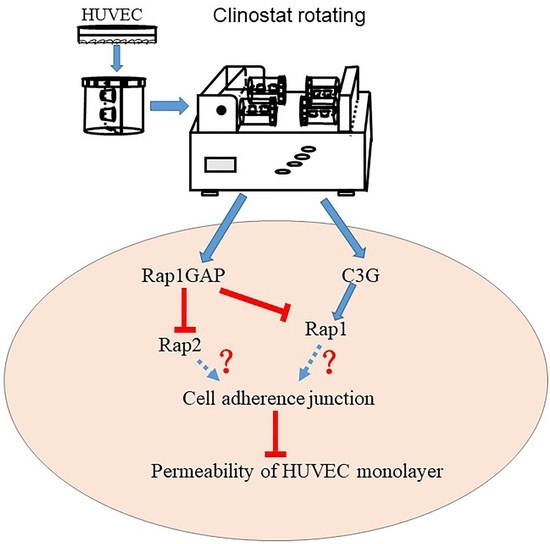
Simulated Microgravity Increases the Permeability of HUVEC Monolayer through Up-Regulation of Rap1GAP and Decreased Rap2 Activation
Abstract
:1. Introduction
2. Results
2.1. Modulation of Endothelium Permeability by Simulated Microgravity
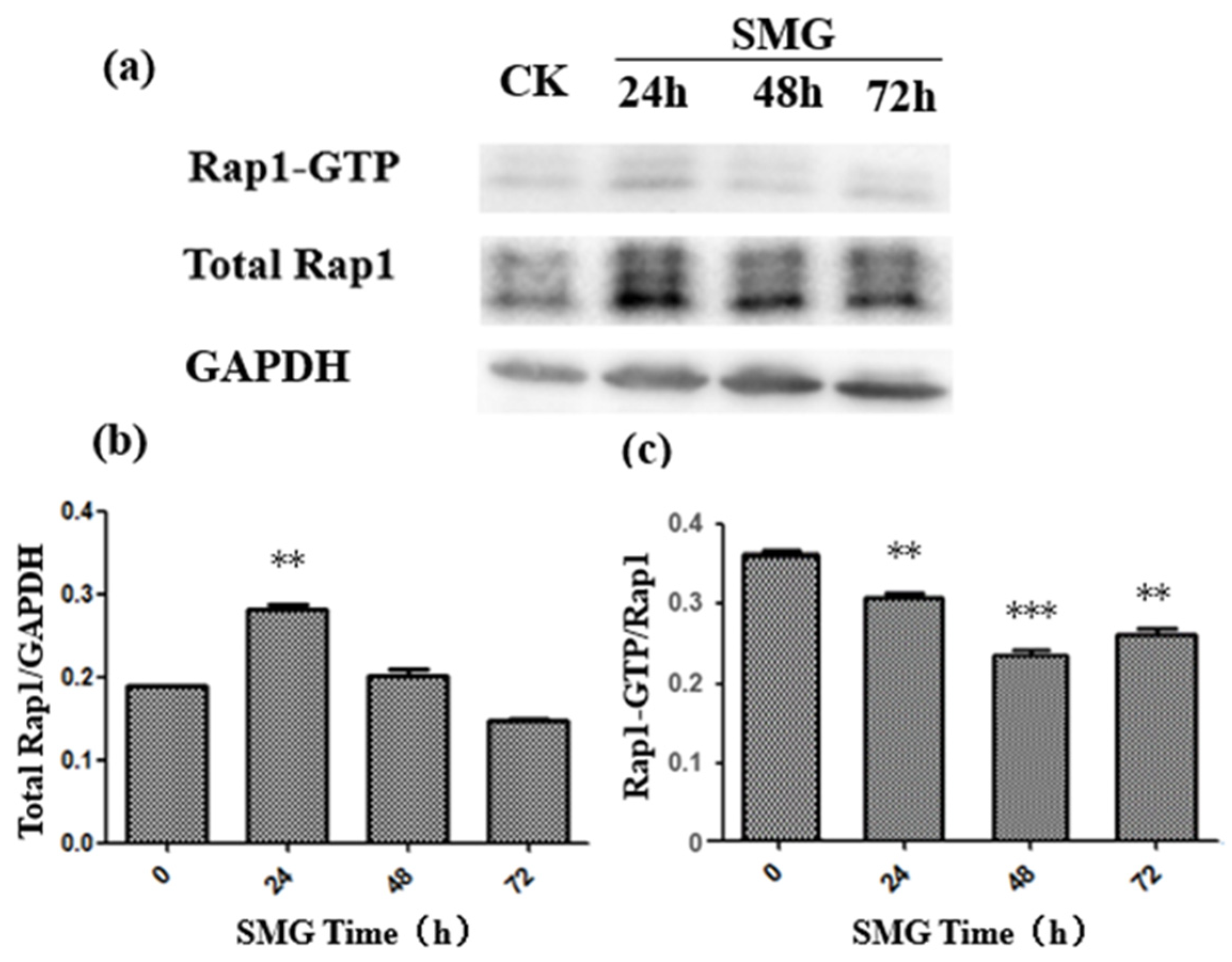
2.2. The Activation of Rap2 Was Down-Regulated under SMG
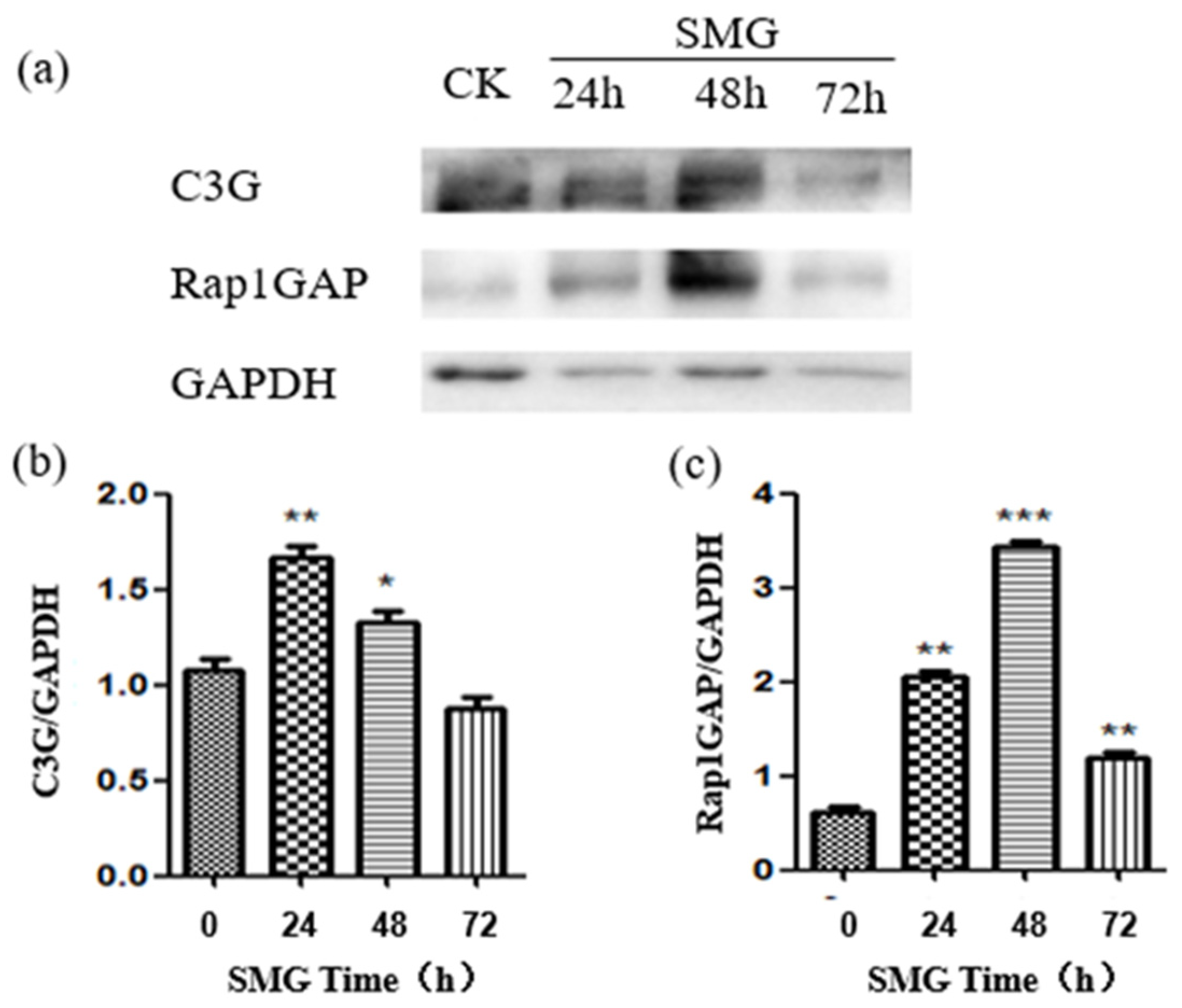
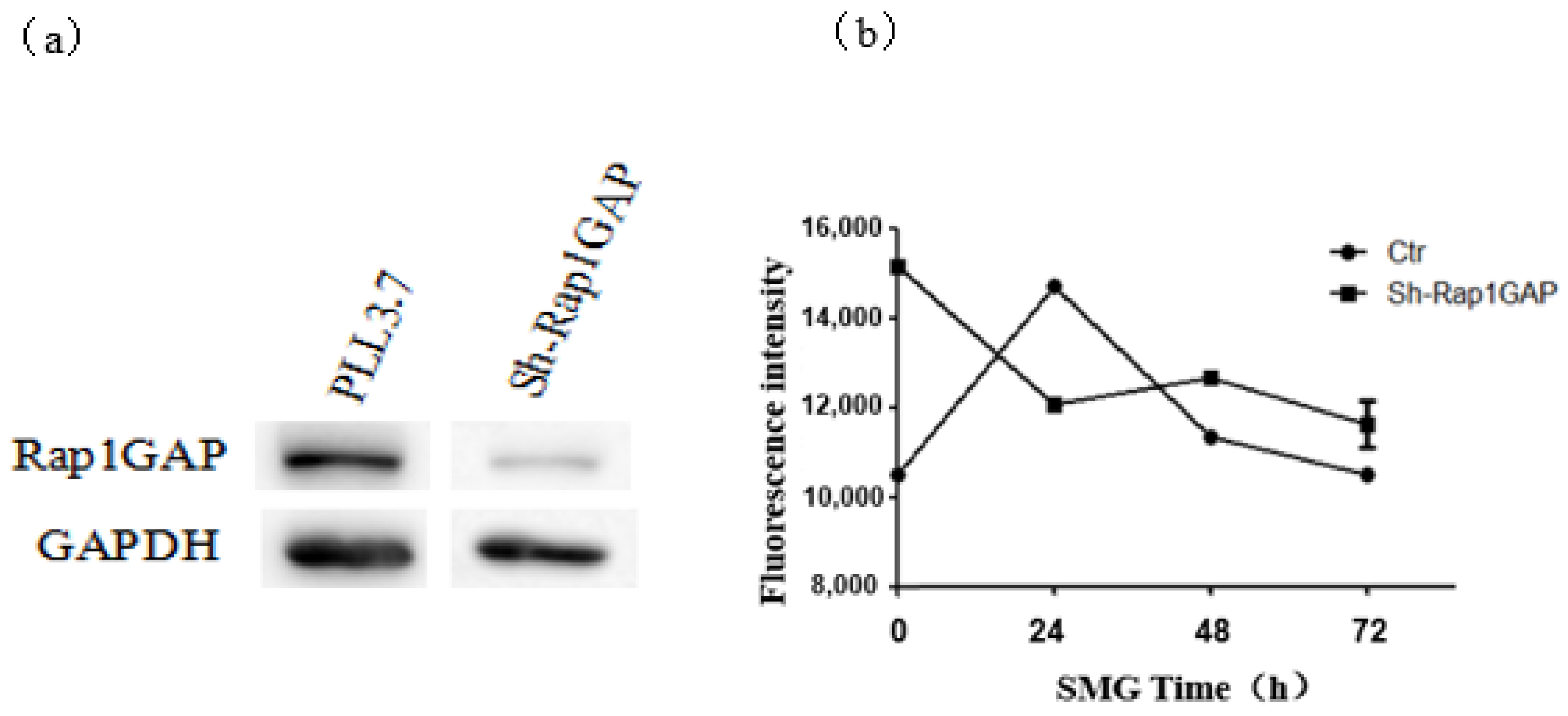
2.3. Rap1GAP Was Down-Regulated under Simulated Microgravity
2.4. The Expression of VE-Cadherin and β-Catenin Was Down-Regulated under Simulated Microgravity
2.5. The Distribution of Actin Filaments Was Modulated by Simulated Microgravity
2.6. The Knock-Down of Rap1GAP Decreased the Permeability of HUVEC Monolayer under Simulated Microgravity
3. Discussion
4. Materials and Methods
4.1. Cultures of HUVEC
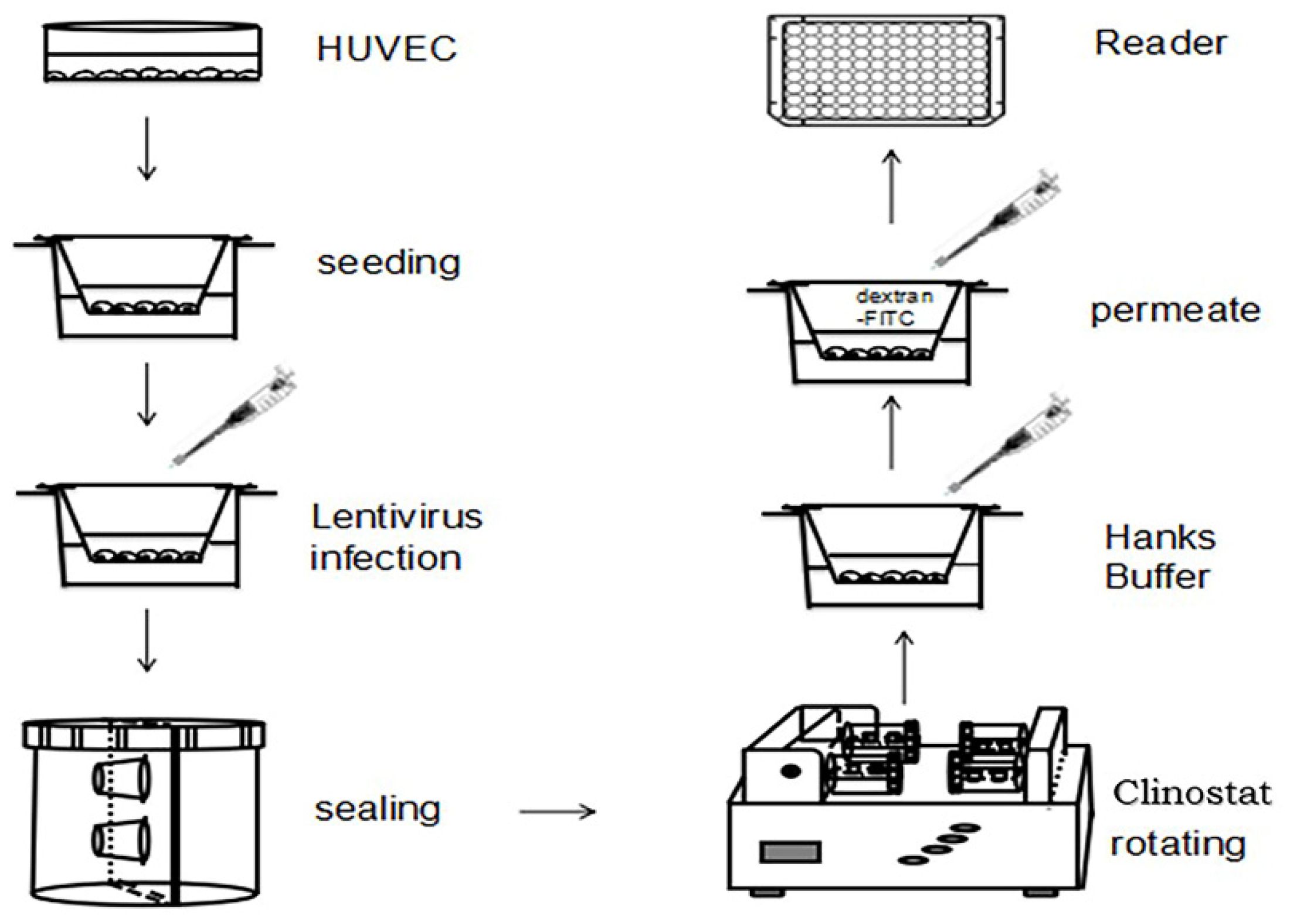
4.2. Cell Culture in Rotating Culture Vessel
4.3. Construction of Interference Vector
4.4. Virus Packaging and Infection
4.5. Screening of HUVEC Cells Expressing shRNAs
4.6. HUVEC Monolayer Permeability Model
4.7. Immunofluorescence Analysis
4.8. Western Blot
4.9. GST Pull-Down Assay for Activation of Rap1 and Rap2
4.10. Data Analysis
5. Conclusions
- (1)
- The simulated microgravity effect can affect the expression level and activation state of Rap1 and Rap2 in the cell, and the SMG condition will affect the adhesion and connection of monolayer HUVEC cells. With the extension of the treatment, the strength of the adhesion and connection between the cells would decrease and then recover, reaching the weakest state at 24 h of SMG.
- (2)
- The simulated microgravity effect has an impact on the expression of four proteins that have an impact on cell adhesion and junction in HUVEC cells: Rap1GAP, C3G, VE-cadherin, and β-catenin. Under the SMG effect, the expression of Rap1GAP and C3G proteins would increase and then decrease, while that of VE-cadherin and β-catenin proteins would decrease and then recover.
- (3)
- Under the simulated microgravity effect, the two proteins Rap1GAP and VE-cadherin would affect the adhesion and connection state of the monolayer HUVEC cells. When the intracellular Rap1GAP was interfered with, the intercellular adhesion would slightly enhance under the SMG effect. When intracellular VE-cadherin was interfered with, the adhesion and junction between cells under the effect of SMG would slightly enhance and then recover.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMG | simulated microgravity effect |
| CK | check or control |
| GEF | guanine nucleotide exchange factor |
| kDA | kilodalton |
| DAPI | 4′,6-diamidine-2-phenylindol |
| RWVS | rotating wall vessel |
| PFA | paraformaldehyde |
| PBS | phosphate buffer saline |
| RIPA | radio immunoprecipitation assay |
| HRP | horseradish peroxidase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
References
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Alexander, G.P.; Francisco, C.M.A.; Martha, V.A.; Hilda, V.R.; Michael, S. The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb. Haemost. 2015, 113, 20–36. [Google Scholar]
- Laura, F.M.; Beatriz, M.R.; Carolina, L.B.; Mariona, G.; Robert, J.C.; Natalia, R.R.; Anaïs, J.; Eva, C.M.; Isabel, C.; Susan, C. Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function. Arter. Thromb. Vasc. Biol. 2012, 32, e90–e102. [Google Scholar]
- Lampugnani, M.G.; Corada, M.; Caveda, L.; Breviario, F.; Ayalon, O.; Geiger, B.; Dejana, E. The molecular organization of endothelial cell to cell junctions: Differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J. Cell Biol. 1995, 129, 203–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Sano, K.; Tanihara, H. Diversity of the cadherin family: Evidence for eight new cadherins in nervous tissue. Cell Regul. 1991, 2, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Lampugnani, M.G.; Zanetti, A.; Breviario, F.; Balconi, G.; Orsenigo, F.; Corada, M.; Spagnuolo, R.; Betson, M.; Braga, V.; Dejana, E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol. Biol. Cell 2002, 13, 1175–1189. [Google Scholar] [CrossRef]
- Iyer, S.; Ferreri, D.M.; DeCocco, N.C.; Minnear, F.L.; Vincent, P.A. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L1143–L1153. [Google Scholar] [CrossRef]
- Gianfranco, B.; Elisabetta, D. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar]
- Hägerling, R.; Hoppe, E.; Dierkes, C.; Stehling, M.; Makinen, T.; Butz, S.; Vestweber, D.; Kiefer, F. Distinct roles of VE-cadherin for development and maintenance of specific lymph vessel beds. EMBO J. 2018, 37, e98271. [Google Scholar] [CrossRef]
- Castro Dias, M.; Mapunda, J.A.; Vladymyrov, M.; Engelhardt, B. Structure andjunctional complexes of endothelial, epithelial and glial brain barriers. Int. J. Mol. Sci. 2019, 20, 5372. [Google Scholar] [CrossRef] [Green Version]
- Grimsley-Myers, C.M.; Isaacson, R.H.; Cadwell, C.M.; Campos, J.; Hernandes, M.S.; Myers, K.R.; Kowalczyk, A.P. VE-cadherin endocytosis controls vascular integrity and patterning during development. J. Cell Biol. 2020, 219, e201909081. [Google Scholar] [CrossRef] [Green Version]
- Chiasson, C.M.; Wittich, K.B.; Vincent, P.A.; Faundez, V.; Kowalczyk, A.P. Kowalczyk P120-Catenin Inhibits VE-Cadherin Internalization through a Rho-independent Mechanism. Mol. Biol. Cell 2009, 20, 1970–1980. [Google Scholar] [CrossRef] [Green Version]
- Lisowska, J.; Rödel, C.J.; Manet, S.; Miroshnikova, Y.A.; Boyault, C.; Planus, E.; De Mets, R.; Lee, H.H.; Destaing, O.; Mertani, H.; et al. The CCM1-CCM2 complex controls complementary functions of ROCK1 and ROCK2 that are required for endothelial integrity. J. Cell Sci. 2018, 131, jcs216093. [Google Scholar] [CrossRef] [Green Version]
- Cain, R.J.; Vanhaesebroeck, B.; Ridley, A.J. The PI3K p110α isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J. Cell Biol. 2010, 188, 863–876. [Google Scholar] [CrossRef] [Green Version]
- Polakis, P.G.; Rubinfeld, B.; Evans, T.; McCormick, F. Purification of a plasma membrane-associated GTPase-activating protein specific for rap1/Krev-1 from HL60 cells. Proc. Natl. Acad. Sci. USA 1991, 88, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Pannekoek, W.-J.; Post, A.; Bos, J.L. Rap1 signaling in endothelial barrier control. Cell Adhes. Migr. 2014, 8, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewat, E.A.; Durmort, C.; Jacquamet, L.; Concord, E.; Gulino-Debrac, D. Architecture of the VE-cadherin hexamer. Architecture of the VE-cadherin hexamer. J. Mol. Biol. 2007, 365, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- van den Berghe, N.; Cool, R.H.; Horn, G.; Wittinghofer, A. Biochemical characterization of C3G: An exchange factor that discriminates between Rap1 and Rap2 and is not inhibited by Rap1A(S17N). Oncogene 1997, 15, 845–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohba, Y.; Mochizuki, N.; Matsuo, K.; Yamashita, S.; Nakaya, M.; Hashimoto, Y.; Hamaguchi, M.; Kurata, T.; Nagashima, K.; Matsuda, M. Rap2 as a slowly responding molecular switch in the Rap1 signaling cascade. Mol. Cell Biol. 2000, 20, 6074–6083. [Google Scholar] [CrossRef] [Green Version]
- Parker, W.H.; Rhea, E.M.; Qu, Z.C.; Hecker, M.R.; May, J.M. Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am. J. Physiol. Cell Physiol. 2016, 311, C652–C662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohba, Y.; Ikuta, K.; Ogura, A.; Matsuda, J.; Mochizuki, N.; Nagashima, K.; Kurokawa, K.; Mayer, B.J.; Maki, K.; Miyazaki, J.; et al. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J. 2001, 20, 3333–3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukova, A.A.; Tian, X.; Tian, Y.; Higginbotham, K.; Birukov, K.G. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol. Biol. Cell 2013, 24, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, H.; Sugimoto, Y.; Matsuzaki, T.; Ikawa, Y.; Noda, M. A ras-related gene with transformation suppressor activity. Cell 1989, 56, 77–84. [Google Scholar] [CrossRef]
- Tsukamoto, N.; Hattori, M.; Yang, H.; Bos, J.L.; Minato, N. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J. Biol. Chem. 1999, 274, 18463–18469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, A.L.; Brown, N.H. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science 2002, 295, 1285–1288. [Google Scholar] [CrossRef]
- Bonello, T.T.; Perez-Vale, K.Z.; Sumigray, K.D.; Peifer, M. Rap1 acts via multiple mechanisms to position Canoe and adherens junctions and mediate apical-basal polarity establishment. Development 2018, 145, dev157941. [Google Scholar] [CrossRef] [Green Version]
- Wittchen, E.S.; Worthylake, R.A.; Kelly, P.; Casey, P.J.; Quilliam, L.A.; Burridge, K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J. Biol. Chem. 2005, 280, 11675–11682. [Google Scholar] [CrossRef] [Green Version]
- Pizon, V.; Chardin, P.; Lerosey, I.; Olofsson, B.; Tavitian, A. Human cDNAs rap1 and rap2 homologous to the drosophila gene Dras3 encode proteins closely related to ras in the ‘effector’ region. Oncogene 1988, 3, 201–204. [Google Scholar]
- Rubinfeld, B.; Munemitsu, S.; Clark, R.; Conroy, L.; Watt, K.; Crosier, W.J.; McCormick, F.; Polakis, P. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21 rap1. Cell 1991, 65, 1033–1042. [Google Scholar] [CrossRef]
- Niola, F.; Zhao, X.; Singh, D.; Sullivan, R.; Castano, A.; Verrico, A.; Lasorella, A. Mesenchymal high-grade glioma is maintained by the ID-RAP1 axis. J. Clin. Investig. 2013, 123, 405–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannekoek, W.J.; Linnemann, J.R.; Brouwer, P.M.; Bos, J.L.; Rehmann, H. Rap1 and Rap2 antagonistically control endothelial barrier resistance. PLoS ONE. 2013, 8, e57903. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Ciana, A.; Pietra, D.; Balduini, C.; Minetti, G.; Torti, M. Rap2, but not Rap1 GTPase is expressed in human red blood cells and is involved in vesiculation. Biochim. Biophys. Acta 2006, 1763, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Di, J.; Qu, D.; Gao, Z.; Zhang, Y.; Zheng, J. Role of Rap2 and its Downstream Effectors in Tumorigenesis. Anti-Cancer Agents Med. Chem. 2015, 15, 1269–1276. [Google Scholar] [CrossRef]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood-brain barrier integrity. NPJ Microgravity 2016, 2, 16019. [Google Scholar] [CrossRef] [Green Version]
- Kapitonova, M.Y.; Kuznetsov, S.L.; Froemming, G.R.; Muid, S.; Nor-Ashikin, M.N.; Otman, S.; Shahir, A.R.; Nawawi, H. Effects of space mission factors on the morphology and Function of endothelial cells. Bull. Exp. Biol. Med. 2013, 154, 796–801. [Google Scholar] [CrossRef]
- Crawford-Young, S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2003, 50, 183–191. [Google Scholar] [CrossRef]
- Szulcek, R.; van Bezu, J.; Boonstra, J.; van Loon, J.J.; van Nieuw Amerongen, G.P. Transient intervals of hyper-gravity enhance endothelial barrier integrity: Impact of mechanical and gravitational forces measured electrically. PLoS ONE 2015, 10, e0144269. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Luissint, A.-C.; Sumagin, R.; Lai, C.; Vielmuth, F.; Wolf, M.F.; Laur, O.; Reiss, K.; Spindler, V.; Stehle, T.; et al. Trans-dimerization of JAM-A regulates Rap2 and is mediated by a domain that is distinct from the cis-dimerization interface. Mol. Biol. Cell 2014, 25, 1574–1585. [Google Scholar] [CrossRef]
- Wehland, M.; Ma, X.; Braun, M.; Hauslage, J.; Hemmersbach, R.; Bauer, J.; Grosse, J.; Infanger, M.; Grimm, D. The impact of altered gravity and vibration on endothelial cells during a parabolic flight. Cell Physiol. Biochem. 2013, 31, 432–451. [Google Scholar] [CrossRef]
- Kang, J.C. Study on the Response of Cytoskeleton System to Mechanical Changes. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 30 June 2015. [Google Scholar]
- Tsygankova, O.M.; Wang, H.; Meinkoth, J.L. Tumor cell migration and invasion are enhanced by depletion of Rap1 GTPase-activating protein (Rap1GAP). J. Biol. Chem. 2013, 288, 24636–24646. [Google Scholar] [CrossRef] [Green Version]
- Ukropec, J.A.; Hollinger, M.K.; Woolkalis, M.J. Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress. Exp. Cell Res. 2002, 273, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Tsygankova, O.M.; Ma, C.; Tang, W.; Korch, C.; Feldman, M.D.; Lv, Y.; Brose, M.S.; Meinkoth, J.L. Downregulation of Rap1GAP in human tumor cells alters cell/matrix and cell/cell adhesion. Mol. Cell Biol. 2010, 30, 3262–3274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwartkruis, F.J.; Wolthuis, R.M.; Nabben, N.M.; Franke, B.; Bos, J.L. Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J. 1998, 17, 5905–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.J.; Yu, J.R.; Nie, J.L.; Wang, C.Y.; Yang, F.; Ding, B.; Gu, Y.; Qu, L.N.; Wang, Y.M.; Li, Y.H. Development of two direction multi-sample cell experimental device for microgravity effects simulation. Space Med. Med. Eng. 2011, 1, 13–16. [Google Scholar]
- Shi, S.L.; Li, Q.; Cao, Q.; Diao, Y.; Zhang, Y.; Yue, L.; Wei, L.J. EMT transcription factors are involved in the altered cell adhesion under simulated microgravity effect or overloading by regulation of E-cadherin. Int. J. Mol. Sci. 2020, 21, 1349. [Google Scholar] [CrossRef] [Green Version]





| Gene Name | Primer Sequence |
|---|---|
| Sh-Rap1GAP-s | 5′-TGCAATGTGGTATTTCAAGAGAATAGAATAGAACCGATCCACATTGCTTTTTTC-3′ |
| Sh-Rap1GAP-a | 5′-TCGAGAAAAAAGCAATGTGGATCGGTTCTATTCTCTTGAAATAGAACCGATCCACATTGCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Li, J.; Li, E.; Guo, W.; He, Y.; Wang, J.; Zhang, Y.; Yue, L.; Wei, L. Simulated Microgravity Increases the Permeability of HUVEC Monolayer through Up-Regulation of Rap1GAP and Decreased Rap2 Activation. Int. J. Mol. Sci. 2022, 23, 630. https://doi.org/10.3390/ijms23020630
Shi S, Li J, Li E, Guo W, He Y, Wang J, Zhang Y, Yue L, Wei L. Simulated Microgravity Increases the Permeability of HUVEC Monolayer through Up-Regulation of Rap1GAP and Decreased Rap2 Activation. International Journal of Molecular Sciences. 2022; 23(2):630. https://doi.org/10.3390/ijms23020630
Chicago/Turabian StyleShi, Shuliang, Jing Li, Erzhuo Li, Wenqi Guo, Yao He, Jinpeng Wang, Yao Zhang, Lei Yue, and Lijun Wei. 2022. "Simulated Microgravity Increases the Permeability of HUVEC Monolayer through Up-Regulation of Rap1GAP and Decreased Rap2 Activation" International Journal of Molecular Sciences 23, no. 2: 630. https://doi.org/10.3390/ijms23020630