The Therapeutic Potential of Zinc-Alpha2-Glycoprotein (AZGP1) in Fibrotic Kidney Disease
Abstract
:1. Introduction
2. Results
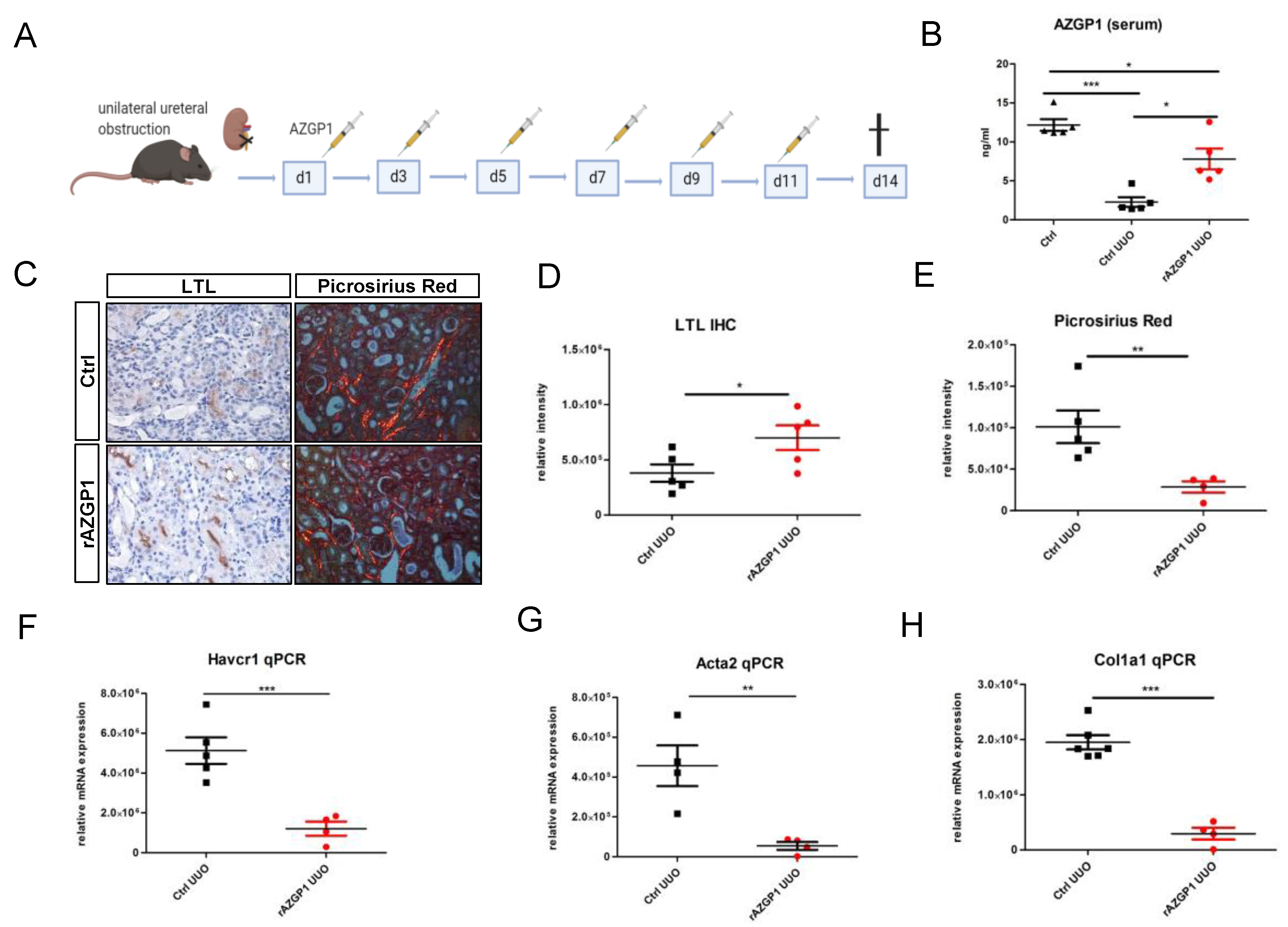
2.1. Administration of Recombinant AZGP1 Attenuates Renal Fibrosis Development
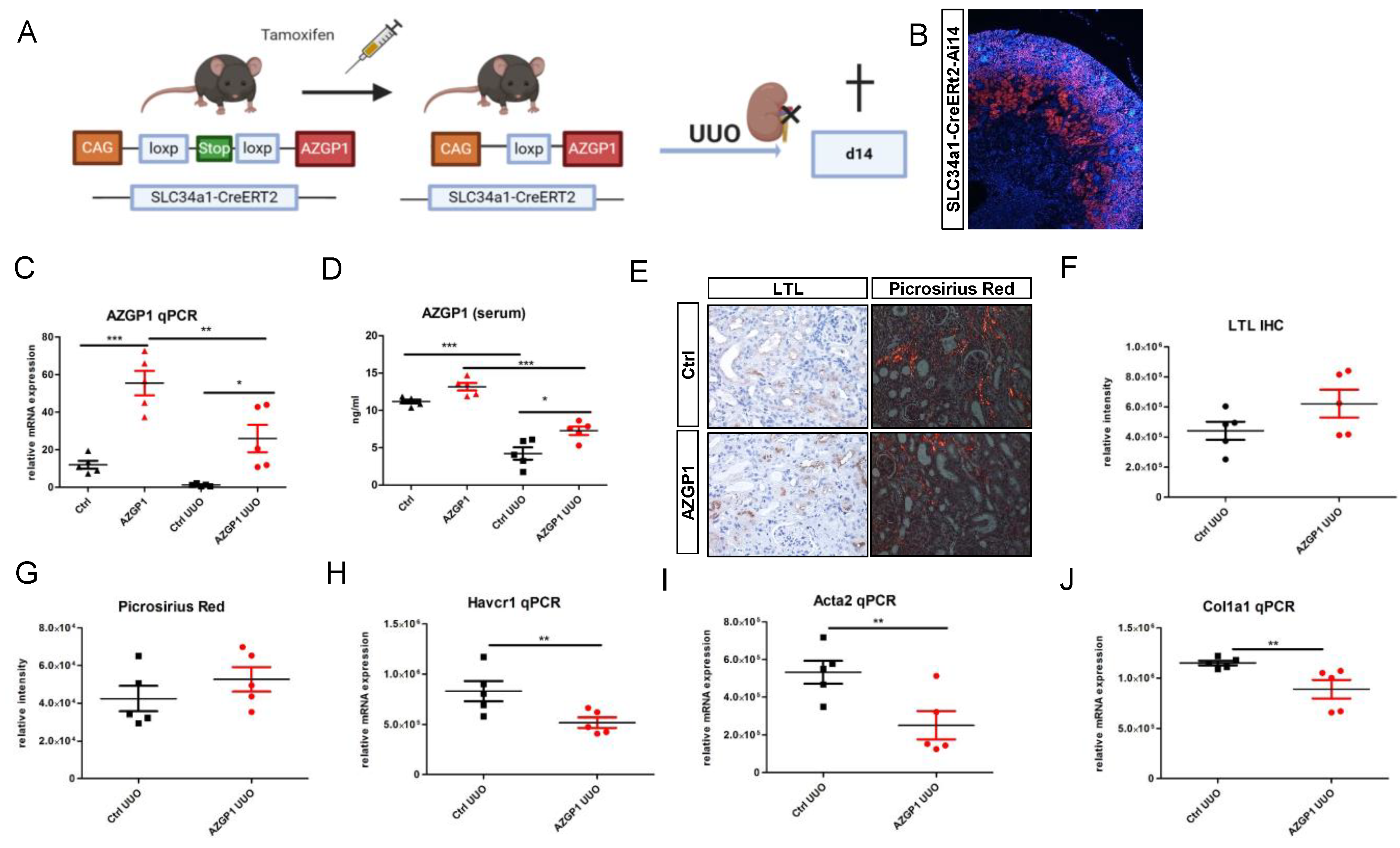
2.2. Transgenic AZGP1 Expression in Proximal Tubule Provides Protection in UUO
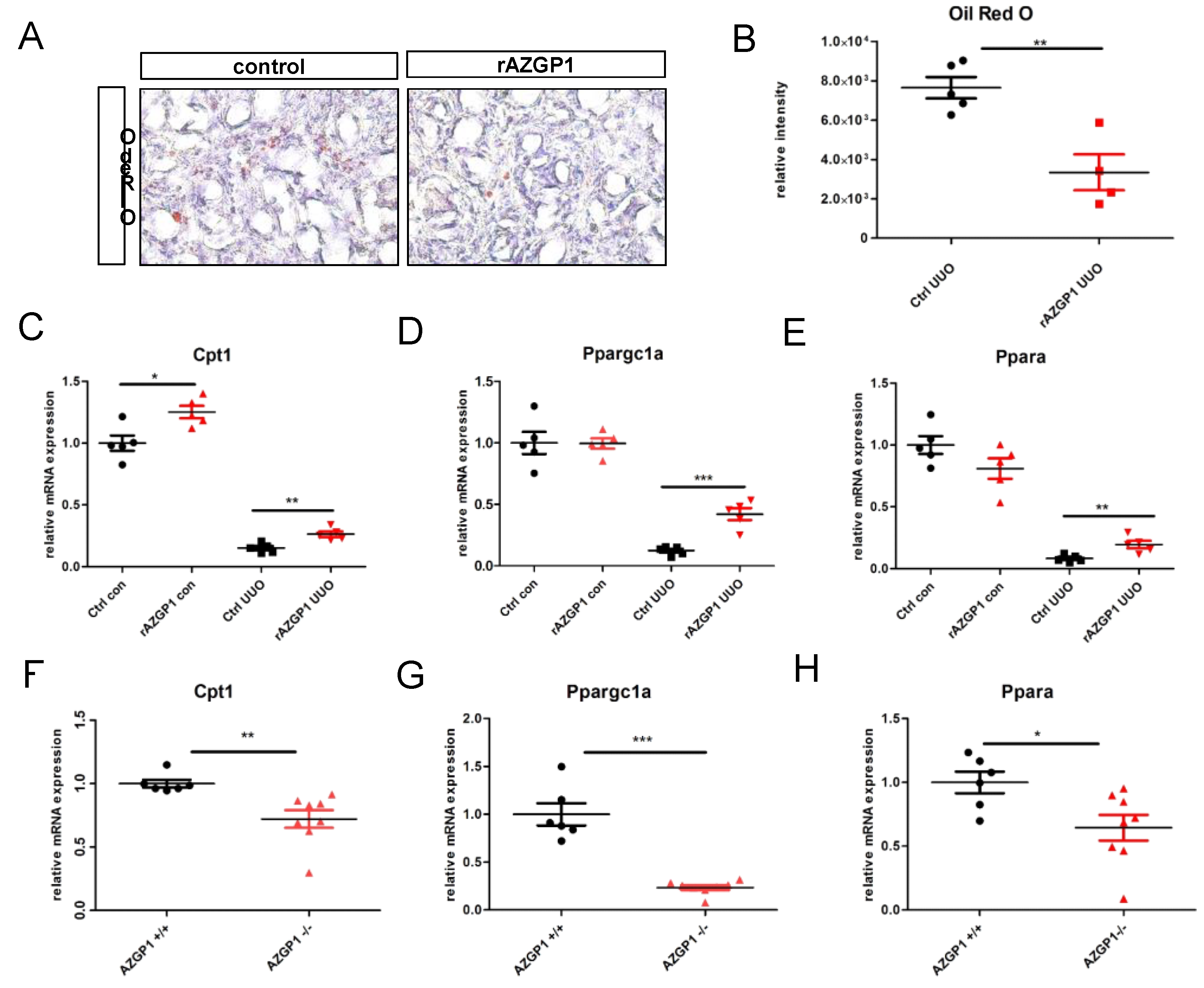
2.3. AZGP1 Reduces Intrarenal Lipid Accumulation and Acts as an Inducer of Key Players in Lipid Metabolism
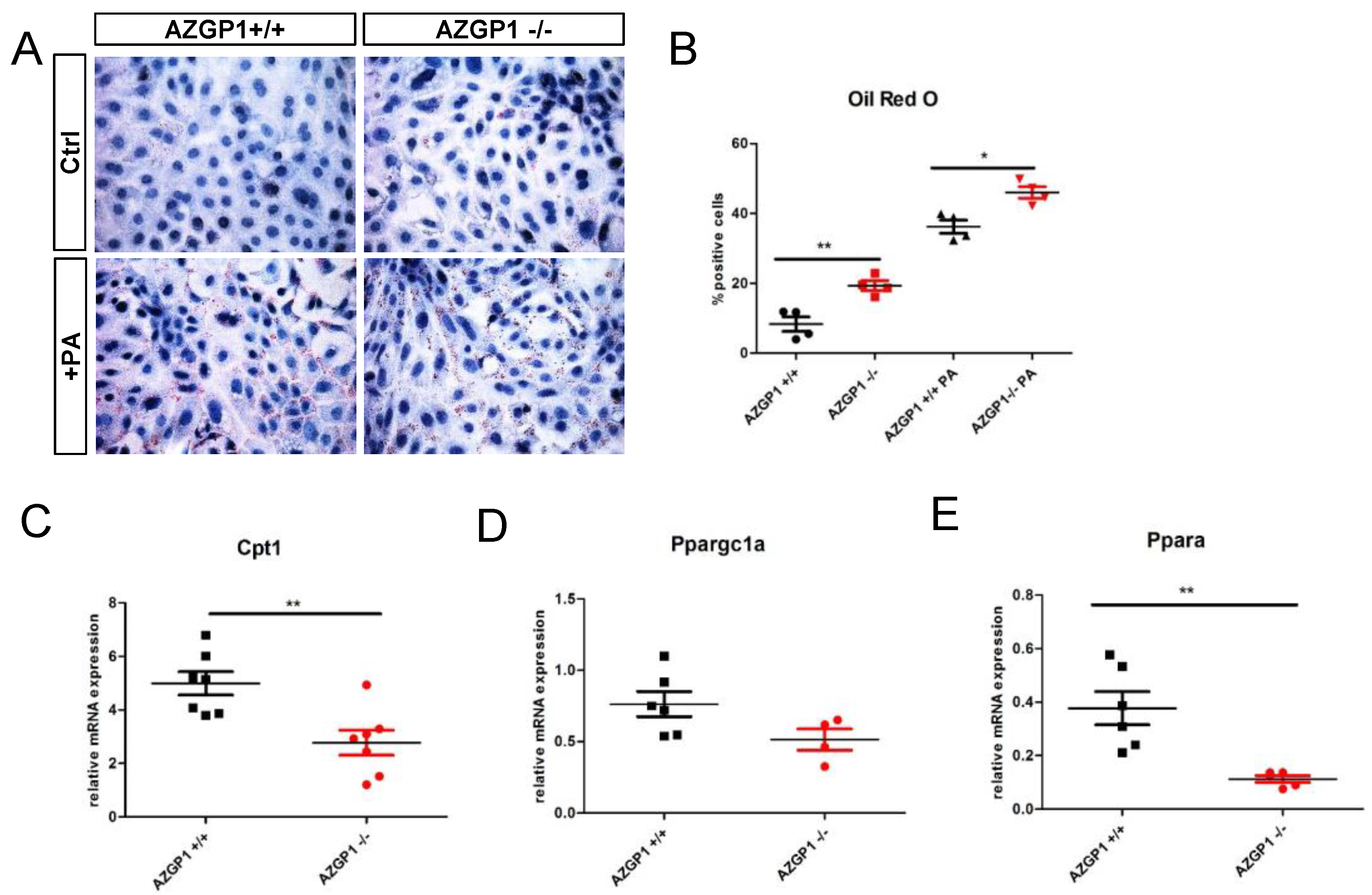
2.4. Altered Lipid Metabolism in AZGP1 Deficient Tubular Cells
3. Discussion
4. Material and Methods
4.1. Transgenic Mice
4.2. Unilateral Ureteral Obstruction (UUO)
4.3. Cell Culture
4.4. Histological and Immunohistochemical Stainings
4.5. Oil Red O Staining
4.6. AZGP1 Measurement in Serum
4.7. Synthesis and Purification of AZGP1
4.8. Quantitative Real-Time PCR
4.9. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Farris, A.B.; Colvin, R.B. Renal interstitial fibrosis. Curr. Opin. Nephrol. Hypertens. 2012, 21, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sörensen-Zender, I.; Bhayana, S.; Susnik, N.; Rolli, V.; Batkai, S.; Baisantry, A.; Bahram, S.; Sen, P.; Teng, B.; Lindner, R.; et al. Zinc-α2-glycoprotein exerts antifibrotic effects in kidney and heart. J. Am. Soc. Nephrol. 2015, 26, 2659–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sörensen-Zender, I.; Beneke, J.; Schmidt, B.M.; Menne, J.; Haller, H.; Schmitt, R. Zinc-alpha2-glycoprotein in patients with acute and chronic kidney disease. BMC Nephrol. 2013, 14, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Zinc α2-glycoprotein: A multidisciplinary protein. Mol. Cancer Res. 2008, 6, 892–906. [Google Scholar] [CrossRef] [Green Version]
- Burgi, W.; Schmid, K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J. Biol. Chem. 1961, 236, 1066–1074. [Google Scholar] [CrossRef]
- Ekman, R.; Johansson, B.G.; Ravnskov, U. Renal handling of Zn α2 glycoprotein as compared with that of albumin and the retinol binding protein. J. Clin. Investig. 1976, 57, 945–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, V.O.; Lobo, J.C.; Stockler-Pinto, M.B.; Farage, N.E.; Velarde, G.C.; Fouque, D.; Leite, M.; Mafra, D. Zinc-α2-glycoprotein: Is there association between this new adipokine and body composition in hemodialysis patients? Ren. Fail. 2012, 34, 1062–1067. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, L.Z.; Zhang, C.Z.; Yi, C.; Liu, L.L.; Zhou, X.; Xie, G.B.; Cai, M.Y.; Li, Y.; Yun, J.P. Decreased expression of zinc-alpha2-glycoprotein in hepatocellular carcinoma associates with poor prognosis. J. Transl. Med. 2012, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Parris, T.Z.; Kovács, A.; Aziz, L.; Hajizadeh, S.; Nemes, S.; Semaan, M.; Forssell-Aronsson, E.; Karlsson, P.; Helou, K. Additive effect of the AZGP1, PIP, S100A8 and UBE2C molecular biomarkers improves outcome prediction in breast carcinoma. Int. J. Cancer 2014, 134, 1617–1629. [Google Scholar] [CrossRef] [Green Version]
- Brysk, M.M.; Lei, G.; Adler-Storthz, K.; Chen, Z.; Brysk, H.; Tyring, S.K.; Arany, I. Zinc-α2-glycoprotein expression as a marker of differentiation in human oral tumors. Cancer Lett. 1999, 137, 117–120. [Google Scholar] [CrossRef]
- Hirai, K.; Hussey, H.J.; Barber, M.D.; Price, S.A.; Tisdale, M.J. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res. 1998, 58, 2359–2365. [Google Scholar] [PubMed]
- Rolli, V.; Radosavljevic, M.; Astier, V.; Macquin, C.; Castan-Laurell, I.; Visentin, V.; Guigné, C.; Carpéné, C.; Valet, P.; Gilfillan, S.; et al. Lipolysis is altered in MHC class I zinc-α2-glycoprotein deficient mice. FEBS Lett. 2007, 581, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Bing, C.; Bao, Y.; Jenkins, J.; Sanders, P.; Manieri, M.; Cinti, S.; Tisdale, M.J.; Trayhurn, P. Zinc-α2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc. Natl. Acad. Sci. USA 2004, 101, 2500–2505. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.T.; Tisdale, M.J. Mechanism of attenuation of skeletal muscle atrophy by zinc- α2-glycoprotein. Endocrinology 2010, 151, 4696–4704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.W.; Liu, H.; Mukherjee, R.; Yun, J.W. Downregulation of fetuin-B and zinc-α2-glycoprotein is linked to impaired fatty acid metabolism in liver cells. Cell. Physiol. Biochem. 2012, 30, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Luo, X.; Li, Z.H.; Wu, J.C.; Luo, S.Z.; Xu, M.Y. Zinc-α2-glycoprotein 1 attenuates non-alcoholic fatty liver disease by negatively regulating tumour necrosis factor-α. World J. Gastroenterol. 2019, 25, 5451–5468. [Google Scholar] [CrossRef]
- Xiao, X.; Li, H.; Qi, X.; Wang, Y.; Xu, C.; Liu, G.; Wen, G.; Liu, J. Zinc alpha2 glycoprotein alleviates palmitic acid-induced intracellular lipid accumulation in hepatocytes. Mol. Cell. Endocrinol. 2017, 439, 155–164. [Google Scholar] [CrossRef]
- Faulconnier, Y.; Boby, C.; Pires, J.; Labonne, C.; Leroux, C. Effects of Azgp1 −/− on mammary gland, adipose tissue and liver gene expression and milk lipid composition in lactating mice. Gene 2019, 692, 201–207. [Google Scholar] [CrossRef]
- Schmitt, R.; Marlier, A.; Cantley, L.G. Zag expression during aging suppresses proliferation after kidney injury. J. Am. Soc. Nephrol. 2008, 19, 2375–2383. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.Y.; Chen, R.; Yu, J.X.; Liu, T.; Qu, Y.; Lu, L.G. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways. Cancer Lett. 2016, 374, 241–249. [Google Scholar] [CrossRef]
- Lin, B.; He, H.; Zhang, Q.; Zhang, J.; Xu, L.; Zhou, L.; Zheng, S.; Wu, L. Long non-coding RNA00844 inhibits MAPK signaling to suppress the progression of hepatocellular carcinoma by targeting AZGP1. Ann. Transl. Med. 2020, 8, 1365. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, K.M.; Song, H.E.; Choi, K.H.; Hwang, J.J.; Yoo, H.J.; Song, J.W. The role of free fatty acids in idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 50, OA4635. [Google Scholar]
- Miguel, V.; Tituaña, J.; Ignacio Herrero, J.; Herrero, L.; Serra, D.; Cuevas, P.; Barbas, C.; Puyol, D.R.; Márquez-Expósito, L.; Ruiz-Ortega, M.; et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Investig. 2021, 131, e140695. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Yuan, Q.; Lv, Y.; Ding, H.; Ke, Q.; Shi, C.; Luo, J.; Jiang, L.; Yang, J.; Zhou, Y. CPT1α maintains phenotype of tubules via mitochondrial respiration during kidney injury and repair. Cell Death Dis. 2021, 12, 792. [Google Scholar] [CrossRef]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Reza Akhavan-Sharif, M.; Khankin, E.V.; Saintgeniez, M.; et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef] [Green Version]
- Proctor, G.; Jiang, T.; Iwahashi, M.; Wang, Z.; Li, J.; Levi, M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 2006, 55, 2502–2509. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Malaga-DIeguez, L.; Chinga, F.; Kang, H.M.; Tao, J.; Reidy, K.; Susztak, K. Deletion of Lkb1 in renal tubular epithelial cells leads to CKD by altering metabolism. J. Am. Soc. Nephrol. 2016, 27, 439–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.W.; Lee, E.K.; Lee, M.K.; Oh, G.T.; Yu, B.P.; Chung, H.Y. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J. Am. Soc. Nephrol. 2018, 29, 1223–1237. [Google Scholar] [CrossRef] [Green Version]
- Jao, T.M.; Nangaku, M.; Wu, C.H.; Sugahara, M.; Saito, H.; Maekawa, H.; Ishimoto, Y.; Aoe, M.; Inoue, T.; Tanaka, T.; et al. ATF6α downregulation of PPARα promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int. 2019, 95, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, P.; Park, J.; Hurtado del Pozo, C.; Li, L.; Doke, T.; Huang, S.; Zhao, J.; Kang, H.M.; Shrestra, R.; Balzer, M.S.; et al. The Nuclear Receptor ESRRA Protects from Kidney Disease by Coupling Metabolism and Differentiation. Cell Metab. 2021, 33, 379–394.e8. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, X.G.; Zhang, S. Druggability of lipid metabolism modulation against renal fibrosis. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, W.; Praddaude, F.; Buleon, M.; Jaafar, A.; Vallet, M.; Rischmann, P.; Galarreta, C.I.; Chevalier, R.L.; Tack, I. Renal functional decline and glomerulotubular injury are arrested but not restored by release of unilateral ureteral obstruction (UUO). Am. J. Physiol. Ren. Physiol. 2013, 304, F432–F439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, R.; Cantley, L.G. The impact of aging on kidney repair. Am. J. Physiol. Ren. Physiol. 2008, 294, F1265–F1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Primer | Sequence |
|---|---|
| Actin | for: AGCCATGTACGTAGCCATCC, rev: CTCTCAGCTGTGGTGGTAA. |
| Havcr1 | for: AAA CCA GAG ATT CCC ACA CG rev: GTCGTG GGT CTT CCT GTA GC |
| Acta2 | for: GTG CTA TGT CGC TCT GGA CTT TGA rev: ATG AAA GAT GGC TGG AAG AGG GTC |
| Col1a1 | for: GTCCCAACCCCCAAAGAC rev: CCCTCGACTCCTACATCTTCTGA |
| AZGP1 | for: ATG GTG CCT GTC CTG CTG TC rev: TCG CAA CCA AAC ATT CCC TG |
| Ppargc1a | for: TAT GGA GTG ACA TAG AGT GTG CT rev: CCA CTT CAA TCC ACC CAG AAA G |
| Cpt1 | for: CTC CGC CTG AGC CAT GAA G rev: CAC CAG TGA TGA TGC CAT TCT |
| Ppara | for: TGC AAA CTT GGA CTT GAA CG rev: GAT CAG CAT CCC GTC TTT GT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sörensen-Zender, I.; Rong, S.; Haller, H.; Schmitt, R. The Therapeutic Potential of Zinc-Alpha2-Glycoprotein (AZGP1) in Fibrotic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 646. https://doi.org/10.3390/ijms23020646
Sörensen-Zender I, Rong S, Haller H, Schmitt R. The Therapeutic Potential of Zinc-Alpha2-Glycoprotein (AZGP1) in Fibrotic Kidney Disease. International Journal of Molecular Sciences. 2022; 23(2):646. https://doi.org/10.3390/ijms23020646
Chicago/Turabian StyleSörensen-Zender, Inga, Song Rong, Hermann Haller, and Roland Schmitt. 2022. "The Therapeutic Potential of Zinc-Alpha2-Glycoprotein (AZGP1) in Fibrotic Kidney Disease" International Journal of Molecular Sciences 23, no. 2: 646. https://doi.org/10.3390/ijms23020646
APA StyleSörensen-Zender, I., Rong, S., Haller, H., & Schmitt, R. (2022). The Therapeutic Potential of Zinc-Alpha2-Glycoprotein (AZGP1) in Fibrotic Kidney Disease. International Journal of Molecular Sciences, 23(2), 646. https://doi.org/10.3390/ijms23020646






