Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases
Abstract
1. Introduction
2. Intestinal Probiotics
3. Native Probiotics and Diseases
3.1. Native Probiotics and Cancer
3.1.1. Native Escherichia coli Nissle 1917 (EcN) and Cancer
3.1.2. Native Akkermansia muciniphila (AM) and Cancer
3.1.3. Native Clostridium butyricum (CB) and Cancer
3.1.4. Native Bifidobacterium spp., Lactic Acid Bacteria and Cancer
3.2. Native Probiotics and Inflammatory Bowel Disease (IBD)
3.2.1. Native Escherichia coli Nissle 1917 (EcN) and IBD
3.2.2. Native Akkermansia muciniphila (AM) and IBD
3.2.3. Native Clostridium butyricum (CB) and IBD
3.2.4. Native Bifidobacterium spp., Lactic acid Bacteria and IBD
3.3. Native Probiotics and Obesity with Associated Diseases
3.3.1. Native Escherichia coli Nissle 1917 (EcN) and Obesity with Associated Diseases
3.3.2. Native Akkermansia muciniphila (AM) and Obesity with Associated Diseases
3.3.3. Native Clostridium butyricum (CB) and Obesity with Associated Diseases
3.3.4. Native Bifidobacterium spp., Lactic Acid Bacteria and Obesity with Associated Diseases
3.4. Summary
4. Modified Probiotics and Diseases
4.1. Modified Escherichia coli Nissle 1917, EcN
4.1.1. Express Direct Therapeutic Factors
4.1.2. Express Adjuvant Therapeutic Factors
4.1.3. EcN or EcN-derivatives as a Targeted Delivery System of Therapeutic Factors
4.2. Modified Akkermansia muciniphila
4.3. Modified Clostridium Butyricum
4.4. Modified Lactic Acid Bacteria and Bifidobacterium spp.
4.4.1. Treatment of Diseases
4.4.2. Probiotic Vaccines
4.5. Summary
5. Challenges and Outlooks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-Producing Human Gut Symbiont, Clostridium Butyricum, and Its Role in Health and Disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, C.; Yu, J.; Qi, Q.; Wang, Q. Bioengineered Escherichia coli Nissle 1917 for Tumour-Targeting Therapy. Microb. Biotechnol. 2020, 13, 629–636. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, T. Engineered Akkermansia muciniphila: A Promising Agent against Diseases (Review). Exp. Ther. Med. 2020, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Díez-Sainz, E.; Milagro, F.I.; Riezu-Boj, J.I.; Lorente-Cebrián, S. Effects of Gut Microbiota-Derived Extracellular Vesicles on Obesity and Diabetes and Their Potential Modulation through Diet. J. Physiol. Biochem. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The Gut Microbiota Influences Anticancer Immunosurveillance and General Health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Liu, Q.; Gai, Y.; Chen, Y.; Lan, X.; Jiang, D. Escherichia coli Nissle 1917 as a Novel Microrobot for Tumor-Targeted Imaging and Therapy. Pharmaceutics 2021, 13, 1226. [Google Scholar] [CrossRef] [PubMed]
- Sonnenborn, U. Escherichia coli Strain Nissle 1917—From Bench to Bedside and Back: History of a Special Escherichia coli Strain with Probiotic Properties. FEMS Microbiol. Lett. 2016, 363, fnw212. [Google Scholar] [CrossRef]
- Hu, R.; Lin, H.; Li, J.; Zhao, Y.; Wang, M.; Sun, X.; Min, Y.; Gao, Y.; Yang, M. Probiotic Escherichia coli Nissle 1917—Derived Outer Membrane Vesicles Enhance Immunomodulation and Antimicrobial Activity in RAW264.7 Macrophages. BMC Microbiol. 2020, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Chen, S.; Guo, S.; Yue, T.; Hou, Q.; Feng, M.; Xu, H.; Liu, Y.; Wang, P.; et al. The Administration of Escherichia coli Nissle 1917 Ameliorates Irinotecan-Induced Intestinal Barrier Dysfunction and Gut Microbial Dysbiosis in Mice. Life Sci. 2019, 231, 116529. [Google Scholar] [CrossRef]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M.; Barreau, F.; Oswald, E.; Saoudi, A. Oral Administration of the Probiotic Strain Escherichia coli Nissle 1917 Reduces Susceptibility to Neuroinflammation and Repairs Experimental Autoimmune Encephalomyelitis-Induced Intestinal Barrier Dysfunction. Front. Immunol. 2017, 8, 1096. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.L.S.; Campos, C.L.V.; Reis, D.C.; Cassali, G.D.; Generoso, S.V.; Cardoso, V.N.; Azevedo, V.; Medeiros, J.D.; Fernandes, G.R.; Nicoli, J.R.; et al. Beneficial Effects Resulting from Oral Administration of Escherichia coli Nissle 1917 on a Chronic Colitis Model. Benef. Microbes 2020, 11, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Maltby, R.; Leatham-Strains Jensen, M.P.; Gibson, T.; Cohen, P.S.; Conway, T. Nutritional Basis for Colonization Resistance by Human Commensal Escherichia coli HS and Nissle 1917 against E. Coli O157:H7 in the Mouse Intestine. PLoS ONE 2013, 8, e53957. [Google Scholar] [CrossRef]
- Schinner, S.A.C.; Mokszycki, M.E.; Adediran, J.; Leatham-Jensen, M.; Conway, T.; Cohen, P.S. Escherichia coli EDL933 Requires Gluconeogenic Nutrients to Successfully Colonize the Intestines of Streptomycin-Treated Mice Precolonized with E. Coli Nissle 1917. Infect. Immun. 2015, 83, 1983–1991. [Google Scholar] [CrossRef]
- Reister, M.; Hoffmeier, K.; Krezdorn, N.; Rotter, B.; Liang, C.; Rund, S.; Dandekar, T.; Sonnenborn, U.; Oelschlaeger, T.A. Complete Genome Sequence of the Gram-Negative Probiotic Escherichia coli Strain Nissle 1917. J. Biotechnol. 2014, 187, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Pasteurised Akkermansia muciniphila as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06780. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next Generation Probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, X.; Sun, Y.; Zhao, J.; Miao, S.; Xiong, Q.; Zhang, Y.; Zhang, G. Comparative Genomic and Functional Analysis of Akkermansia muciniphila and Closely Related Species. Genes Genom. 2019, 41, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like Proteins of Akkermansia muciniphila Modulate Host Immune Responses and Gut Barrier Function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Durand, S.; Daillère, R.; Iribarren, K.; Lemaitre, F.; Derosa, L.; Aprahamian, F.; Bossut, N.; Nirmalathasan, N.; Madeo, F.; et al. Oral Administration of Akkermansia muciniphila Elevates Systemic Antiaging and Anticancer Metabolites. Aging 2021, 13, 6375–6405. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Corleto, J.; Ruegger, P.M.; Logan, G.D.; Peacock, B.B.; Mendonca, S.; Yamaki, S.; Adamson, T.; Ermel, R.; McKemy, D.; et al. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci. Rep. 2019, 9, 20324. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Zhang, H.; Wu, S.; Qi, G. Supplemental Clostridium Butyricum Modulates Lipid Metabolism Through Shaping Gut Microbiota and Bile Acid Profile of Aged Laying Hens. Front. Microbiol. 2020, 11, 600. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Xia, J.; Wang, B.; Liu, H.; Yang, L.; Wang, Y.; Ling, Z. Role of Probiotics in the Treatment of Minimal Hepatic Encephalopathy in Patients with HBV-Induced Liver Cirrhosis. J. Int. Med. Res. 2018, 46, 3596–3604. [Google Scholar] [CrossRef]
- Vieira, A.T.; Fukumori, C.; Ferreira, C.M. New Insights into Therapeutic Strategies for Gut Microbiota Modulation in Inflammatory Diseases. Clin. Transl. Immunol. 2016, 5, e87. [Google Scholar] [CrossRef]
- Patil, P.; Bhandary, S.K.; Haridas, V.; Sarathkumar, E.; Shetty, P. Is Butyrate a Natural Alternative to Dexamethasone in the Management of COVID-19? F1000 Res. 2021, 10, 273. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, W.-R.; Wang, X.-H.; Li, G.-Q.; Xu, L.-Q.; Cui, X.; Liu, Y.; Zuo, X.-L. Clostridium Butyricum Alleviates Intestinal Low-Grade Inflammation in TNBS-Induced Irritable Bowel Syndrome in Mice by Regulating Functional Status of Lamina Propria Dendritic Cells. World J. Gastroenterol. 2019, 25, 5469–5482. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The Gut Microbiota and Inflammatory Bowel Disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of Dietary Supplementation of Probiotic, Clostridium Butyricum, on Growth Performance, Immune Response, Intestinal Barrier Function, and Digestive Enzyme Activity in Broiler Chickens Challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Cao, G.T.; Ferket, P.R.; Liu, T.T.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Chen, A.G. Effects of Probiotic, Clostridium Butyricum, on Growth Performance, Immune Function, and Cecal Microflora in Broiler Chickens. Poult. Sci. 2012, 91, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Brunt, J.; Cross, K.L.; Peck, M.W. Apertures in the Clostridium Sporogenes Spore Coat and Exosporium Align to Facilitate Emergence of the Vegetative Cell. Food Microbiol. 2015, 51, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Bi, X.; Nguyen, L.V.; Matsuda, K.; Pham, H.V.; Phan, C.T.T.; Khu, D.T.K.; Ichimura, H. Effects of Short-Term Probiotic Ingestion on Immune Profiles and Microbial Translocation among HIV-1-Infected Vietnamese Children. Int. J. Mol. Sci. 2017, 18, 2185. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, C.; Chen, D.; Liu, S. Potential of Three Probiotic Lactobacilli in Transforming Star Fruit Juice into Functional Beverages. Food Sci. Nutr. 2018, 6, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Roos, S.; Eeckhaut, V.; MacKenzie, D.A.; Derde, M.; Verstraete, W.; Marzorati, M.; Possemiers, S.; Vanhoecke, B.; Van Immerseel, F.; et al. Incorporating a Mucosal Environment in a Dynamic Gut Model Results in a More Representative Colonization by Lactobacilli. Microb. Biotechnol. 2012, 5, 106–115. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the Probiotic Potential of Lactic Acid Bacteria from African Traditional Fermented Foods and Beverages. Food Nutr. Res. 2016, 60, 29630. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Zhang, H.; Xie, Y.; Xiong, L.; Liu, H.; Wang, F. Effects of a Probiotic on the Growth Performance, Intestinal Flora, and Immune Function of Chicks Infected with Salmonella Pullorum. Poult. Sci. 2020, 99, 5316–5323. [Google Scholar] [CrossRef]
- Zou, Y.-J.; Xu, J.-J.; Wang, X.; Zhu, Y.-H.; Wu, Q.; Wang, J.-F. Lactobacillus Johnsonii L531 Ameliorates Escherichia coli-Induced Cell Damage via Inhibiting NLRP3 Inflammasome Activity and Promoting ATG5/ATG16L1-Mediated Autophagy in Porcine Mammary Epithelial Cells. Vet. Sci. 2020, 7, 112. [Google Scholar] [CrossRef]
- Kim, J.E.; Sharma, A.; Sharma, G.; Lee, S.Y.; Shin, H.S.; Rudra, D.; Im, S.-H. Lactobacillus Pentosus Modulates Immune Response by Inducing IL-10 Producing Tr1 Cells. Immune Netw. 2019, 19, e39. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, S.; Tamagnini, I.; Mora, D.; Minuzzo, M.; Scarafoni, A.; Arioli, S.; Hellman, J.; Karp, M.; Parini, C. Implication of an Outer Surface Lipoprotein in Adhesion of Bifidobacterium Bifidum to Caco-2 Cells. Appl. Environ. Microbiol. 2008, 74, 4695–4702. [Google Scholar] [CrossRef] [PubMed]
- Cheikhyoussef, A.; Pogori, N.; Chen, H.; Tian, F.; Chen, W.; Tang, J.; Zhang, H. Antimicrobial Activity and Partial Characterization of Bacteriocin-like Inhibitory Substances (BLIS) Produced by Bifidobacterium Infantis BCRC 14602. Food Control 2009, 20, 553–559. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as Vitamin Suppliers to Their Host: A Gut Microbiota Perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile Resistance Mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Ranjan, A.; Srivastava, A.K.; Singh, M.; Shukla, A.K.; Atri, N.; Mishra, A.; Singh, A.K.; Singh, S.K. Cytotoxic and Apoptotic Inducing Activity of Amoora Rohituka Leaf Extracts in Human Breast Cancer Cells. J. Ayurveda Integr. Med. 2020, 11, 383–390. [Google Scholar] [CrossRef]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic Analysis of Colorectal Cancer Datasets Identifies Cross-Cohort Microbial Diagnostic Signatures and a Link with Choline Degradation. Nat. Med. 2019, 25, 667–678. [Google Scholar] [CrossRef]
- Li, R.; Helbig, L.; Fu, J.; Bian, X.; Herrmann, J.; Baumann, M.; Stewart, A.F.; Müller, R.; Li, A.; Zips, D.; et al. Expressing Cytotoxic Compounds in Escherichia coli Nissle 1917 for Tumor-Targeting Therapy. Res. Microbiol. 2019, 170, 74–79. [Google Scholar] [CrossRef]
- Alizadeh, S.; Esmaeili, A.; Omidi, Y. Anti-Cancer Properties of Escherichia coli Nissle 1917 against HT-29 Colon Cancer Cells through Regulation of Bax/Bcl-XL and AKT/PTEN Signaling Pathways. Iran. J. Basic Med. Sci. 2020, 23, 886–893. [Google Scholar] [CrossRef]
- Shi, L.; Sheng, J.; Wang, M.; Luo, H.; Zhu, J.; Zhang, B.; Liu, Z.; Yang, X. Combination Therapy of TGF-β Blockade and Commensal-Derived Probiotics Provides Enhanced Antitumor Immune Response and Tumor Suppression. Theranostics 2019, 9, 4115–4129. [Google Scholar] [CrossRef] [PubMed]
- Nougayrède, J.-P.; Chagneau, C.V.; Motta, J.-P.; Bossuet-Greif, N.; Belloy, M.; Taieb, F.; Gratadoux, J.-J.; Thomas, M.; Langella, P.; Oswald, E. A Toxic Friend: Genotoxic and Mutagenic Activity of the Probiotic Strain Escherichia coli Nissle 1917. mSphere 2021, 6, e0062421. [Google Scholar] [CrossRef]
- Vernocchi, P.; Gili, T.; Conte, F.; Del Chierico, F.; Conta, G.; Miccheli, A.; Botticelli, A.; Paci, P.; Caldarelli, G.; Nuti, M.; et al. Network Analysis of Gut Microbiome and Metabolome to Discover Microbiota-Linked Biomarkers in Patients Affected by Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2020, 21, 8730. [Google Scholar] [CrossRef]
- Lapidot, Y.; Amir, A.; Nosenko, R.; Uzan-Yulzari, A.; Veitsman, E.; Cohen-Ezra, O.; Davidov, Y.; Weiss, P.; Bradichevski, T.; Segev, S.; et al. Alterations in the Gut Microbiome in the Progression of Cirrhosis to Hepatocellular Carcinoma. mSystems 2020, 5, e00153-20. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, X.; Chen, S.; Fu, X.; Ma, G.; Zhang, A. Akkermansia muciniphila Enhances the Antitumor Effect of Cisplatin in Lewis Lung Cancer Mice. J. Immunol. Res. 2020, 2020, 2969287. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sheng, J.; Chen, G.; Zhu, P.; Shi, C.; Li, B.; Park, C.; Wang, J.; Zhang, B.; Liu, Z.; et al. Combining IL-2-Based Immunotherapy with Commensal Probiotics Produces Enhanced Antitumor Immune Response and Tumor Clearance. J. Immunother. Cancer 2020, 8, e000973. [Google Scholar] [CrossRef]
- Daisley, B.A.; Chanyi, R.M.; Abdur-Rashid, K.; Al, K.F.; Gibbons, S.; Chmiel, J.A.; Wilcox, H.; Reid, G.; Anderson, A.; Dewar, M.; et al. Abiraterone Acetate Preferentially Enriches for the Gut Commensal Akkermansia muciniphila in Castrate-Resistant Prostate Cancer Patients. Nat. Commun. 2020, 11, 4822. [Google Scholar] [CrossRef]
- Panebianco, C.; Adamberg, K.; Jaagura, M.; Copetti, M.; Fontana, A.; Adamberg, S.; Kolk, K.; Vilu, R.; Andriulli, A.; Pazienza, V. Influence of Gemcitabine Chemotherapy on the Microbiota of Pancreatic Cancer Xenografted Mice. Cancer Chemother. Pharmacol. 2018, 81, 773–782. [Google Scholar] [CrossRef]
- Teng, L.; Wang, K.; Chen, W.; Wang, Y.; Bi, L. HYR-2 Plays an Anti-Lung Cancer Role by Regulating PD-L1 and Akkermansia muciniphila. Pharmacol. Res. 2020, 160, 105086. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Zhong, Y.N.; Zhao, F.; Hao, Z.; Xu, Y.; Lai, R.; Shen, G.; Yin, X. Effect and Mechanism of Vitamin D on the Development of Colorectal Cancer Based on Intestinal Flora Disorder. J. Gastroenterol. Hepatol. 2020, 35, 1023–1031. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, J.; Zhang, Y.; Guo, Y.; Chang, Q.; Chen, L.; Wang, Y.; Wang, S.; Song, Y.; et al. Sini Decoction Ameliorates Colorectal Cancer and Modulates the Composition of Gut Microbiota in Mice. Front. Pharmacol. 2021, 12, 609992. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, W.; Lan, T.; Yang, W.; Yu, D.; Fang, X.; Wu, H. A Purified Aspartic Protease from Akkermansia muciniphila Plays an Important Role in Degrading Muc2. Int. J. Mol. Sci. 2019, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, J.; Wu, H.; Yu, D.; Fang, X. Akkermansia muciniphila Aspartic Protease Amuc_1434* Inhibits Human Colorectal Cancer LS174T Cell Viability via TRAIL-Mediated Apoptosis Pathway. Int. J. Mol. Sci. 2020, 21, 3385. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8+ T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Fan, L.; Xu, C.; Ge, Q.; Lin, Y.; Wong, C.C.; Qi, Y.; Ye, B.; Lian, Q.; Zhuo, W.; Si, J.; et al. A. Muciniphila Suppresses Colorectal Tumorigenesis by Inducing TLR2/NLRP3-Mediated M1-Like TAMs. Cancer Immunol. Res. 2021, 9, 1111–1124. [Google Scholar] [CrossRef]
- Luo, Z.-W.; Xia, K.; Liu, Y.-W.; Liu, J.-H.; Rao, S.-S.; Hu, X.-K.; Chen, C.-Y.; Xu, R.; Wang, Z.-X.; Xie, H. Extracellular Vesicles from Akkermansia muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8+ T Cells and Macrophages. Int. J. Nanomed. 2021, 16, 2949–2963. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut Microbiome Affects the Response to Anti-PD-1 Immunotherapy in Patients with Hepatocellular Carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Salgia, N.J.; Bergerot, P.G.; Maia, M.C.; Dizman, N.; Hsu, J.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti-PD-1 Immune Checkpoint Inhibitors. Eur. Urol. 2020, 78, 498–502. [Google Scholar] [CrossRef]
- Xu, X.; Lv, J.; Guo, F.; Li, J.; Jia, Y.; Jiang, D.; Wang, N.; Zhang, C.; Kong, L.; Liu, Y.; et al. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front. Microbiol. 2020, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopès, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota Triggers STING-Type I IFN-Dependent Monocyte Reprogramming of the Tumor Microenvironment. Cell 2021, 184, 5338–5356.e21. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, W.; Wan, X.; Deng, T. Clostridium Butyricum Modulates Gut Microbiota and Reduces Colitis Associated Colon Cancer in Mice. Int. Immunopharmacol. 2020, 88, 106862. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium Butyricum, a Butyrate-Producing Probiotic, Inhibits Intestinal Tumor Development through Modulating Wnt Signaling and Gut Microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Li, D.; Chen, M.; Liu, J.; Feng, C.; He, Q.; Zhao, J.; Zhang, L.; Chen, J.; et al. The Efficacy and Safety of Clostridium Butyricum and Bacillus Coagulans in Helicobacter Pylori Eradication Treatment: An Open-Label, Single-Arm Pilot Study. Medicine 2020, 99, e22976. [Google Scholar] [CrossRef]
- Shin, D.-S.; Rhee, K.-J.; Eom, Y.-B. Effect of Probiotic Clostridium Butyricum NCTC 7423 Supernatant on Biofilm Formation and Gene Expression of Bacteroides Fragilis. J. Microbiol. Biotechnol. 2020, 30, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-W.; Li, R.-Q.; An, J.-X.; Xie, T.-Q.; Han, Z.-Y.; Xu, R.; Fang, Y.; Zhang, X.-Z. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, e2004529. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Dai, X.; Li, K.; Gui, G.; Liu, J.; Yang, H. Clostridium Butyricum Partially Regulates the Development of Colitis-Associated Cancer through MiR-200c. Cell Mol. Biol. 2017, 63, 59–66. [Google Scholar] [CrossRef]
- Tian, Y.; Li, M.; Song, W.; Jiang, R.; Li, Y.Q. Effects of Probiotics on Chemotherapy in Patients with Lung Cancer. Oncol. Lett. 2019, 17, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Xie, Q.; Ma, L.; An, G.; Xiao, J.; Li, J.; Liu, X.; Gao, P.; Liang, J.; Li, Y. Synergistic Anti-Tumour Effects of Clostridium Butyricum in Combination with Apatinib in CT26 Colorectal Tumour-Bearing Mice. Anticancer Drugs 2019, 30, 991–997. [Google Scholar] [CrossRef]
- Tomita, Y.; Ikeda, T.; Sakata, S.; Saruwatari, K.; Sato, R.; Iyama, S.; Jodai, T.; Akaike, K.; Ishizuka, S.; Saeki, S.; et al. Association of Probiotic Clostridium Butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol. Res. 2020, 8, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Ho, P.-Y.; Chen, W.-J.; Wu, S.-H.; Pan, M.-H. Lactobacillus Fermentum V3 Ameliorates Colitis-Associated Tumorigenesis by Modulating the Gut Microbiome. Am. J. Cancer Res. 2020, 10, 1170–1181. [Google Scholar]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The Relationship between the Structural Characteristics of Lactobacilli-EPS and Its Ability to Induce Apoptosis in Colon Cancer Cells in Vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.-Q.; Mesmari, N.; Dahmani, S.; Al Dhaheri, A.S.; Kizhakkayil, J. In Vitro Investigation of Bioactivities of Solid-State Fermented Lupin, Quinoa and Wheat Using Lactobacillus spp. Food Chem. 2019, 275, 50–58. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Asadzadeh Aghdaei, H.; Dabiri, H.; Akhavan Sepahi, A.; Modarressi, M.H.; Nazemalhosseini Mojarad, E. The Association between Fecal Microbiota and Different Types of Colorectal Polyp as Precursors of Colorectal Cancer. Microb. Pathog. 2018, 124, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Nagata, S.; Saito, M.; Shimizu, T.; Yamashiro, Y.; Matsuki, T.; Asahara, T.; Nomoto, K. Effects of the Enteral Administration of Bifidobacterium Breve on Patients Undergoing Chemotherapy for Pediatric Malignancies. Support. Care Cancer 2010, 18, 751–759. [Google Scholar] [CrossRef]
- Raj, R.; Das, S. Development and Application of Anticancer Fluorescent CdS Nanoparticles Enriched Lactobacillus Bacteria as Therapeutic Microbots for Human Breast Carcinoma. Appl. Microbiol. Biotechnol. 2017, 101, 5439–5451. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From Biomarker to Biological Function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Damaskos, D.; Kolios, G. Probiotics and Prebiotics in Inflammatory Bowel Disease: Microflora “on the Scope”. Br. J. Clin. Pharmacol. 2008, 65, 453–467. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Mikami, Y.; Kanai, T. Bacteriotherapy for Inflammatory Bowel Disease. Inflamm. Regen. 2021, 41, 3. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M. Clinical Use of E. Coli Nissle 1917 in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2008, 14, 1012–1018. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukás, M.; Fixa, B.; Kascák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining Remission of Ulcerative Colitis with the Probiotic Escherichia coli Nissle 1917 Is as Effective as with Standard Mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef]
- Behrouzi, A.; Mazaheri, H.; Falsafi, S.; Tavassol, Z.H.; Moshiri, A.; Siadat, S.D. Intestinal Effect of the Probiotic Escherichia coli Strain Nissle 1917 and Its OMV. J. Diabetes Metab. Disord. 2020, 19, 597–604. [Google Scholar] [CrossRef]
- Waidmann, M.; Bechtold, O.; Frick, J.-S.; Lehr, H.-A.; Schubert, S.; Dobrindt, U.; Loeffler, J.; Bohn, E.; Autenrieth, I.B. Bacteroides Vulgatus Protects against Escherichia coli-Induced Colitis in Gnotobiotic Interleukin-2-Deficient Mice. Gastroenterology 2003, 125, 162–177. [Google Scholar] [CrossRef]
- Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Rodríguez-Sojo, M.J.; Rodríguez-Cabezas, M.E.; Olivares, M.; García, F.; Gálvez, J.; Morón, R.; Rodríguez-Nogales, A. Intestinal Anti-Inflammatory Effects of Probiotics in DNBS-Colitis via Modulation of Gut Microbiota and MicroRNAs. Eur. J. Nutr. 2021, 60, 2537–2551. [Google Scholar] [CrossRef]
- Bian, Z.; Li, L.; Cui, J.; Zhang, H.; Liu, Y.; Zhang, C.-Y.; Zen, K. Role of MiR-150-Targeting c-Myb in Colonic Epithelial Disruption during Dextran Sulphate Sodium-Induced Murine Experimental Colitis and Human Ulcerative Colitis. J. Pathol. 2011, 225, 544–553. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Fernández-Caballero, J.A.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. The Administration of Escherichia coli Nissle 1917 Ameliorates Development of DSS-Induced Colitis in Mice. Front. Pharmacol. 2018, 9, 468. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The Influence of the Gut Microbiota on the Bioavailability of Oral Drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, M.-J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal Anti-Inflammatory Effects of Outer Membrane Vesicles from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Geerlings, S.Y.; Kostopoulos, I.; de Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Belzer, C.; de Vos, W.M. Microbes Inside—From Diversity to Function: The Case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sugihara, K.; Gillilland, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic Acid-Bilirubin Nanomedicine for Targeted Modulation of Dysregulated Intestinal Barrier, Microbiome and Immune Responses in Colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Earley, H.; Lennon, G.; Balfe, Á.; Coffey, J.C.; Winter, D.C.; O’Connell, P.R. The Abundance of Akkermansia muciniphila and Its Relationship with Sulphated Colonic Mucins in Health and Ulcerative Colitis. Sci. Rep. 2019, 9, 15683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, P.; Wu, X.; Lu, G.; Marcella, C.; Ji, X.; Ji, G.; Zhang, F. Alterations of Akkermansia muciniphila in the Inflammatory Bowel Disease Patients with Washed Microbiota Transplantation. Appl. Microbiol. Biotechnol. 2020, 104, 10203–10215. [Google Scholar] [CrossRef]
- Zhai, R.; Xue, X.; Zhang, L.; Yang, X.; Zhao, L.; Zhang, C. Strain-Specific Anti-Inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front. Cell Infect. Microbiol. 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Piątek-Guziewicz, A.; Zagrodzki, P.; Mach, T.; Zwolińska-Wcisło, M. Fecal Levels of Lactic, Succinic and Short-Chain Fatty Acids in Patients with Ulcerative Colitis and Crohn Disease: A Pilot Study. J. Clin. Med. 2021, 10, 4701. [Google Scholar] [CrossRef]
- Araki, Y.; Andoh, A.; Takizawa, J.; Takizawa, W.; Fujiyama, Y. Clostridium Butyricum, a Probiotic Derivative, Suppresses Dextran Sulfate Sodium-Induced Experimental Colitis in Rats. Int. J. Mol. Med. 2004, 13, 577–580. [Google Scholar] [CrossRef]
- Scott, N.A.; Andrusaite, A.; Andersen, P.; Lawson, M.; Alcon-Giner, C.; Leclaire, C.; Caim, S.; Le Gall, G.; Shaw, T.; Connolly, J.P.R.; et al. Antibiotics Induce Sustained Dysregulation of Intestinal T Cell Immunity by Perturbing Macrophage Homeostasis. Sci. Transl. Med. 2018, 10, eaao4755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Q.; Ding, T.T.; Zhao, J.-S.; Yang, X.; Zhang, H.-X.; Zhang, J.-J.; Cui, Y.-L. Therapeutic Effects of Clostridium Butyricum on Experimental Colitis Induced by Oxazolone in Rats. World J. Gastroenterol. 2009, 15, 1821–1828. [Google Scholar] [CrossRef]
- Hayashi, A.; Sato, T.; Kamada, N.; Mikami, Y.; Matsuoka, K.; Hisamatsu, T.; Hibi, T.; Roers, A.; Yagita, H.; Ohteki, T.; et al. A Single Strain of Clostridium Butyricum Induces Intestinal IL-10-Producing Macrophages to Suppress Acute Experimental Colitis in Mice. Cell Host Microbe 2013, 13, 711–722. [Google Scholar] [CrossRef]
- Kanai, T.; Mikami, Y.; Hayashi, A. A Breakthrough in Probiotics: Clostridium Butyricum Regulates Gut Homeostasis and Anti-Inflammatory Response in Inflammatory Bowel Disease. J. Gastroenterol. 2015, 50, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zeng, L.; Li, L.-J.; Mo, L.-H.; Xie, R.-D.; Feng, B.-S.; Zheng, P.-Y.; Liu, Z.-G.; Liu, Z.-J.; Yang, P.-C. Specific Immunotherapy Ameliorates Ulcerative Colitis. Allergy Asthma Clin. Immunol. 2016, 12, 37. [Google Scholar] [CrossRef]
- Araki, Y.; Fujiyama, Y.; Andoh, A.; Koyama, S.; Kanauchi, O.; Bamba, T. The Dietary Combination of Germinated Barley Foodstuff plus Clostridium Butyricum Suppresses the Dextran Sulfate Sodium-Induced Experimental Colitis in Rats. Scand. J. Gastroenterol. 2000, 35, 1060–1067. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, G.; Chen, C.; Zeng, X.; Ye, H. Prebiotics Effects in Vitro of Polysaccharides from Tea Flowers on Gut Microbiota of Healthy Persons and Patients with Inflammatory Bowel Disease. Int. J. Biol. Macromol. 2020, 158, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Liu, T.; Fu, A.; Ruan, S.; Zhong, H.; Tang, J.; Zhao, M.; Li, Y.; Zhu, S.; Cai, H.; et al. Novel Gut Microbiota Patterns Involved in the Attenuation of Dextran Sodium Sulfate-Induced Mouse Colitis Mediated by Glycerol Monolaurate via Inducing Anti-Inflammatory Responses. mBio 2021, 12, e0214821. [Google Scholar] [CrossRef]
- Mu, J.; Xu, J.; Wang, L.; Chen, C.; Chen, P. Anti-Inflammatory Effects of Purple Sweet Potato Anthocyanin Extract in DSS-Induced Colitis: Modulation of Commensal Bacteria and Attenuated Bacterial Intestinal Infection. Food Funct. 2021, 12, 11503–11514. [Google Scholar] [CrossRef]
- Ghavami, S.B.; Yadegar, A.; Aghdaei, H.A.; Sorrentino, D.; Farmani, M.; Mir, A.S.; Azimirad, M.; Balaii, H.; Shahrokh, S.; Zali, M.R. Immunomodulation and Generation of Tolerogenic Dendritic Cells by Probiotic Bacteria in Patients with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 6266. [Google Scholar] [CrossRef]
- Wang, N.; Wang, S.; Xu, B.; Liu, F.; Huo, G.; Li, B. Alleviation Effects of Bifidobacterium Animalis Subsp. Lactis XLTG11 on Dextran Sulfate Sodium-Induced Colitis in Mice. Microorganisms 2021, 9, 2093. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, F.; Ma, H.; Chen, S. Exopolysaccharide-Producing Bifidobacterium Adolescentis Strains with Similar Adhesion Property Induce Differential Regulation of Inflammatory Immune Response in Treg/Th17 Axis of DSS-Colitis Mice. Nutrients 2019, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium Bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-ΚB-Independent Manner. Int. J. Mol. Sci. 2021, 22, 8070. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, Q.; Etareri Evivie, S.; Liu, D.; Dong, J.; Ping, L.; Liu, F.; Li, B.; Huo, G. Bifidobacterium Dentium N8 with Potential Probiotic Characteristics Prevents LPS-Induced Intestinal Barrier Injury by Alleviating the Inflammatory Response and Regulating the Tight Junction in Caco-2 Cell Monolayers. Food Funct. 2021, 12, 7171–7184. [Google Scholar] [CrossRef] [PubMed]
- Leccese, G.; Bibi, A.; Mazza, S.; Facciotti, F.; Caprioli, F.; Landini, P.; Paroni, M. Probiotic Lactobacillus and Bifidobacterium Strains Counteract Adherent-Invasive Escherichia coli (AIEC) Virulence and Hamper IL-23/Th17 Axis in Ulcerative Colitis, but Not in Crohn’s Disease. Cells 2020, 9, 1824. [Google Scholar] [CrossRef]
- Engevik, M.A.; Herrmann, B.; Ruan, W.; Engevik, A.C.; Engevik, K.A.; Ihekweazu, F.; Shi, Z.; Luck, B.; Chang-Graham, A.L.; Esparza, M.; et al. Bifidobacterium Dentium-Derived y-Glutamylcysteine Suppresses ER-Mediated Goblet Cell Stress and Reduces TNBS-Driven Colonic Inflammation. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of Diabetes and Obesity: Epidemiological Perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic Adaptation to a High-Fat Diet Is Associated with a Change in the Gut Microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Fernández-Tomé, S. Gut Mucosal and Adipose Tissues as Health Targets of the Immunomodulatory Mechanisms of Probiotics. Trends Food Sci. Technol. 2021, 112, 764–779. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Ukena, S.N.; Singh, A.; Dringenberg, U.; Engelhardt, R.; Seidler, U.; Hansen, W.; Bleich, A.; Bruder, D.; Franzke, A.; Rogler, G.; et al. Probiotic Escherichia coli Nissle 1917 Inhibits Leaky Gut by Enhancing Mucosal Integrity. PLoS ONE 2007, 2, e1308. [Google Scholar] [CrossRef]
- Alvarez, C.-S.; Badia, J.; Bosch, M.; Giménez, R.; Baldomà, L. Outer Membrane Vesicles and Soluble Factors Released by Probiotic Escherichia coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight Junction Proteins in Intestinal Epithelial Cells. Front. Microbiol. 2016, 7, 1981. [Google Scholar] [CrossRef]
- Guo, S.; Chen, S.; Ma, J.; Ma, Y.; Zhu, J.; Ma, Y.; Liu, Y.; Wang, P.; Pan, Y. Escherichia coli Nissle 1917 Protects Intestinal Barrier Function by Inhibiting NF-ΚB-Mediated Activation of the MLCK-P-MLC Signaling Pathway. Mediat. Inflamm. 2019, 2019, 5796491. [Google Scholar] [CrossRef] [PubMed]
- Veltman, K.; Hummel, S.; Cichon, C.; Sonnenborn, U.; Schmidt, M.A. Identification of Specific MiRNAs Targeting Proteins of the Apical Junctional Complex That Simulate the Probiotic Effect of E. Coli Nissle 1917 on T84 Epithelial Cells. Int. J. Biochem. Cell Biol. 2012, 44, 341–349. [Google Scholar] [CrossRef]
- Alvarez, C.-S.; Giménez, R.; Cañas, M.-A.; Vera, R.; Díaz-Garrido, N.; Badia, J.; Baldomà, L. Extracellular Vesicles and Soluble Factors Secreted by Escherichia coli Nissle 1917 and ECOR63 Protect against Enteropathogenic E. Coli-Induced Intestinal Epithelial Barrier Dysfunction. BMC Microbiol. 2019, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, L.D.; Geier, M.S.; Chua, A.E.; Butler, R.N.; Cummins, A.G.; Sander, G.R.; Howarth, G.S. Probiotic Factors Partially Prevent Changes to Caspases 3 and 7 Activation and Transepithelial Electrical Resistance in a Model of 5-Fluorouracil-Induced Epithelial Cell Damage. Support. Care Cancer 2012, 20, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Belda, E.; Prifti, E.; Everard, A.; Kayser, B.D.; Bouillot, J.-L.; Chevallier, J.-M.; Pons, N.; Le Chatelier, E.; Ehrlich, S.D.; et al. Akkermansia muciniphila Abundance Is Lower in Severe Obesity, but Its Increased Level after Bariatric Surgery Is Not Associated with Metabolic Health Improvement. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E446–E459. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Davis, J.A.; Collier, F.; Mohebbi, M.; Stuart, A.L.; Loughman, A.; Pasco, J.A.; Jacka, F.N. Obesity, Akkermansia muciniphila, and Proton Pump Inhibitors: Is There a Link? Obes. Res. Clin. Pract. 2020, 14, 524–530. [Google Scholar] [CrossRef]
- Verhoog, S.; Taneri, P.E.; Roa Díaz, Z.M.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium Prausnitzii: A Systematic Review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, K.; Zhang, Y.; Han, X.; Zhao, A.; Liu, J.; Qu, C.; Ge, K.; Huang, F.; Hernandez, B.; et al. Food Withdrawal Alters the Gut Microbiota and Metabolome in Mice. FASEB J. 2018, 32, 4878–4888. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Liu, H.-X.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Wan, Y.-J.Y. Obesity Treatment by Epigallocatechin-3-Gallate-Regulated Bile Acid Signaling and Its Enriched Akkermansia muciniphila. FASEB J. 2018, 32, fj201800370R. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Rastelli, M.; Morissette, A.; Suriano, F.; Le Roy, T.; Pilon, G.; Delzenne, N.M.; Marette, A.; Van Hul, M.; Cani, P.D. Rhubarb Supplementation Prevents Diet-Induced Obesity and Diabetes in Association with Increased Akkermansia muciniphila in Mice. Nutrients 2020, 12, 2932. [Google Scholar] [CrossRef]
- Lee, B.-H.; Chen, C.-H.; Hsu, Y.-Y.; Chuang, P.-T.; Shih, M.-K.; Hsu, W.-H. Polysaccharides Obtained from Cordyceps Militaris Alleviate Hyperglycemia by Regulating Gut Microbiota in Mice Fed a High-Fat/Sucrose Diet. Foods 2021, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, P.; Zhang, P.; Chang, Y.; Cui, M.; Duan, J. Protein-Bound β-Glucan from Coriolus Versicolor Has Potential for Use Against Obesity. Mol. Nutr. Food Res. 2019, 63, e1801231. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Zhuang, L.; Chen, X.; Min, H.; Song, S.; Liang, Q.; Li, A.-D.; Gao, Q. Puerarin Prevents High-Fat Diet-Induced Obesity by Enriching Akkermansia muciniphila in the Gut Microbiota of Mice. PLoS ONE 2019, 14, e0218490. [Google Scholar] [CrossRef]
- Nishiyama, M.; Ohtake, N.; Kaneko, A.; Tsuchiya, N.; Imamura, S.; Iizuka, S.; Ishizawa, S.; Nishi, A.; Yamamoto, M.; Taketomi, A.; et al. Increase of Akkermansia muciniphila by a Diet Containing Japanese Traditional Medicine Bofutsushosan in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 839. [Google Scholar] [CrossRef]
- Fujisaka, S.; Usui, I.; Nawaz, A.; Igarashi, Y.; Okabe, K.; Furusawa, Y.; Watanabe, S.; Yamamoto, S.; Sasahara, M.; Watanabe, Y.; et al. Bofutsushosan Improves Gut Barrier Function with a Bloom of Akkermansia muciniphila and Improves Glucose Metabolism in Mice with Diet-Induced Obesity. Sci. Rep. 2020, 10, 5544. [Google Scholar] [CrossRef]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Zhang, Y.; Ni, J.; Hou, X.; et al. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, e2100536. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Bose, S.; Lim, S.; Seo, J.; Shin, J.; Lee, D.; Chung, W.-H.; Song, E.-J.; Nam, Y.-D.; Kim, H. Beneficial Effects of Newly Isolated Akkermansia muciniphila Strains from the Human Gut on Obesity and Metabolic Dysregulation. Microorganisms 2020, 8, 1413. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential Modulation by Akkermansia muciniphila and Faecalibacterium Prausnitzii of Host Peripheral Lipid Metabolism and Histone Acetylation in Mouse Gut Organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia muciniphila Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease by Regulating the Metabolism of L-Aspartate via Gut-Liver Axis. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004–e03019. [Google Scholar] [CrossRef]
- Bodogai, M.; O’Connell, J.; Kim, K.; Kim, Y.; Moritoh, K.; Chen, C.; Gusev, F.; Vaughan, K.; Shulzhenko, N.; Mattison, J.A.; et al. Commensal Bacteria Contribute to Insulin Resistance in Aging by Activating Innate B1a Cells. Sci. Transl. Med. 2018, 10, eaat4271. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Depommier, C.; Van Hul, M.; Everard, A.; Delzenne, N.M.; De Vos, W.M.; Cani, P.D. Pasteurized Akkermansia muciniphila Increases Whole-Body Energy Expenditure and Fecal Energy Excretion in Diet-Induced Obese Mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef]
- Obanda, D.N.; Husseneder, C.; Raggio, A.M.; Page, R.; Marx, B.; Stout, R.W.; Guice, J.; Coulon, D.; Keenan, M.J. Abundance of the Species Clostridium Butyricum in the Gut Microbiota Contributes to Differences in Obesity Phenotype in Outbred Sprague-Dawley CD Rats. Nutrition 2020, 78, 110893. [Google Scholar] [CrossRef]
- Weng, H.; Endo, K.; Li, J.; Kito, N.; Iwai, N. Induction of Peroxisomes by Butyrate-Producing Probiotics. PLoS ONE 2015, 10, e0117851. [Google Scholar] [CrossRef]
- Shang, H.; Sun, J.; Chen, Y.Q. Clostridium Butyricum CGMCC0313.1 Modulates Lipid Profile, Insulin Resistance and Colon Homeostasis in Obese Mice. PLoS ONE 2016, 11, e0154373. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yang, H.; Heng, C.; Wang, H.; Chen, S.; Hu, Y.; Jiang, Z.; Yu, Q.; Wang, Z.; Qian, S.; et al. Amelioration of Non-Alcoholic Fatty Liver Disease by Sodium Butyrate Is Linked to the Modulation of Intestinal Tight Junctions in Db/Db Mice. Food Funct. 2020, 11, 10675–10689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, Y.; Liu, H.; Gao, J.; Nie, W. Clostridium Butyricum Reduce Lipogenesis through Bacterial Wall Components and Butyrate. Appl. Microbiol. Biotechnol. 2014, 98, 7549–7557. [Google Scholar] [CrossRef]
- Chen, J.-C.; Lee, W.-J.; Tsou, J.-J.; Liu, T.-P.; Tsai, P.-L. Effect of Probiotics on Postoperative Quality of Gastric Bypass Surgeries: A Prospective Randomized Trial. Surg. Obes. Relat. Dis. 2016, 12, 57–61. [Google Scholar] [CrossRef]
- Shang, X.; Zhang, X.; Du, C.; Ma, Z.; Jin, S.; Ao, N.; Yang, J.; Du, J. Clostridium Butyricum Alleviates Gut Microbiota Alteration-Induced Bone Loss after Bariatric Surgery by Promoting Bone Autophagy. J. Pharmacol. Exp. Ther. 2021, 377, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-Diabetic Effects of Clostridium Butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-Producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 7046. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shan, K.; Pan, L.-L.; Feng, N.; Lv, Z.; Sun, Y.; Li, J.; Wu, C.; Zhang, H.; Chen, W.; et al. Clostridium Butyricum CGMCC0313.1 Protects against Autoimmune Diabetes by Modulating Intestinal Immune Homeostasis and Inducing Pancreatic Regulatory T Cells. Front. Immunol. 2017, 8, 1345. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Ling, Z.; Yu, X.; Chen, W.; Li, H.; Jin, J.; Pang, M.; Zhang, H.; Yu, J.; et al. Clostridium Butyricum Attenuates Cerebral Ischemia/Reperfusion Injury in Diabetic Mice via Modulation of Gut Microbiota. Brain Res. 2016, 1642, 180–188. [Google Scholar] [CrossRef]
- Da Silva, C.C.; Monteil, M.A.; Davis, E.M. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child. Obes. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Castañeda-Márquez, A.C.; Díaz-Benítez, C.E.; Bahena-Roman, M.; Campuzano-Benítez, G.E.; Galván-Portillo, M.; Campuzano-Rincón, J.C.; Lagunas-Martínez, A.; Bermudez-Morales, V.H.; Orbe-Orihuela, Y.C.; Peralta-Romero, J.; et al. Lactobacillus paracasei as a Protective Factor of Obesity Induced by an Unhealthy Diet in Children. Obes. Res. Clin. Pract. 2020, 14, 271–278. [Google Scholar] [CrossRef]
- Carreras, N.L.; Martorell, P.; Chenoll, E.; Genovés, S.; Ramón, D.; Aleixandre, A. Anti-Obesity Properties of the Strain Bifidobacterium Animalis Subsp. Lactis CECT 8145 in Zücker Fatty Rats. Benef. Microbes 2018, 9, 629–641. [Google Scholar] [CrossRef]
- Huo, Y.; Lu, X.; Wang, X.; Wang, X.; Chen, L.; Guo, H.; Zhang, M.; Li, Y. Bifidobacterium Animalis Subsp. Lactis A6 Alleviates Obesity Associated with Promoting Mitochondrial Biogenesis and Function of Adipose Tissue in Mice. Molecules 2020, 25, 1490. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Zeng, X.; Tan, F.; Yi, R.; Pan, Y.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus Plantarum KFY04 Prevents Obesity in Mice through the PPAR Pathway and Alleviates Oxidative Damage and Inflammation. Food Funct. 2020, 11, 5460–5472. [Google Scholar] [CrossRef]
- Schellekens, H.; Torres-Fuentes, C.; van de Wouw, M.; Long-Smith, C.M.; Mitchell, A.; Strain, C.; Berding, K.; Bastiaanssen, T.F.S.; Rea, K.; Golubeva, A.V.; et al. Bifidobacterium Longum Counters the Effects of Obesity: Partial Successful Translation from Rodent to Human. EBioMedicine 2021, 63, 103176. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.-B.; Wen, J.; Zhao, Y.-C.; Tian, S.-J.; Zhang, X.-Y.; Wang, D.-H. Bifidobacterium Pseudolongum Reduces Triglycerides by Modulating Gut Microbiota in Mice Fed High-Fat Food. J. Steroid Biochem. Mol. Biol. 2020, 198, 105602. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Tang, M.-W.; Tan, F.; Zhou, X.-R.; Fan, L.; Xie, Y.-X.; Zhao, X. Anti-Obesity Effect of Lactobacillus Plantarum CQPC01 by Modulating Lipid Metabolism in High-Fat Diet-Induced C57BL/6 Mice. J. Food Biochem. 2020, 44, e13491. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yan, H.; Lu, Y.; Li, X.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Anti-Obesity Effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on High-Fat and High-Fructose Diet Mice Base on Inflammatory Response Alleviation and Gut Microbiota Regulation. Eur. J. Nutr. 2020, 59, 2709–2728. [Google Scholar] [CrossRef]
- Won, S.-M.; Chen, S.; Lee, S.Y.; Lee, K.E.; Park, K.W.; Yoon, J.-H. Lactobacillus sakei ADM14 Induces Anti-Obesity Effects and Changes in Gut Microbiome in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 12, 3703. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-M.; Han, S.-K.; Kim, J.-K.; Oh, S.-J.; Jang, H.-B.; Kim, D.-H. Lactobacillus sakei Alleviates High-Fat-Diet-Induced Obesity and Anxiety in Mice by Inducing AMPK Activation and SIRT1 Expression and Inhibiting Gut Microbiota-Mediated NF-ΚB Activation. Mol. Nutr. Food Res. 2019, 63, e1800978. [Google Scholar] [CrossRef]
- Yang, G.; Hong, E.; Oh, S.; Kim, E. Non-Viable Lactobacillus Johnsonii JNU3402 Protects against Diet-Induced Obesity. Foods 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Murga, M.L.; Olivares, M.; Sanz, Y. Bifidobacterium Pseudocatenulatum CECT 7765 Reverses the Adverse Effects of Diet-Induced Obesity through the Gut-Bone Axis. Bone 2020, 141, 115580. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, H.; Gao, F.; Qian, Z.; Mao, W.; Yin, Y.; Tan, J.; Chen, D. Antidiabetic Effects of Selenium-Enriched Bifidobacterium Longum DD98 in Type 2 Diabetes Model of Mice. Food Funct. 2020, 11, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Ben Othman, M.; Sakamoto, K. Effect of Inactivated Bifidobacterium Longum Intake on Obese Diabetes Model Mice (TSOD). Food Res. Int. 2020, 129, 108792. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic Effects of Lactobacillus reuteri DSM 17938 in People with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-C.; Tsai, W.-H.; Jheng, Y.-P.; Su, S.-L.; Wang, S.-Y.; Lin, C.-C.; Chen, Y.-H.; Chang, W.-W. The Beneficial Effects of Lactobacillus reuteri ADR-1 or ADR-3 Consumption on Type 2 Diabetes Mellitus: A Randomized, Double-Blinded, Placebo-Controlled Trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus Alleviates Type 2 Diabetes by Regulating Hepatic Glucose, Lipid Metabolism and Gut Microbiota in Mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium Strains and Galactooligosaccharides Improve Intestinal Barrier Function in Obese Adults but Show No Synergism When Used Together as Synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Groele, L.; Szajewska, H.; Szypowska, A. Effects of Lactobacillus rhamnosus GG and Bifidobacterium Lactis Bb12 on Beta-Cell Function in Children with Newly Diagnosed Type 1 Diabetes: Protocol of a Randomised Controlled Trial. BMJ Open 2017, 7, e017178. [Google Scholar] [CrossRef]
- Hossain, M.; Park, D.-S.; Rahman, M.S.; Ki, S.-J.; Lee, Y.R.; Imran, K.M.; Yoon, D.; Heo, J.; Lee, T.-J.; Kim, Y.-S. Bifidobacterium Longum DS0956 and Lactobacillus rhamnosus DS0508 Culture-Supernatant Ameliorate Obesity by Inducing Thermogenesis in Obese-Mice. Benef. Microbes 2020, 11, 361–373. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Huang, P.-H. Metabolic Engineering of Probiotic Escherichia coli for Cytolytic Therapy of Tumors. Sci. Rep. 2021, 11, 5853. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, H.; Liu, F.; Chen, Y.; Tang, S.; Ji, W.; Tang, J.; Liu, Z.; Sun, Y.; Hu, S.; et al. Escherichia coli Nissle 1917 Engineered to Express Tum-5 Can Restrain Murine Melanoma Growth. Oncotarget 2017, 8, 85772–85782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Xia, L.; Zhang, X.; Ding, X.; Yan, F.; Wu, F. Escherichia coli Nissle 1917 Targets and Restrains Mouse B16 Melanoma and 4T1 Breast Tumors through Expression of Azurin Protein. Appl. Environ. Microbiol. 2012, 78, 7603–7610. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Hong, Y.-H. In Situ Delivery of Biobutyrate by Probiotic Escherichia coli for Cancer Therapy. Sci. Rep. 2021, 11, 18172. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Yang, R.; Zhu, Z.; Zhang, L.; Wu, Z.; Sun, X. An Engineered Probiotic Secreting Sj16 Ameliorates Colitis via Ruminococcaceae/Butyrate/Retinoic Acid Axis. Bioeng. Transl. Med. 2021, 6, e10219. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.-Y.; Zhang, D.; Zhang, Y.-D.; Li, Z.-H.; Liu, X.; Wu, F.; Chen, G.-Q. Construction of a Sustainable 3-Hydroxybutyrate-Producing Probiotic Escherichia coli for Treatment of Colitis. Cell Mol. Immunol. 2021, 18, 2344–2357. [Google Scholar] [CrossRef]
- Cui, M.; Sun, T.; Li, S.; Pan, H.; Liu, J.; Zhang, X.; Li, L.; Li, S.; Wei, C.; Yu, C.; et al. NIR Light-Responsive Bacteria with Live Bio-Glue Coatings for Precise Colonization in the Gut. Cell Rep. 2021, 36, 109690. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pang, G.; Zhang, T.; Sun, T.; Zhang, L.; Kang, R.; Xue, X.; Pan, H.; Yang, C.; Zhang, X.; et al. Optotheranostic Nanosystem with Phone Visual Diagnosis and Optogenetic Microbial Therapy for Ulcerative Colitis At-Home Care. ACS Nano 2021, 15, 7040–7052. [Google Scholar] [CrossRef]
- Ma, J.; Li, C.; Wang, J.; Gu, J. Genetically Engineered Escherichia coli Nissle 1917 Secreting GLP-1 Analog Exhibits Potential Antiobesity Effect in High-Fat Diet-Induced Obesity Mice. Obesity 2020, 28, 315–322. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Chen, Z.; Guo, Y.; McMillan, C.; Flynn, C.R.; Davies, S.S. Two-Week Administration of Engineered Escherichia coli Establishes Persistent Resistance to Diet-Induced Obesity Even without Antibiotic Pre-Treatment. Appl. Microbiol. Biotechnol. 2019, 103, 6711–6723. [Google Scholar] [CrossRef]
- Geldart, K.G.; Kommineni, S.; Forbes, M.; Hayward, M.; Dunny, G.M.; Salzman, N.H.; Kaznessis, Y.N. Engineered E. Coli Nissle 1917 for the Reduction of Vancomycin-Resistant Enterococcus in the Intestinal Tract. Bioeng. Transl. Med. 2018, 3, 197–208. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Koh, E.; Wong, A.; March, J.C.; Bentley, W.E.; Lee, Y.S.; Chang, M.W. Engineered Probiotic Escherichia coli Can Eliminate and Prevent Pseudomonas Aeruginosa Gut Infection in Animal Models. Nat. Commun. 2017, 8, 15028. [Google Scholar] [CrossRef]
- May-Zhang, L.S.; Chen, Z.; Dosoky, N.S.; Yancey, P.G.; Boyd, K.L.; Hasty, A.H.; Linton, M.F.; Davies, S.S. Administration of N-Acyl-Phosphatidylethanolamine Expressing Bacteria to Low Density Lipoprotein Receptor-/- Mice Improves Indices of Cardiometabolic Disease. Sci. Rep. 2019, 9, 420. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Chaudhari, A.; Kumar, G.N. Amelioration of Cadmium- and Mercury-Induced Liver and Kidney Damage in Rats by Genetically Engineered Probiotic Escherichia coli Nissle 1917 Producing Pyrroloquinoline Quinone with Oral Supplementation of Citric Acid. Nutrition 2016, 32, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.S.; Raghuvanshi, R.; Kumar, G.N. Genetically Engineered Escherichia coli Nissle 1917 Synbiotic Counters Fructose-Induced Metabolic Syndrome and Iron Deficiency. Appl. Microbiol. Biotechnol. 2017, 101, 4713–4723. [Google Scholar] [CrossRef]
- Somabhai, C.A.; Raghuvanshi, R.; Nareshkumar, G. Genetically Engineered Escherichia coli Nissle 1917 Synbiotics Reduce Metabolic Effects Induced by Chronic Consumption of Dietary Fructose. PLoS ONE 2016, 11, e0164860. [Google Scholar] [CrossRef]
- Kurtz, C.B.; Millet, Y.A.; Puurunen, M.K.; Perreault, M.; Charbonneau, M.R.; Isabella, V.M.; Kotula, J.W.; Antipov, E.; Dagon, Y.; Denney, W.S.; et al. An Engineered E. Coli Nissle Improves Hyperammonemia and Survival in Mice and Shows Dose-Dependent Exposure in Healthy Humans. Sci. Transl. Med. 2019, 11, eaau7975. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, M.K.; Vockley, J.; Searle, S.L.; Sacharow, S.J.; Phillips, J.A.; Denney, W.S.; Goodlett, B.D.; Wagner, D.A.; Blankstein, L.; Castillo, M.J.; et al. Safety and Pharmacodynamics of an Engineered E. Coli Nissle for the Treatment of Phenylketonuria: A First-in-Human Phase 1/2a Study. Nat. Metab. 2021, 3, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Liu, Y.; Su, T.; Lin, C.; Shao, L.; Li, L.; Li, W.; Niu, G.; Yu, J.; et al. Engineering a Probiotic Strain of Escherichia coli to Induce the Regression of Colorectal Cancer through Production of 5-Aminolevulinic Acid. Microb. Biotechnol. 2021, 14, 2130–2139. [Google Scholar] [CrossRef]
- Huang, C.; Wang, F.-B.; Liu, L.; Jiang, W.; Liu, W.; Ma, W.; Zhao, H. Hypoxic Tumor Radiosensitization Using Engineered Probiotics. Adv. Healthc. Mater. 2021, 10, e2002207. [Google Scholar] [CrossRef] [PubMed]
- Canale, F.P.; Basso, C.; Antonini, G.; Perotti, M.; Li, N.; Sokolovska, A.; Neumann, J.; James, M.J.; Geiger, S.; Jin, W.; et al. Metabolic Modulation of Tumours with Engineered Bacteria for Immunotherapy. Nature 2021, 598, 662–666. [Google Scholar] [CrossRef]
- Duan, F.; March, J.C. Interrupting Vibrio Cholerae Infection of Human Epithelial Cells with Engineered Commensal Bacterial Signaling. Biotechnol. Bioeng. 2008, 101, 128–134. [Google Scholar] [CrossRef]
- Duan, F.; March, J.C. Engineered Bacterial Communication Prevents Vibrio Cholerae Virulence in an Infant Mouse Model. Proc. Natl. Acad. Sci. USA 2010, 107, 11260–11264. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, M.; Song, X.; Zhang, Z.; Zhang, Z.; Chen, Z.; Li, X. Bacterial Microbots for Acid-Labile Release of Hybrid Micelles to Promote the Synergistic Antitumor Efficacy. Acta Biomater. 2018, 78, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhao, L.; Song, X.; Tang, M.; Mo, C.; Li, X. Doxorubicin-Conjugated Escherichia coli Nissle 1917 Swimmers to Achieve Tumor Targeting and Responsive Drug Release. J. Control. Release 2017, 268, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Praveschotinunt, P.; Duraj-Thatte, A.M.; Gelfat, I.; Bahl, F.; Chou, D.B.; Joshi, N.S. Engineered E. Coli Nissle 1917 for the Delivery of Matrix-Tethered Therapeutic Domains to the Gut. Nat. Commun. 2019, 10, 5580. [Google Scholar] [CrossRef] [PubMed]
- Kan, A.; Gelfat, I.; Emani, S.; Praveschotinunt, P.; Joshi, N.S. Plasmid Vectors for in Vivo Selection-Free Use with the Probiotic E. Coli Nissle 1917. ACS Synth. Biol. 2021, 10, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Kraśko, J.A.; Žilionytė, K.; Darinskas, A.; Strioga, M.; Rjabceva, S.; Zalutsky, I.; Derevyanko, M.; Kulchitsky, V.; Lubitz, W.; Kudela, P.; et al. Bacterial Ghosts as Adjuvants in Syngeneic Tumour Cell Lysate-Based Anticancer Vaccination in a Murine Lung Carcinoma Model. Oncol. Rep. 2017, 37, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Hao, L.; Liu, X.; Borrás-Hidalgo, O.; Zhang, Y. Enhanced Anti-Proliferative Efficacy of Epothilone B Loaded with Escherichia coli Nissle 1917 Bacterial Ghosts on the HeLa Cells by Mitochondrial Pathway of Apoptosis. Drug Dev. Ind. Pharm. 2018, 44, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, P.; Zhang, Z.; Liu, Y.; Chen, M.; Li, S.; Li, X. Bacterial Navigation for Tumor Targeting and Photothermally-Triggered Bacterial Ghost Transformation for Spatiotemporal Drug Release. Acta Biomater. 2021, 131, 172–184. [Google Scholar] [CrossRef]
- Montanaro, J.; Inic-Kanada, A.; Ladurner, A.; Stein, E.; Belij, S.; Bintner, N.; Schlacher, S.; Schuerer, N.; Mayr, U.B.; Lubitz, W.; et al. Escherichia coli Nissle 1917 Bacterial Ghosts Retain Crucial Surface Properties and Express Chlamydial Antigen: An Imaging Study of a Delivery System for the Ocular Surface. Drug Des. Dev. Ther. 2015, 9, 3741–3754. [Google Scholar] [CrossRef][Green Version]
- Rosenthal, J.A.; Huang, C.-J.; Doody, A.M.; Leung, T.; Mineta, K.; Feng, D.D.; Wayne, E.C.; Nishimura, N.; Leifer, C.; DeLisa, M.P.; et al. Mechanistic Insight into the TH1-Biased Immune Response to Recombinant Subunit Vaccines Delivered by Probiotic Bacteria-Derived Outer Membrane Vesicles. PLoS ONE 2014, 9, e112802. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Jiang, B.; Jin, D.; Yang, Y.; Zhang, M.; Zhang, D.; Zhao, H.; Xu, M.; Song, H.; Wu, W.; et al. Engineered Recombinant Escherichia coli Probiotic Strains Integrated with F4 and F18 Fimbriae Cluster Genes in the Chromosome and Their Assessment of Immunogenic Efficacy in Vivo. ACS Synth. Biol. 2020, 9, 412–426. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, W.; He, L.; Chen, Y.; Ding, X.; Sun, Y.; Hu, S.; Yang, H.; Huang, W.; Zhang, Y.; et al. E. Coli Nissle 1917-Derived Minicells for Targeted Delivery of Chemotherapeutic Drug to Hypoxic Regions for Cancer Therapy. Theranostics 2018, 8, 1690–1705. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-Tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]
- Sturm, A.; Rilling, K.; Baumgart, D.C.; Gargas, K.; Abou-Ghazalé, T.; Raupach, B.; Eckert, J.; Schumann, R.R.; Enders, C.; Sonnenborn, U.; et al. Escherichia coli Nissle 1917 Distinctively Modulates T-Cell Cycling and Expansion via Toll-like Receptor 2 Signaling. Infect. Immun. 2005, 73, 1452–1465. [Google Scholar] [CrossRef]
- Ali, M.K.; Liu, Q.; Liang, K.; Li, P.; Kong, Q. Bacteria-Derived Minicells for Cancer Therapy. Cancer Lett. 2020, 491, 11–21. [Google Scholar] [CrossRef] [PubMed]
- van Passel, M.W.J.; Kant, R.; Zoetendal, E.G.; Plugge, C.M.; Derrien, M.; Malfatti, S.A.; Chain, P.S.G.; Woyke, T.; Palva, A.; de Vos, W.M.; et al. The Genome of Akkermansia muciniphila, a Dedicated Intestinal Mucin Degrader, and Its Use in Exploring Intestinal Metagenomes. PLoS ONE 2011, 6, e16876. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Behrouzi, A.; Shahriary, A.; Ahmadi Badi, S.; Davari, M.; Khatami, S.; Rahimi Jamnani, F.; Fateh, A.; Vaziri, F.; Siadat, S.D. Comparative Study of Effect of Akkermansia muciniphila and Its Extracellular Vesicles on Toll-like Receptors and Tight Junction. Gastroenterol. Hepatol. Bed Bench 2019, 12, 163–168. [Google Scholar] [PubMed]
- Kang, C.-S.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz Azizi Raftar, S.; Ashrafian, F.; Yadegar, A.; Lari, A.; Moradi, H.R.; Shahriary, A.; Azimirad, M.; Alavifard, H.; Mohsenifar, Z.; Davari, M.; et al. The Protective Effects of Live and Pasteurized Akkermansia muciniphila and Its Extracellular Vesicles against HFD/CCl4-Induced Liver Injury. Microbiol. Spectr. 2021, 9, e0048421. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, D.; Zhuo, J.; Lin, Z.; Yang, M.; Xu, X. The Gut-Liver Axis in Immune Remodeling: New Insight into Liver Diseases. Int. J. Biol. Sci. 2020, 16, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubfar, R.; Behrouzi, A.; Zare Banadkoki, E.; Ashrafian, F.; Lari, A.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; Khatami, S.; Siadat, S.D. Effect of Akkermansia muciniphila, Faecalibacterium Prausnitzii, and Their Extracellular Vesicles on the Serotonin System in Intestinal Epithelial Cells. Probiotics Antimicrob. Proteins 2021, 13, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of Serotonin Signaling/Metabolism by Akkermansia muciniphila and Its Extracellular Vesicles through the Gut-Brain Axis in Mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Chen, C.-Y.; Liu, Z.-Z.; Luo, Z.-W.; Rao, S.-S.; Jin, L.; Wan, T.-F.; Yue, T.; Tan, Y.-J.; Yin, H.; et al. Extracellular Vesicles from Child Gut Microbiota Enter into Bone to Preserve Bone Mass and Strength. Adv. Sci. 2021, 8, 2004831. [Google Scholar] [CrossRef]
- Morishita, M.; Horita, M.; Higuchi, A.; Marui, M.; Katsumi, H.; Yamamoto, A. Characterizing Different Probiotic-Derived Extracellular Vesicles as a Novel Adjuvant for Immunotherapy. Mol. Pharm. 2021, 18, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, Z.; Liang, Q.; Shen, H.; Zhao, Z.; Chen, Z.; He, R.; Feng, S.; Cao, D.; Gan, G.; et al. Overexpression of PEGF Improved the Gut Protective Function of Clostridium Butyricum Partly through STAT3 Signal Pathway. Appl. Microbiol. Biotechnol. 2021, 105, 5973–5991. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Zhang, Q.; Xu, Z.; Wang, J.; Sun, H. Oral Engineered Bifidobacterium Longum Expressing RhMnSOD to Suppress Experimental Colitis. Int. Immunopharmacol. 2018, 57, 25–32. [Google Scholar] [CrossRef]
- Wei, P.; Yang, Y.; Ding, Q.; Li, X.; Sun, H.; Liu, Z.; Huang, J.; Gong, Y. Oral Delivery of Bifidobacterium Longum Expressing α-Melanocyte-Stimulating Hormone to Combat Ulcerative Colitis. J. Med. Microbiol. 2016, 65, 160–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, Z.; Huang, Z.; Sao, C.; Huang, Y.; Zhang, F.; Yang, J.; Lian, J.; Zeng, Z.; Luo, W.; Zeng, W.; et al. Bifidobacterium as an Oral Delivery Carrier of Interleukin-12 for the Treatment of Coxsackie Virus B3-Induced Myocarditis in the Balb/c Mice. Int. Immunopharmacol. 2012, 12, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Long, R.T.; Zeng, W.S.; Chen, L.Y.; Guo, J.; Lin, Y.Z.; Huang, Q.S.; Luo, S.Q. Bifidobacterium as an Oral Delivery Carrier of Oxyntomodulin for Obesity Therapy: Inhibitory Effects on Food Intake and Body Weight in Overweight Mice. Int. J. Obes. 2010, 34, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Yang, Y.; Li, T.; Ding, Q.; Sun, H. A Engineered Bifidobacterium Longum Secreting a Bioative Penetratin-Glucagon-like Peptide 1 Fusion Protein Enhances Glucagon-like Peptide 1 Absorption in the Intestine. J. Microbiol. Biotechnol. 2015, in press. [Google Scholar]
- Oh, J.-H.; Schueler, K.L.; Stapleton, D.S.; Alexander, L.M.; Yen, C.-L.E.; Keller, M.P.; Attie, A.D.; van Pijkeren, J.-P. Secretion of Recombinant Interleukin-22 by Engineered Lactobacillus reuteri Reduces Fatty Liver Disease in a Mouse Model of Diet-Induced Obesity. mSphere 2020, 5, e00183-20. [Google Scholar] [CrossRef]
- Hendrikx, T.; Duan, Y.; Wang, Y.; Oh, J.-H.; Alexander, L.M.; Huang, W.; Stärkel, P.; Ho, S.B.; Gao, B.; Fiehn, O.; et al. Bacteria Engineered to Produce IL-22 in Intestine Induce Expression of REG3G to Reduce Ethanol-Induced Liver Disease in Mice. Gut 2019, 68, 1504–1515. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, X.; Miao, Y.; Han, Y.; Wei, J.; Chen, T. Therapeutic Effect of GLP-1 Engineered Strain on Mice Model of Alzheimer’s Disease and Parkinson’s Disease. AMB Express 2020, 10, 80. [Google Scholar] [CrossRef]
- Buford, T.W.; Sun, Y.; Roberts, L.M.; Banerjee, A.; Peramsetty, S.; Knighton, A.; Verma, A.; Morgan, D.; Torres, G.E.; Li, Q.; et al. Angiotensin (1-7) Delivered Orally via Probiotic, but Not Subcutaneously, Benefits the Gut-Brain Axis in Older Rats. Geroscience 2020, 42, 1307–1321. [Google Scholar] [CrossRef]
- Esposito, G.; Pesce, M.; Seguella, L.; Lu, J.; Corpetti, C.; Del Re, A.; De Palma, F.D.E.; Esposito, G.; Sanseverino, W.; Sarnelli, G. Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice. Int. J. Mol. Sci. 2021, 22, 2945. [Google Scholar] [CrossRef]
- Jia, S.; Huang, X.; Li, H.; Zheng, D.; Wang, L.; Qiao, X.; Jiang, Y.; Cui, W.; Tang, L.; Li, Y.; et al. Immunogenicity Evaluation of Recombinant Lactobacillus casei W56 Expressing Bovine Viral Diarrhea Virus E2 Protein in Conjunction with Cholera Toxin B Subunit as an Adjuvant. Microb. Cell Fact. 2020, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, L.; Ma, S.; Wang, X.; Wang, Y.; Xiao, Y.; Jiang, Y.; Qiao, X.; Tang, L.; Xu, Y.; et al. Immunogenicity of EGFP-Marked Recombinant Lactobacillus casei against Transmissible Gastroenteritis Virus and Porcine Epidemic Diarrhea Virus. Viruses 2017, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; et al. Oral Recombinant Lactobacillus vaccine Targeting the Intestinal Microfold Cells and Dendritic Cells for Delivering the Core Neutralizing Epitope of Porcine Epidemic Diarrhea Virus. Microb. Cell Fact. 2018, 17, 20. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, X.; Jiang, Y.; Tang, L.; Xu, Y.; Qiao, X.; Min, L.; Wen, C.; Ma, G.; Li, Y. Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets. Viruses 2018, 10, 106. [Google Scholar] [CrossRef]
- Duan, K.; Hua, X.; Wang, Y.; Wang, Y.; Chen, Y.; Shi, W.; Tang, L.; Li, Y.; Liu, M. Oral Immunization with a Recombinant Lactobacillus Expressing CK6 Fused with VP2 Protein against IPNV in Rainbow Trout (Oncorhynchus Mykiss). Fish Shellfish Immunol. 2018, 83, 223–231. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, X.; Ren, X.; Duan, K.; Gao, S.; Sun, J.; Feng, Y.; Zhou, Y.; Guan, X.; Li, D.; et al. Oral Immunization with Recombinant Lactobacillus casei Displayed AHA1-CK6 and VP2 Induces Protection against Infectious Pancreatic Necrosis in Rainbow Trout (Oncorhynchus Mykiss). Fish Shellfish Immunol. 2020, 100, 18–26. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Y.; Wang, Z.; Bai, J.; Jia, S.; Feng, B.; Jiang, Y.; Cui, W.; Tang, L.; Li, Y.; et al. Oral Immunization of Mice with a Probiotic Lactobacillus casei Constitutively Expressing the α-Toxoid Induces Protective Immunity against Clostridium Perfringens α-Toxin. Virulence 2019, 10, 166–179. [Google Scholar] [CrossRef]
- Yu, M.; Qi, R.; Chen, C.; Yin, J.; Ma, S.; Shi, W.; Wu, Y.; Ge, J.; Jiang, Y.; Tang, L.; et al. Immunogenicity of Recombinant Lactobacillus casei-Expressing F4 (K88) Fimbrial Adhesin FaeG in Conjunction with a Heat-Labile Enterotoxin A (LTAK63) and Heat-Labile Enterotoxin B (LTB) of Enterotoxigenic Escherichia coli as an Oral Adjuvant in Mice. J. Appl. Microbiol. 2017, 122, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yue, J.; Deng, Y.; Yang, S.; Zhu, L.; Xu, Z. Immunogenicity of engineered Lactobacillus plantarum expressing porcine epidemic diarrhea virus S1 gene. Chin. J. Biotechnol. 2021, 37, 2779–2785. [Google Scholar] [CrossRef]
- Pan, N.; Liu, B.; Bao, X.; Zhang, H.; Sheng, S.; Liang, Y.; Pan, H.; Wang, X. Oral Delivery of Novel Recombinant Lactobacillus Elicit High Protection against Staphylococcus Aureus Pulmonary and Skin Infections. Vaccines 2021, 9, 984. [Google Scholar] [CrossRef]
- Wei, W.; Wiggins, J.; Hu, D.; Vrbanac, V.; Bowder, D.; Mellon, M.; Tager, A.; Sodroski, J.; Xiang, S.-H. Blocking HIV-1 Infection by Chromosomal Integrative Expression of Human CD4 on the Surface of Lactobacillus acidophilus ATCC 4356. J. Virol. 2019, 93, e01830-18. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Jin, B.; Monis, P.; Saint, C. A Genetic and Metabolic Approach to Redirection of Biochemical Pathways of Clostridium Butyricum for Enhancing Hydrogen Production. Biotechnol. Bioeng. 2013, 110, 338–342. [Google Scholar] [CrossRef]
- Cai, G.; Jin, B.; Saint, C.; Monis, P. Genetic Manipulation of Butyrate Formation Pathways in Clostridium Butyricum. J. Biotechnol. 2011, 155, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yun, J.; Zhang, H.; Magocha, T.A.; Zabed, H.; Xue, Y.; Fokum, E.; Sun, W.; Qi, X. Genetically Engineered Strains: Application and Advances for 1,3-Propanediol Production from Glycerol. Food Technol. Biotechnol. 2018, 56, 3–15. [Google Scholar] [CrossRef]
- Kuehne, S.A.; Minton, N.P. ClosTron-Mediated Engineering of Clostridium. Bioengineered 2012, 3, 247–254. [Google Scholar] [CrossRef]
- Yu, M.; Du, Y.; Jiang, W.; Chang, W.-L.; Yang, S.-T.; Tang, I.-C. Effects of Different Replicons in Conjugative Plasmids on Transformation Efficiency, Plasmid Stability, Gene Expression and n-Butanol Biosynthesis in Clostridium Tyrobutyricum. Appl. Microbiol. Biotechnol. 2012, 93, 881–889. [Google Scholar] [CrossRef]
- Heap, J.T.; Pennington, O.J.; Cartman, S.T.; Carter, G.P.; Minton, N.P. The ClosTron: A Universal Gene Knock-out System for the Genus Clostridium. J. Microbiol. Methods 2007, 70, 452–464. [Google Scholar] [CrossRef]
- Ng, Y.K.; Ehsaan, M.; Philip, S.; Collery, M.M.; Janoir, C.; Collignon, A.; Cartman, S.T.; Minton, N.P. Expanding the Repertoire of Gene Tools for Precise Manipulation of the Clostridium Difficile Genome: Allelic Exchange Using PyrE Alleles. PLoS ONE 2013, 8, e56051. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Luo, H.; Wang, Y.; Wang, Y.; Tu, T.; Qin, X.; Su, X.; Bai, Y.; Yao, B.; et al. Exploiting Heterologous and Endogenous CRISPR-Cas Systems for Genome Editing in the Probiotic Clostridium Butyricum. Biotechnol. Bioeng. 2021, 118, 2448–2459. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Shimizu, H.; Akiyama, Y.; Taniguchi, S. In Situ Delivery and Production System of Trastuzumab ScFv with Bifidobacterium. Biochem. Biophys. Res. Commun. 2017, 493, 306–312. [Google Scholar] [CrossRef]
- Wei, C.; Xun, A.Y.; Wei, X.X.; Yao, J.; Wang, J.Y.; Shi, R.Y.; Yang, G.H.; Li, Y.X.; Xu, Z.L.; Lai, M.G.; et al. Bifidobacteria Expressing Tumstatin Protein for Antitumor Therapy in Tumor-Bearing Mice. Technol. Cancer Res. Treat. 2016, 15, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.Z.; Rodrigues, N.d.C.; Gonzaga, M.I.; Paiolo, J.C.C.; de Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci Junior, E.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella Copri and Bacteroides Vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, F.; Shao, L.; Cheng, Y.; Li, L. Dysbiosis of the Urinary Microbiota Associated with Urine Levels of Proinflammatory Chemokine Interleukin-8 in Female Type 2 Diabetic Patients. Front. Immunol. 2017, 8, 1032. [Google Scholar] [CrossRef]
- Tang-Fichaux, M.; Chagneau, C.V.; Bossuet-Greif, N.; Nougayrède, J.-P.; Oswald, É.; Branchu, P. The Polyphosphate Kinase of Escherichia coli Is Required for Full Production of the Genotoxin Colibactin. mSphere 2020, 5, e01195-20. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 733–745. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila Exacerbates Gut Inflammation in Salmonella Typhimurium-Infected Gnotobiotic Mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; van Mil, S.W.C.; Müller, M.; Kleerebezem, M.; van der Meer, R. Gut Microbiota Facilitates Dietary Heme-Induced Epithelial Hyperproliferation by Opening the Mucus Barrier in Colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef]
- Ghoddusi, H.B.; Sherburn, R. Preliminary Study on the Isolation of Clostridium Butyricum Strains from Natural Sources in the UK and Screening the Isolates for Presence of the Type E Botulinal Toxin Gene. Int. J. Food Microbiol. 2010, 142, 202–206. [Google Scholar] [CrossRef]
- Abe, Y.; Negasawa, T.; Monma, C.; Oka, A. Infantile Botulism Caused by Clostridium Butyricum Type E Toxin. Pediatr. Neurol. 2008, 38, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Mizuma, M.; Nakagawa, K.; Aoki, S.; Miura, T.; Takadate, T.; Ariake, K.; Maeda, S.; Kawaguchi, K.; Masuda, K.; et al. Probiotic-Related Bacteremia after Major Hepatectomy for Biliary Cancer: A Report of Two Cases. Surg. Case Rep. 2021, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Mays, Z.J.S.; Chappell, T.C.; Nair, N.U. Quantifying and Engineering Mucus Adhesion of Probiotics. ACS Synth. Biol. 2020, 9, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Ortega Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 10224. [Google Scholar] [CrossRef] [PubMed]

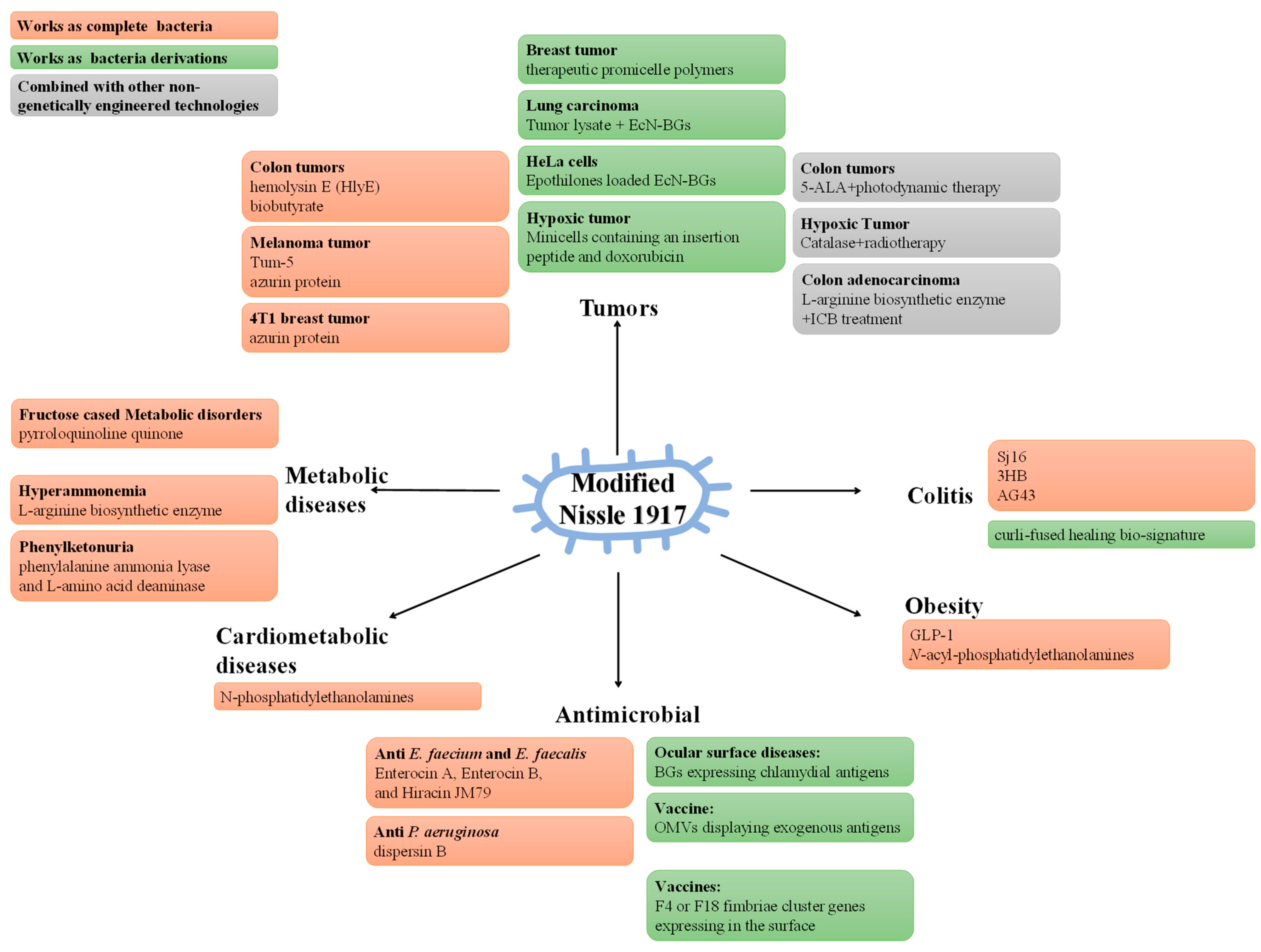
| Strategy | Mechanisms | Functions/Benefits | Reference | |
|---|---|---|---|---|
| Express direct therapeutic factors | express HlyE | the cytotoxicity of released HlyE | against tumors | [192] |
| express Tum-5 | the anti-angiogenesis effects of released Tum-5 | against tumors | [193] | |
| express azurin | azurin selectively kills cancer cells | against tumors | [194] | |
| express colibactin | the cytotoxicity of released colibactin | against tumors | [45] | |
| express glidobactin | the cytotoxicity of released glidobactin | against tumors | [45] | |
| express luminmide | the cytotoxicity of released luminmide | against tumors | [45] | |
| express butyrate | the cytotoxicity of released butyrate | against tumors | [195] | |
| express Sj16 | via Ruminococcaceae/butyrate/retinoic acid axis | against colitis | [196] | |
| express 3HB | regulate gut microbiota, relieve architectural changes, inflammatory cell infiltrations, and epithelial injuries | against colitis | [197] | |
| express autotransporter 43 adhesins antigen | use optogenetics to activate secretion | against colitis/precise spatiotemporal colonization | [198] | |
| express IL-10 | phone visual diagnosis and optogenetics based secretion | against colitis/mobile health service | [199] | |
| express GLP-1 analog | GLP-1 analog diminishes food intake | against obesity | [200] | |
| express N-acyl-phosphatidylethanolamines | N-acyl-phosphatidylethanolamines induce satiety | against obesity | [201] | |
| express antimicrobial peptides | antimicrobial peptides target and kill Enterococcus | against vancomycin-resistant Enterococcus | [202] | |
| develop a synthetic genetic system | sense and kill Pseudomonas aeruginosa | against Pseudomonas aeruginosa | [203] | |
| express N-acyl-phosphatidylethanolamines | reduce body weight, liver inflammation, fibrosis and atherosclerotic necrosis | against cardiovascular metabolic disease | [204] | |
| express PQQ and other metabolizing enzymes | relieve oxidative stress | against heavy metal toxicity/iron deficiency/fructose-induced dyslipidemia/hyperglycemia/hepatic steatosis | [205,206,207] | |
| express l-arg biosynthetic enzyme | exhausts ammonia for L-arginine biosynthesis | against hyperammonemia | [208] | |
| express phenylalanine ammonia lyase and L-amino acid deaminase | consume phenylalanine within the gastrointestinal tract | against PKU | [209] | |
| Express adjuvant therapeutic factors | express 5-ALA | 5-ALA contributes to photodynamic therapy | against cancer | [210] |
| express CAT | increase the production of O2 contributing to radiotherapy | against cancer | [211] | |
| produce high local concentrations of arginine | the enhancement on T-cell anti-tumor activity of L-arginine in tumors | against cancer | [212] | |
| express cholera autoinducer 1 | use the qurom sensing of bacteria | against Vibrio cholerae | [213,214] | |
| Targeted delivery system | connect therapeutic promicelle polymers on bacteria | connection material responsively to acidic tumor microenvironment to release drug | against cancer | [215,216] |
| secrete curli-fused healing bio-signature | curli fibers bind firmly to inflammation site and transport therapeutic factors | against colitis | [217,218] | |
| use EcN-GBs as an adjuvant | induce cellular immune responses | against LLC | [219] | |
| EcN-GBs load Epothilones | the cytotoxicity of released Epothilones | against cancer | [220] | |
| modified EcN-GBs temporal and spatial release of contained drugs | using photothermal effect of nanorods to modulate release | release modulation | [221] | |
| express chlamydial antigens | induce cellular immune responses | against ocular surface diseases | [222] | |
| display exogenous antigens (ClyA fusion chimera) on the surface of EcN-OMVs | induce cellular immune responses | recombinant subunit vaccines deliver | [223] | |
| express F4 or F18 fimbriae in the surface of EcN | induce cellular immune responses | live vaccine application | [224] | |
| EcN-derived minicells loaded with a low-pH insertion peptide and doxorubicin | greater drug loading and therapeutic index contribute to doxorubicin tumor regression | against cancer | [225] | |
| Probiotics | Strategy | Mechanisms | Functions/Benefits | Reference | |
|---|---|---|---|---|---|
| Akkermansia muciniphila | OMVS delivery/treatment | regulate inflammation, energy homeostasis, intestinal barrier | against obesity | [230] | |
| OMVS delivery/treatment | activate the AMPK pathway and increase TJ gene expressions | against leaky gut | [231,232] | ||
| OMVS delivery/treatment | regulate inflammation, epithelial stability | against DSS-induced colitis | [233] | ||
| OMVS delivery/treatment | reduce inflammation, reverse the activation of hepatic stellate cells and normalize serum glucose, lipid profiles, liver enzymes | against HFD/CCl4-induced liver fibrosis | [234] | ||
| OMVS delivery/treatment | promote osteogenesis and inhibit osteoclastogenesis | against osteoporosis | [238] | ||
| OMVS delivery/treatment | regulate CD8+ T cells and macrophages | against prostate cancer | [62] | ||
| OMVS delivery/treatment | induce serotonin biosynthesis | promote serotonin | [236] | ||
| Clostridium butyricum | OMVS delivery/treatment | enhance proinflammatory cytokine production | stimulate the immune system | [239] | |
| overexpress epidermal growth factor | activate STAT3 signal pathway and inhibit inflammation | gut protection | [240] | ||
| Bifidobacterium longum | express RhMnSOD | regulate inflammatory cytokines | against DSS-induced colitis | [241] | |
| express α-melanocyte-stimulating hormones | inhibit NF-κB p65 expression | against DSS-induced colitis | [242] | ||
| express interleukin-12 | upregulate the expression of Th1 cytokines (IFN-γ and TNF-α) | against coxsackie virus B3-induced myocarditis | [243] | ||
| express oxyntomodulin | reduces food intake, body weight and blood lipid levels | against obesity | [244] | ||
| express GLP-1 | improve the efficiency of glucose control | against type 2 diabetes | [245] | ||
| Lactic acid bacteria | Limosilactobacillus reuteri | express IL-22 | regulate REG3 via STAT3 | against nonalcoholic fatty liver | [246] |
| Limosilactobacillus reuteri | express IL-22 | induce expression of REG3G | against ethanol-induced steatohepatitis | [247] | |
| Lactococcus lactis | express GLP-1 | downregulate TLR4/NF-κB, upregulated the AKT/GSK3β signaling pathway and reverse disturbed microbiota | against Alzheimer/Parkinson | [248] | |
| Lacticaseibacillus paracasei subsp. paracasei | express angiotensin | increase beneficial circulating neurotransmitters and reduce neuro-inflammatory gene expression | benefits the gut-brain axis | [249] | |
| Lacticaseibacillus paracasei subsp. paracasei | express palmitoylethanolamide | block mucosal immune cell infiltration and the release of pro-inflammatory cytokines | against colitis | [250] | |
| Lacticaseibacillus casei | express bovine viral diarrhea virus E2 protein | induce cellular immune responses | against BVDV | [251] | |
| Lacticaseibacillus casei | express antigenic site of TGEV S protein and major antigen site of PEDV S protein | induce cellular immune responses | against TGEV and PEDV | [252,253,254] | |
| Lacticaseibacillus casei | express IPNV protein antigen VP2 | induce cellular immune responses | against IPNV | [255,256] | |
| Lacticaseibacillus casei | express toxoid of Clostridium perfringens α-toxin | induce cellular immune responses | against Clostridium perfringens | [257] | |
| Lacticaseibacillus casei | express the F4 fimbrial adhesin main subunit | induce cellular immune responses | against F4+ enterotoxigenic Escherichia coli | [258] | |
| Lactiplantibacillus plantarum subsp. plantarum | express PEDV S1 protein | induce cellular immune responses | against PEDV | [259] | |
| Lactiplantibacillus plantarum subsp. plantarum | express Staphylococcus aureus nontoxic mutated α-hemolysins | induce cellular immune responses | against Staphylococcus aureus | [260] | |
| Lactobacillus acidophilus | express Human CD4 on the surface | capture and neutralize HIV-1 | against HIV-1 | [261] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Zhao, Z.; Zhao, Z.; Chen, Y.; Zhang, L. Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases. Int. J. Mol. Sci. 2022, 23, 594. https://doi.org/10.3390/ijms23020594
Shen H, Zhao Z, Zhao Z, Chen Y, Zhang L. Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases. International Journal of Molecular Sciences. 2022; 23(2):594. https://doi.org/10.3390/ijms23020594
Chicago/Turabian StyleShen, Haokun, Zitong Zhao, Zengjue Zhao, Yuyi Chen, and Linghua Zhang. 2022. "Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases" International Journal of Molecular Sciences 23, no. 2: 594. https://doi.org/10.3390/ijms23020594
APA StyleShen, H., Zhao, Z., Zhao, Z., Chen, Y., & Zhang, L. (2022). Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases. International Journal of Molecular Sciences, 23(2), 594. https://doi.org/10.3390/ijms23020594





