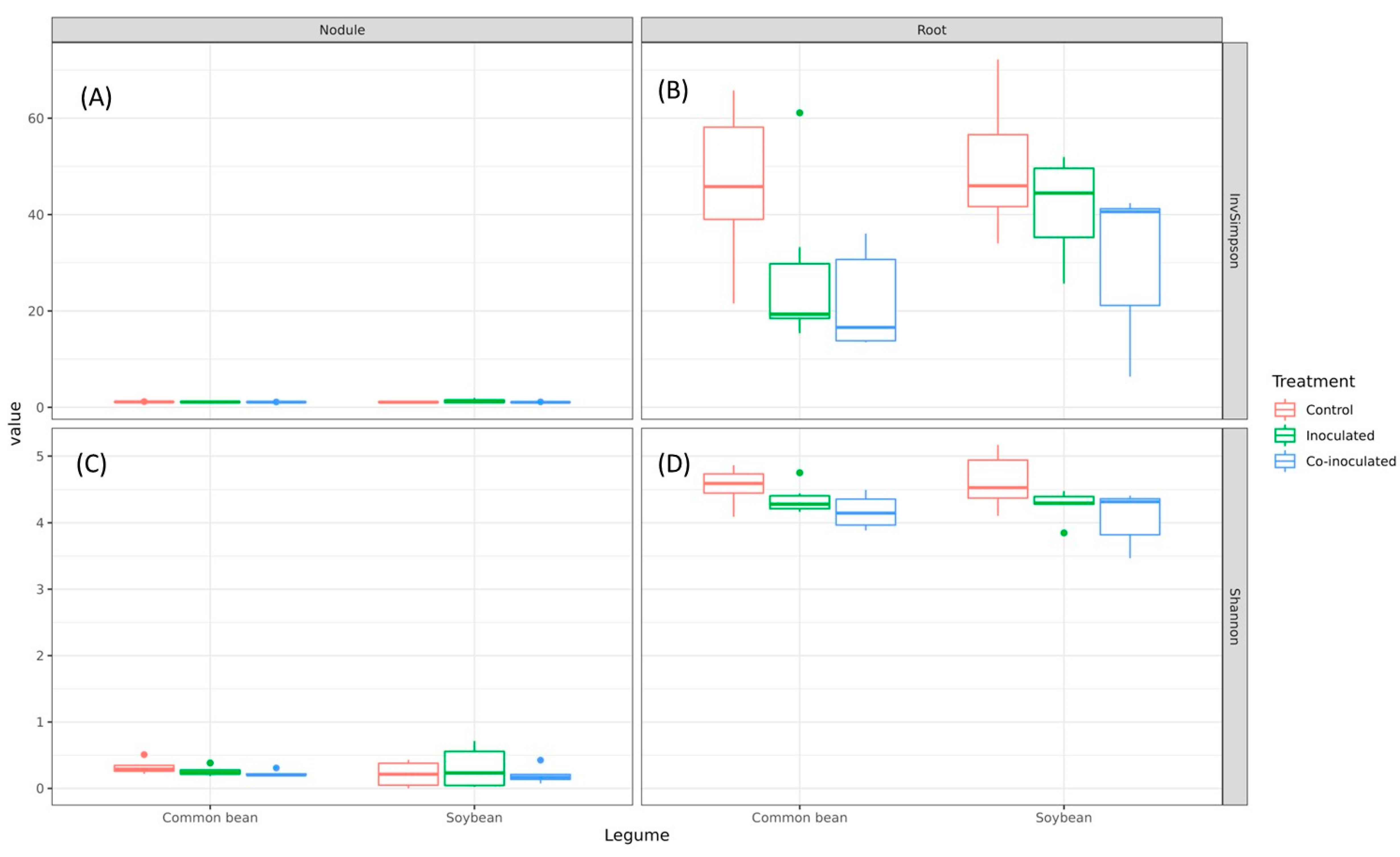
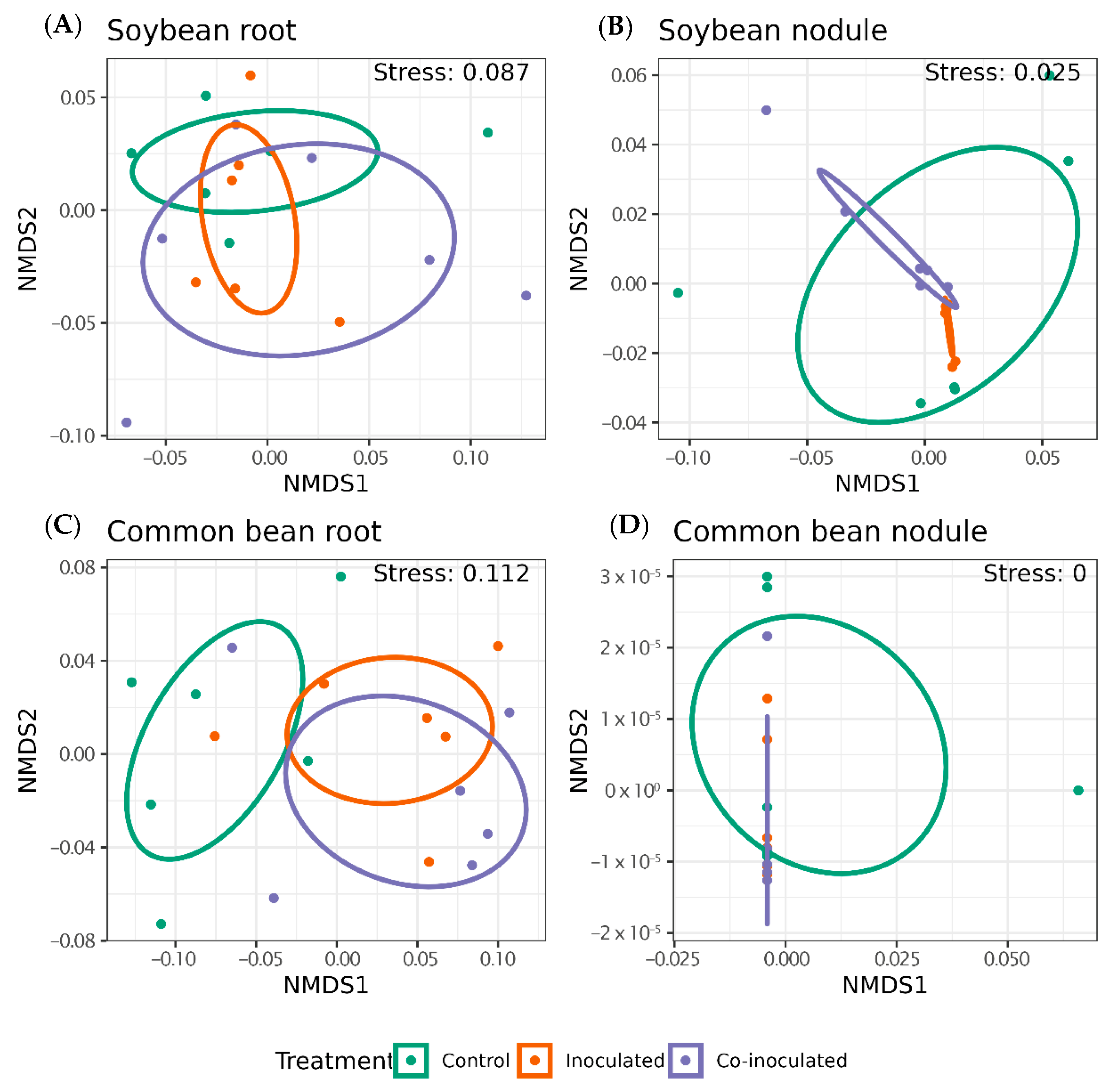
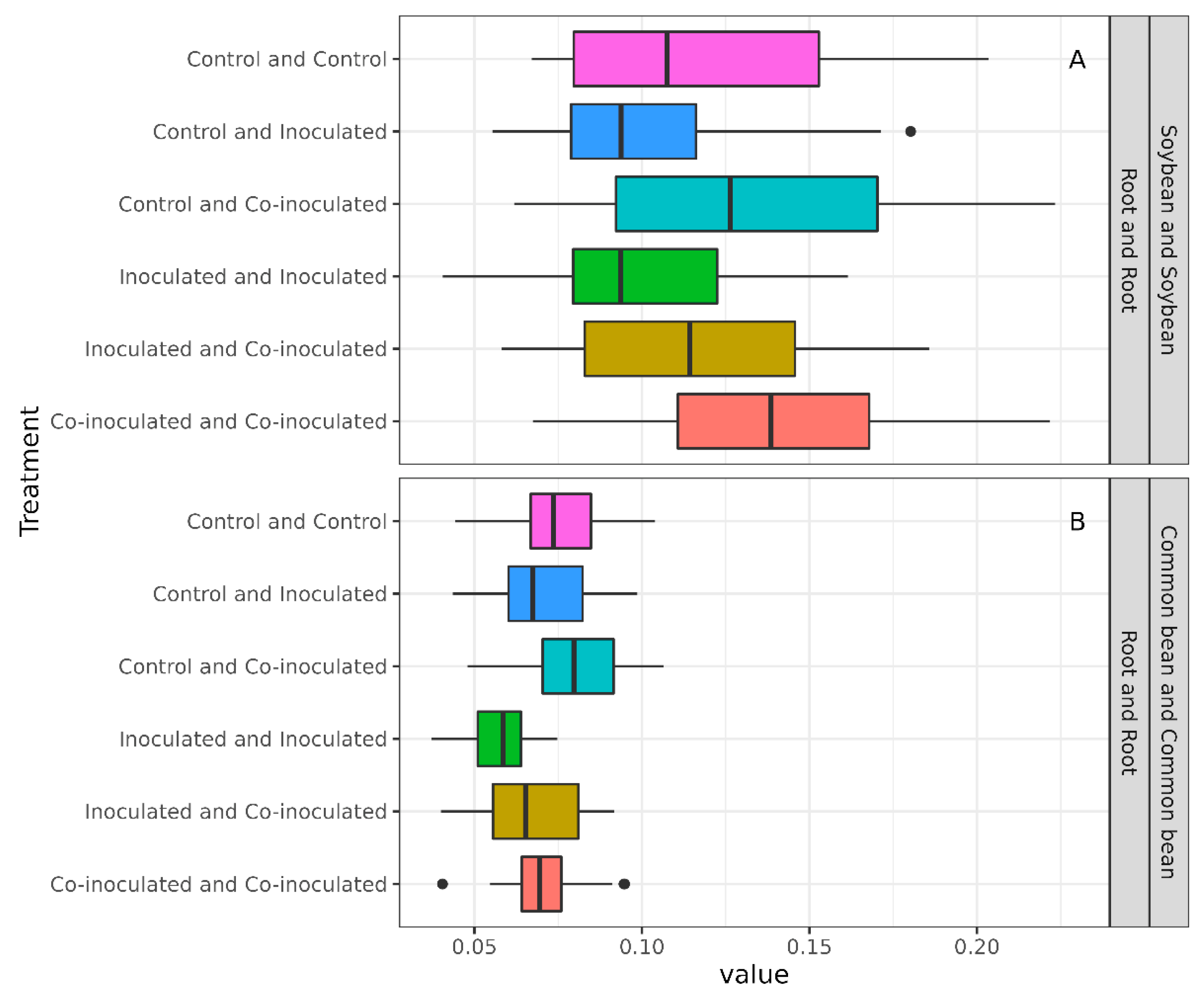
2.7. Diversity of Microbiomes from Soybean and Common-Bean Nodules and Roots
3. Discussion
4. Material and Methods
4.1. Plant Growth Conditions
4.1.1. Soybean
4.1.2. Common Bean
4.2. DNA Extraction, Sequencing, and Microbiome Analysis
4.2.1. Samples Preparation
4.2.2. DNA Extraction
4.2.3. Preparation of 16S rRNA Amplicon Libraries and Sequencing
4.2.4. Bioinformatics and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bruijn, F.J. (Ed.) The quest for biological nitrogen fixation in cereals: A perspective and prospective. In Biological Nitrogen Fixation; John Wiley & Son: Hoboken, NJ, USA, 2015; Volume 2, pp. 1087–1101. [Google Scholar] [CrossRef]
- Ormeño-Orrillo, E.; Hungria, M.; Martinez-Romero, E. Dinitrogen-fixing prokaryotes. In The Prokaryotes: Prokaryotic Physiology and Biochemistry; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 427–451. [Google Scholar]
- Herridge, D.F.; Giller, K.E.; Jensen, E.S.; Peoples, M.B. Quantifying country-to-global scale nitrogen fixation for crop legumes. II. Coefficients, templates and estimates for soybean, groundnut and pulses. Plant Soil 2022, 474, 1–15. [Google Scholar] [CrossRef]
- Hungria, M.; Mendes, I.C. Nitrogen fixation with soybean: The perfect symbiosis. In Biological Nitrogen Fixation; De Bruijn, F.J., Ed.; John Wiley & Son: Hoboken, NJ, USA, 2015; Volume 2, pp. 1005–1019. [Google Scholar]
- Peoples, M.B.; Giller, K.E.; Jensen, E.S.; Herridge, D.F. Quantifying country-to-global scale nitrogen fxation for grain legumes: I. Reliance on nitrogen fxation of soybean, groundnut and pulses. Plant Soil 2021, 469, 1–14. [Google Scholar] [CrossRef]
- Hardarson, G. Methods for enhancing symbiotic nitrogenfixation. Plant Soil 1993, 152, 1–17. [Google Scholar] [CrossRef]
- Graham, P.H. Some problems of nodulation and symbiotic nitrogen fixation in Phaseolus vulgaris L.: A review. Field Crops Res. 1981, 4, 93–112. [Google Scholar] [CrossRef]
- Hungria, M.; Andrade, D.S.; Chueire, L.M.O.; Probanza, A.; Guttierrez-Mañero, F.J.; Megías, M. Isolation and characterization of new efficient and competitive bean (Phaseolus vulgaris L.) rhizobia from Brazil. Soil Biol. Biochem. 2000, 32, 1515–1528. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Mendes, I.C. Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol. Fertil. Soils 2003, 39, 88–93. [Google Scholar] [CrossRef]
- Gomes, D.F.; Ormeño-Orrillo, E.; Hungria, M. Biodiversity, symbiotic efficiency and genomics of Rhizobium tropici and related species. In Biological Nitrogen Fixation; De Bruijn, F.J., Ed.; John Wiley & Son: Hoboken, NJ, USA, 2015; Volume 2, pp. 747–756. [Google Scholar] [CrossRef]
- Mendes, I.C.; Vargas, M.A.T.; Hungria, M. Establishment of Bradyrhizobium japonicum and B. elkanii in a Brazilian Cerrados oxisol. Biol. Fertil. Soils 2004, 40, 28–35. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Hungria, M. Recovery of soybean inoculant strains from uncropped soils in Brazil. Field Crops Res. 2002, 79, 139–152. [Google Scholar] [CrossRef]
- Galli-Terasawa, L.V.; Glienke-Blanco, C.; Hungria, M. Diversity of soybean rhizobial population adapted to a Cerrados soil. World J. Microbiol. Biotechnol. 2003, 19, 933–939. [Google Scholar] [CrossRef]
- Grange, L.; Hungria, M. Genetic diversity of indigenous common bean (Phaseolus vulgaris) rhizobia in two Brazilian ecosystems. Soil Biol. Biochem 2004, 36, 1389–1398. [Google Scholar] [CrossRef]
- Alberton, O.; Kaschuk, G.; Hungria, M. Sampling effects on the assessment of genetic diversity of rhizobia associated with soybean and common bean. Soil Biol. Biochem. 2006, 38, 1298–1307. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Barcellos, F.G.; Thompson, F.L.; Hungria, M. Multilocus sequence analysis of Brazilian Rhizobium strains microsymbionts of common beans (Phaseolus vulgaris) reveals unexpected taxonomic diversity. Res. Microbiol. 2009, 160, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Ormeño-Orrillo, E.; Dall’Agnol, R.F.; Graham, P.H.; Martínez-Romero, E.; Hungria, M. Novel Rhizobium lineages isolated from root nodules of common bean (Phaseolus vulgaris L.) in Andean and Mesoamerican areas. Res. Microbiol. 2013, 164, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.; Chibeba, A.M.; Mercante, F.M.; Hungria, M. Polyphasic characterization of rhizobia microsymbionts of common bean [Phaseolus vulgaris (L.)] isolated in Mato Grosso do Sul, a hotspot of Brazilian biodiversity. Symbiosis 2018, 76, 163–176. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Babalola, O.O. Perspectives for sustainable agriculture from the microbiome in plant rhizosphere. Plant Biotechnol. Rep. 2021, 15, 259–278. [Google Scholar] [CrossRef]
- Santos, L.F.; Olivares, F.L. Plant microbiome structure and benefits for sustainable agriculture. Curr. Plant Biol. 2021, 26, 100198. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.C.; Rocha-Granados, M.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Arif, I.; Batool, M.; Schenk, P.M. Plant microbiome engineering: Expected benefits for improved crop growth and resilience. Trends Biotechnol. 2020, 38, 1385–1396. [Google Scholar] [CrossRef]
- Basile, L.A.; Lepek, V.C. Legume–rhizobium dance: An agricultural tool that could be improved? Microb. Biotechnol. 2021, 14, 1897–1917. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Campos, L.J.M.; Menna, P.; Brandi, F.; Ramos, Y.G. Seed pre-inoculation with Bradyrhizobium as time-optimizing option for large-scale soybean cropping systems. Agron. J. 2020, 112, 5222–5236. [Google Scholar] [CrossRef]
- Sharaf, H.; Rodrigues, R.R.; Moon, J.; Zhang, B.; Mills, K.; Williams, M.A. Unprecedented bacterial community richness in soybean nodules vary with cultivar and water status. Microbiome 2019, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.B.; Mendes, L.W.; Oliveira, L.M.S.; Melo, V.M.M.; Antunes, J.E.L.; Araujo, F.F.; Hungria, M.; Araujo, A.S.F. Nodule microbiome from cowpea and lima bean grown in composted tannery sludge-treated soil. Appl. Soil Ecol. 2020, 151, 103542. [Google Scholar] [CrossRef]
- Bakhtiyarifar, M.; Enayatizamir, N.; Khanlou, K.M. Biochemical and molecular investigation of non-rhizobial endophytic bacteria as potential biofertilisers. Arch. Microbiol. 2021, 203, 513–521. [Google Scholar] [CrossRef]
- Preyanga, R.; Anandham, R.; Krishnamoorthy, R.; Senthilkumar, M.; Gopal, N.O.; Vellaikumar, A.; Meena, S. Groundnut (Arachis hypogaea) nodule Rhizobium and passenger endophytic bacterial cultivable diversity and their impact on plant growth promotion. Rhizosphere 2021, 17, 100309. [Google Scholar] [CrossRef]
- Delamuta, J.R.M.; Scherer, A.J.; Ribeiro, R.A.; Hungria, M. Genetic diversity of Agrobacterium species isolated from nodules of common bean and soybean in Brazil, Mexico, Ecuador and Mozambique, and description of the new species Agrobacterium fabacearum sp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 4233–4244. [Google Scholar] [CrossRef]
- Schaedel, B.M.; Hidrobo, G.; Grossman, J. From microns to meters: Exploring advances in legume microbiome diversity for agroecosystem. Front. Sustain. Food 2021, 5, 668195. [Google Scholar] [CrossRef]
- Crosbie, D.B.; Mahmoudi, M.; Radl, V.; Schloter, M.; Kemen, E.; Marín, M. Microbiome profiling reveals that Pseudomonas antagonizes parasitic nodule colonization of cheater rhizobia in Lotus. New Phytol. 2022, 234, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Minami, M.; Yamamoto, A.; Akao, S. Estimation of the bacterial community diversity of soybean-nodulating bradyrhizobia isolated from Rj-genotype soybeans. Soil Sci. Plant Nutr. 2008, 54, 718–724. [Google Scholar] [CrossRef]
- Mayhood, P.; Mirza, B.S. Soybean root nodule and rhizosphere microbiome: Distribution of rhizobial and nonrhizobial endophytes. Appl. Environ. Microbiol. 2021, 87, e02884-20. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 2015, 6, 811–817. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; Sena, J.V.S.; Poggere, G.; Reis, A.R.; Corrêa, R.S. Meta-analysis reveals benefits of co-inoculation of soybean with Azospirillum brasilense and Bradyrhizobium spp. in Brazil. Appl. Soil Ecol. 2021, 163, 103913. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding impact of Azospirillum brasilense strains Ab-V5 and Ab-V6 on the Brazilian agriculture: Lessons that farmers are receptive to adopt new microbial inoculants. Rev. Bras. Cienc. Solo 2021, 45, e0200128. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech. 2015, 5, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.I.; Dakora, F.D. Rhizobia as a source of plant growth-promoting molecules: Potential applications and possible operational mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Aserse, A.A.; Räsänen, L.A.; Aseffa, F.; Hailemariam, A.; Lindström, K. Diversity of sporadic symbionts and nonsymbiotic endophytic bacteria isolated from nodules of woody, shrub, and food legumes in Ethiopia. Appl. Microbiol. Biotechnol. 2013, 97, 10117–10134. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, W.; Zong, L.; Yang, J.; Jiao, S.; Lin, Y.; Wang, E.; Wei, G. Two cultivated legume plants reveal the enrichment process of the microbiome in the rhizocompartments. Mol. Ecol. 2017, 26, 1641–1651. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, H. Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz. J. Microbiol. 2018, 49, 269–278. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, Y., Jr.; Chen, W.F.; Wang, E.T.; Tian, C.F.; Li, Q.Q.; Zhang, Y.Z.; Sui, X.H.; Chen, W.X. Biodiversity and biogeography of rhizobia associated with soybean plants grown in the North China Plain. Appl. Environ. Microbiol. 2011, 77, 6331–6342. [Google Scholar] [CrossRef]
- Cardoso, J.D.; Hungria, M.; Andrade, D.S. Polyphasic approach for the characterization of rhizobial symbionts effective in fixing N2 with common bean (Phaseolus vulgaris L.). Appl. Microbiol. Biotechnol. 2012, 93, 2035–2049. [Google Scholar] [CrossRef]
- Moura, F.T.; Ribeiro, R.A.; Helene, L.C.F.; Nogueira, M.A.; Hungria, M. So many rhizobial partners, so little nitrogen fixed: The intriguing symbiotic promiscuity of common bean (Phaseolus vulgaris L.). Symbiosis 2022, 86, 169–185. [Google Scholar] [CrossRef]
- Dall´Agnol, R.F.; Bournaud, C.; De Faria, S.M.; Béna, G.; Moulin, L.; Hungria, M. Genetic diversity of symbiotic Paraburkholderia species isolated from nodules of Mimosa pudica (L.) and Phaseolus vulgaris (L.) grown in soils of the Brazilian Atlantic Forest (Mata Atlântica). FEMS Microbiol. Ecol. 2017, 93, fix027. [Google Scholar] [CrossRef]
- Efstathiadou, E.; Ntatsi, G.; Savvas, D.; Tampakak, A.P. Genetic characterization at the species and symbiovar level of indigenous rhizobial isolates nodulating Phaseolus vulgaris in Greece. Sci. Rep. 2021, 11, 8674. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.; Fischer, D.; Rouws, L.F.M.; Fernandes-Júnior, P.I.; Hofmann, A.; Kublik, S.; Schloter, M.; Xavier, G.R.; Radl, V. Cowpea nodules harbor non-rhizobial bacterial communities that are shaped by soil type rather than plant genotype. Front. Plant Sci. 2016, 7, 2064. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Mendoza-Suárez, M.; Andersen, S.U.; Poole, P.S.; Sánchez-Cañizares, C. Competition, nodule occupancy, and persistence of inoculant strains: Key factors in the Rhizobium-legume symbioses. Front. Plant Sci. 2021, 12, 690567. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Gutiérrez, J.P.; Lopez-Lavalle, L.A.B. Seed-transmitted bacteria and fungi dominate juvenile plant microbiomes. Front. Microbiol. 2021, 12, 737616. [Google Scholar] [CrossRef]
- Medina-Paz, F.; Herrera-Estrella, L.; Heil, M. All set before flowering: A 16S gene amplicon-based analysis of the root microbiome recruited by common bean (Phaseolus vulgaris) in its centre of domestication. Plants 2022, 11, 1631. [Google Scholar] [CrossRef]
- Barraza, A.; Vizuet-De-Rueda, J.C.; Alvarez-Venegas, R. Highly diverse root endophyte bacterial community is driven by growth substrate and is plant genotype-independent in common bean (Phaseolus vulgaris L.). PeerJ. 2020, 8, e9423. [Google Scholar] [CrossRef] [PubMed]
- Barelli, L.; Waller, A.S.; Behie, S.W.; Bidochka, M.J. Plant microbiome analysis after Metarhizium amendment reveals increases in abundance of plant growth-promoting organisms and maintenance of diseasesuppressive soil. PLoS ONE 2020, 15, e0231150. [Google Scholar] [CrossRef] [PubMed]
- Rascovan, N.; Carbonetto, B.; Perrig, D.; Díaz, M.; Canciani, W.; Abalo, M.; Alloati, J.; González-Anta, G.; Vazquez, M.P. Integrated analysis of root microbiomes of soybean and wheat from agricultural fields. Sci. Rep. 2016, 6, 28084. [Google Scholar] [CrossRef] [PubMed]
- Stopnisek, N.; Shade, A. Persistent microbiome members in the common bean rhizosphere: An integrated analysis of space, time, and plant genotype. ISME J. 2021, 15, 2708–2722. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Wan, T.T.; Zhao, H.H.; Wang, W. Effects of the biocontrol agent Bacillus amyloliquefaciens SN16-1 on the rhizosphere bacterial community and growth of tomato. J. Phytopathol. 2018, 166, 324–332. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Yang, R.; Zhao, W.; Zhu, D.; Zhang, X.; Huang, Z. Bacillus amyloliquefaciens FH-1 signifcantly afects cucumber seedlings and the rhizosphere bacterial community but not soil. Sci. Rep. 2021, 11, 12055. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, D.; Liu, X.; Gao, G.; Meng, F.; Liu, B.; Xing, P.; Jiang, X.; Ma, M.; Cao, F.; et al. Profound change in soil microbial assembly process and co-occurrence pattern in co-inoculation of Bradyrhizobium japonicum 5038 and Bacillus aryabhattai MB35-5 on soybean. Front. Microbiol. 2022, 13, 846359. [Google Scholar] [CrossRef]
- Coniglio, A.; Larama, G.; Molina, R.; Mora, V.; Torres, D.; Marin, A.; Avila, A.I.; Noircarlan, C.L.; Erijman, L.; Figuerola, E.L.; et al. Modulation of maize rhizosphere microbiota composition by inoculation with Azospirillum argentinense Az39 (Formerly A. brasilense Az39). J. Soil Sci. Plant Nutr. 2022, 22, 3553–3567. [Google Scholar] [CrossRef]
- Baudoin, F.; Nazaret, S.; Mougel, C.; Ranjard, L.; Moënne-Loccoz, Y. Impact of inoculation with the phytostimulatory PGPR Azospirillum lipoferum CRT1 on the genetic structure of the rhizobacterial community of field-grown maize. Soil Biol. Biochem. 2009, 1, 409–413. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 2017, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.; Mirza, B.S.; Imran, A.; Zaheer, A.; Yasmin, S.; Mubeen, F.; Mclean, J.E.; Mirza, M.S. llumina sequencing of 16S rRNA tag shows disparity in rhizobial and nonrhizobial diversity associated with root nodules of mung bean (Vigna radiata L.) growing in different habitats in Pakistan. Microbiol. Res. 2020, 231, 126356. [Google Scholar] [CrossRef] [PubMed]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere signaling: Insights into plant–rhizomicrobiome interactions for sustainable agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Tringe, S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef]
- O’Hara, G.W.; Hungria, M.; Woomer, P.; Howieson, J.G. Counting rhizobia. In Working with Rhizobia; Howieson, J.G., Dilworth, M.J., Eds.; Australian Center for International Agricultural Research (ACIAR): Canberra, Australia, 2016; pp. 109–124. [Google Scholar]
- Hungria, M.; O’Hara, G.W.; Zilli, J.E.; Araujo, R.S.; Deaker, R.; Howieson, J.G. Isolation and growth of rhizobia. In Working with Rhizobia; Howieson, J.G., Dilworth, M.J., Eds.; ACIAR: Canberra, Australia, 2016; pp. 39–60. [Google Scholar]
- Fukami, J.; Abrantes, J.L.F.; Del Cerro, P.; Nogueira, M.A.; Ollero, F.J.; Megías, M.; Hungria, M. Revealing different strategies of quorum sensing in Azospirillum brasilense strains Ab-V5 and Ab-V6. Arch. Microbiol. 2018, 200, 47–56. [Google Scholar] [CrossRef]
- Chibeba, A.M.; Kyei-Boahen, S.; Guimarães, M.F.; Nogueira, M.A.; Hungria, M. Isolation, characterization and selection of indigenous Bradyrhizobium strains with outstanding symbiotic performance to increase soybean yields in Mozambique. Agric. Ecosyst. Environ. 2017, 246, 291–305. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of genus 16S ribosomal RNA gene PCR primers for classical and next-genustion sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Yourstone, S.; Mieczkowski, P.; Jones, C.D.; Dangl, J.L. Practical innovations for high-throughput amplicon sequencing. Nat. Methods 2013, 10, 999–1002. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. Vsearch: A versatile open source tool for metagenomics. PeerJ. 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 September 2022).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 1 September 2022).
- Krassowski, M.; Arts, M.; Lagger, C. Krassowski/Complex-Upset: v1.3.3. Zenodo. 2020. Available online: http://doi.org/10.5281/zenodo.3700590 (accessed on 1 September 2022).
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfiser, H. UpSet: Visualization of Intersecting sets. IEEE Trans. Visual. Comp. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef] [PubMed]

| Replicate | Nodules | Roots | ||||
|---|---|---|---|---|---|---|
| Control 1 | Inoculated | Co-Inoculated | Control | Inoculated | Co-Inoculated | |
| 1 | 97.76 | 99.30 | 96.33 | 4.59 | 13.49 | 41.00 |
| 2 | 99.62 | 99.16 | 98.18 | 10.51 | 11.31 | 11.37 |
| 3 | 98.36 | 99.77 | 97.55 | 8.64 | 18.14 | 37.55 |
| 4 | 98.52 | 99.09 | 92.25 | 4.41 | 10.08 | 9.92 |
| 5 | 99.93 | 99.58 | 94.55 | 9.40 | 8.50 | 12.08 |
| 6 | 91.55 | 98.33 | 96.45 | 5.61 | 5.89 | 8.66 |
| Mean 2 | 97.62 ab | 99.20 a | 95.88 b | 7.19 b | 11.24 ab | 20.10 a |
| SD 3 | 3.08 | 0.50 | 2.17 | 2.65 | 4.25 | 14.94 |
| Replicate | Nodules | Roots | ||||
|---|---|---|---|---|---|---|
| Control 1 | Inoculated | Co-Inoculated | Control | Inoculated | Co-Inoculated | |
| 1 | 90.51 | 92.95 | 94.99 | 9.65 | 25.67 | 24.29 |
| 2 | 95.57 | 89.69 | 95.79 | 11.23 | 24.29 | 26.02 |
| 3 | 89.67 | 89.29 | - 2 | 21.81 | 13.08 | 16.23 |
| 4 | 94.18 | 91.03 | 88.46 | 12.04 | 18.98 | 18.48 |
| 5 | 95.83 | 92.68 | 89.64 | 16.77 | 26.70 | 31.37 |
| 6 | 81.51 | 86.75 | 96.51 | 9.42 | 25.76 | 30.69 |
| Mean 3 | 91.21 a | 90.40 a | 93.08 a | 13.49 b | 22.41 a | 24.51 a |
| SD 4 | 5.41 | 2.33 | 38.15 | 4.87 | 5.34 | 6.20 |
| Plant | Tissue | Treatment | InvSimpson | Shannon | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Soybean | Nodule | Control | 1.09 | 0.08 | 0.21 | 0.17 |
| Inoculated | 1.31 | 0.37 | 0.31 | 0.28 | ||
| Co-inoculated | 1.06 | 0.04 | 0.24 | 0.11 | ||
| Root | Control | 49.86 | 12.48 | 4.62 | 0.38 | |
| Inoculated | 41.30 | 9.47 | 4.27 | 0.20 | ||
| Co-inoculated | 30.71 | 14.53 | 4.09 | 0.38 | ||
| Common bean | Nodule | Control | 1.13 | 0.03 | 0.33 | 0.10 |
| Inoculated | 1.10 | 0.02 | 0.26 | 0.07 | ||
| Co-inoculated | 1.09 | 0.01 | 0.23 | 0.04 | ||
| Root | Control | 46.79 | 15.76 | 4.56 | 0.26 | |
| Inoculated | 27.92 | 15.99 | 4.36 | 0.20 | ||
| Co-inoculated | 21.66 | 9.63 | 4.18 | 0.23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, F.R.; Alves, L.C.; da Silva, J.F.M.; Ribeiro, R.A.; Pauli, G.; Nogueira, M.A.; Hungria, M. Microbiome of Nodules and Roots of Soybean and Common Bean: Searching for Differences Associated with Contrasting Performances in Symbiotic Nitrogen Fixation. Int. J. Mol. Sci. 2022, 23, 12035. https://doi.org/10.3390/ijms231912035
Bender FR, Alves LC, da Silva JFM, Ribeiro RA, Pauli G, Nogueira MA, Hungria M. Microbiome of Nodules and Roots of Soybean and Common Bean: Searching for Differences Associated with Contrasting Performances in Symbiotic Nitrogen Fixation. International Journal of Molecular Sciences. 2022; 23(19):12035. https://doi.org/10.3390/ijms231912035
Chicago/Turabian StyleBender, Flávia Raquel, Leonardo Cardoso Alves, João Fernando Marques da Silva, Renan Augusto Ribeiro, Giuliano Pauli, Marco Antonio Nogueira, and Mariangela Hungria. 2022. "Microbiome of Nodules and Roots of Soybean and Common Bean: Searching for Differences Associated with Contrasting Performances in Symbiotic Nitrogen Fixation" International Journal of Molecular Sciences 23, no. 19: 12035. https://doi.org/10.3390/ijms231912035
APA StyleBender, F. R., Alves, L. C., da Silva, J. F. M., Ribeiro, R. A., Pauli, G., Nogueira, M. A., & Hungria, M. (2022). Microbiome of Nodules and Roots of Soybean and Common Bean: Searching for Differences Associated with Contrasting Performances in Symbiotic Nitrogen Fixation. International Journal of Molecular Sciences, 23(19), 12035. https://doi.org/10.3390/ijms231912035







