Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress
Abstract
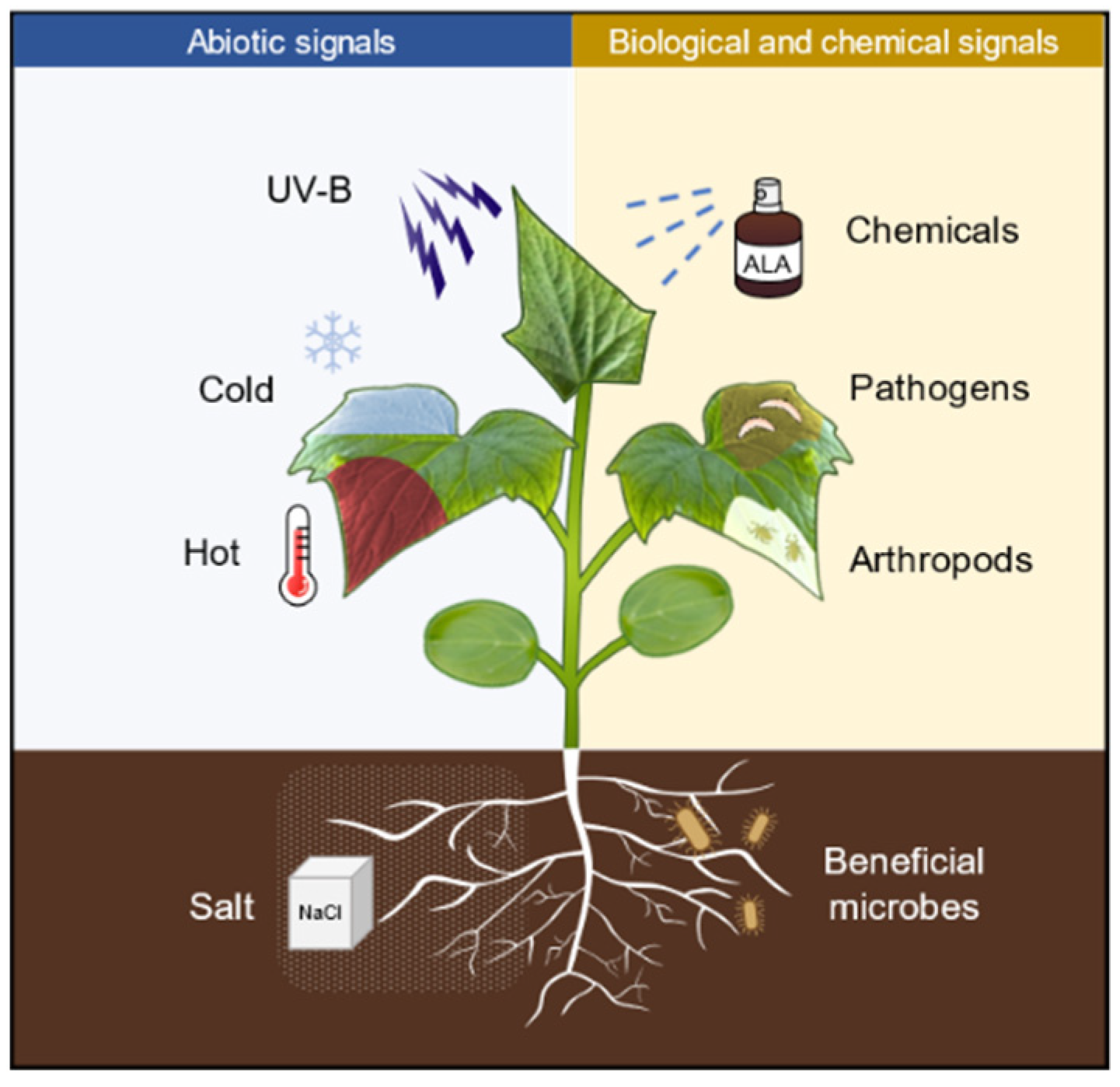
:1. Introduction
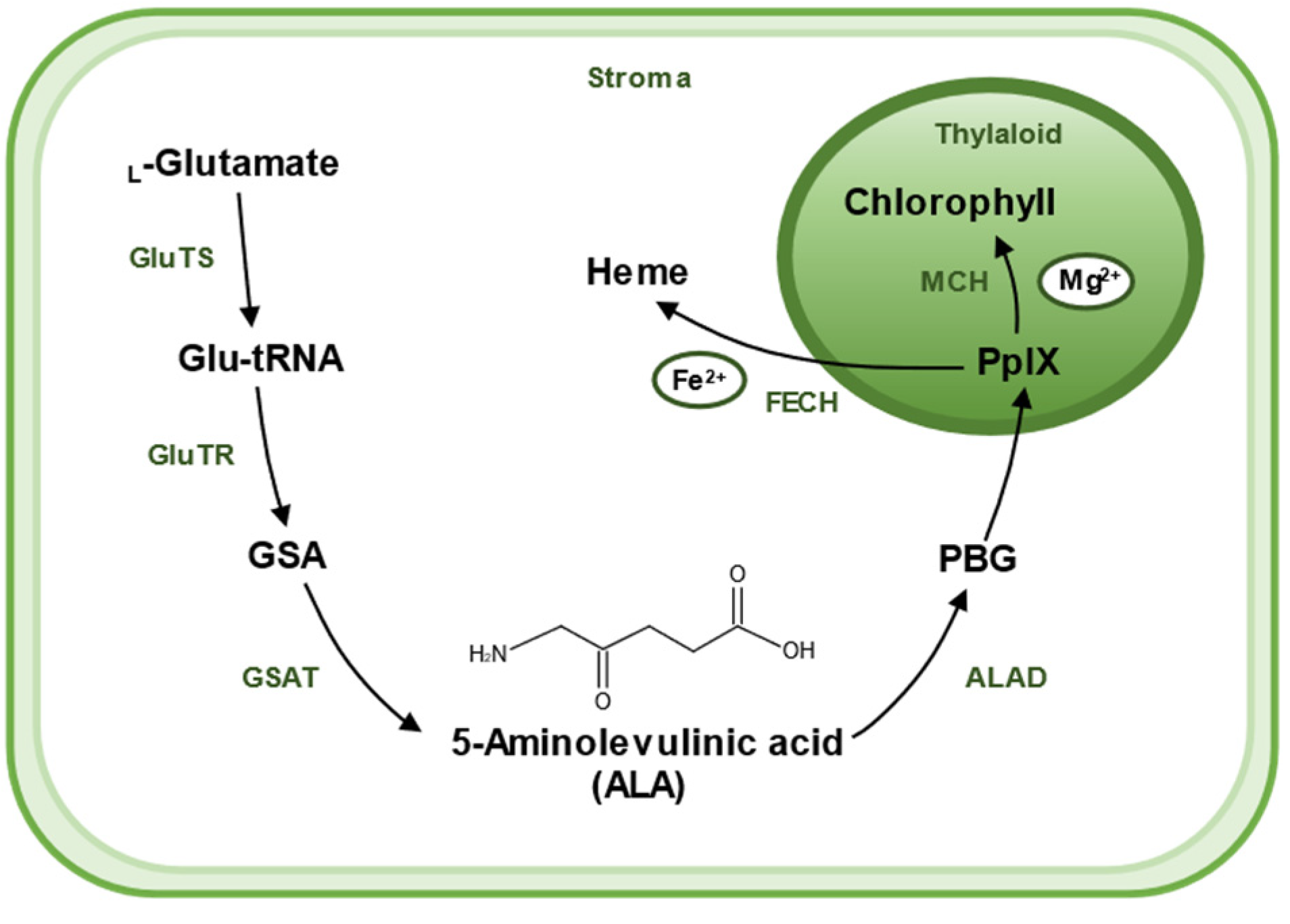
2. Biosynthesis of 5-Aminolevulinic Acid
3. ALA Priming Enhances Plant Resistance to Abiotic Stresses
3.1. ALA Priming Alleviates Salt Stress
3.2. ALA Priming Increases Plant Tolerance to Extreme Temperature
3.3. ALA Priming Mitigates Drought-Induced Damage
3.4. ALA Priming Attenuates UV-B-Induced Damage
4. Application of ALA in Agriculture and Medicine
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense Priming: An Adaptive Part of Induced Resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Valle, A.; López-Calleja, A.C.; Alvarez-Venegas, R. Enhancement of Pathogen Resistance in Common Bean Plants by Inoculation With Rhizobium etli. Front. Plant Sci. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Kim, J.I.; Baek, D.; Park, H.C.; Chun, H.J.; Oh, D.H.; Lee, M.K.; Cha, J.Y.; Kim, W.Y.; Kim, M.C.; Chung, W.S.; et al. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol. Plant 2013, 6, 337–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Yekondi, S.; Chen, P.-W.; Tsai, C.-H.; Yu, C.-W.; Wu, K.; Zimmerli, L. Environmental History Modulates Arabidopsis Pattern-Triggered Immunity in a HISTONE ACETYLTRANSFERASE1–Dependent Manner. Plant Cell 2014, 26, 2676–2688. [Google Scholar] [CrossRef] [Green Version]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.; Hangqi, S.; Jungui, X.; Yajing, G.; Wenjian, S.; Jin, H. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017, 137, 58–72. [Google Scholar]
- Gupta, D.; Prasad, S.M. Priming with 5-aminolevulinic acid (ALA) attenuates UV-B induced damaging effects in two varieties of Cajanus cajan L. seedlings by regulating photosynthetic and antioxidant systems. S. Afr. J. Bot. 2021, 138, 129–140. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Tanou, G.; Filippou, P.; Belghazi, M.; Job, D.; Diamantidis, G.; Fotopoulos, V.; Molassiotis, A. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 2012, 72, 585–599. [Google Scholar] [CrossRef]
- Li, M.; Yu, G.; Ma, J.; Liu, P. Interactions of importers in long-distance transmission of wound-induced jasmonate. Plant Signal. Behav. 2021, 16, 1886490. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Lee, S.; Park, S.; van Kleeff, P.J.M.; Schuurink, R.C.; Ryu, C.-M. Transient Expression of Whitefly Effectors in Nicotiana benthamiana Leaves Activates Systemic Immunity Against the Leaf Pathogen Pseudomonas syringae and Soil-Borne Pathogen Ralstonia solanacearum. Front. Ecol. Evol. 2018, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Carrasco, G.; Martínez-Aguilar, K.; Alvarez-Venegas, R. Transgenerational Defense Priming for Crop Protection against Plant Pathogens: A Hypothesis. Front. Plant Sci. 2017, 8, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef]
- Sasaki, K.; Watanabe, M.; Tanaka, T.; Tanaka, T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2002, 58, 23–29. [Google Scholar] [CrossRef]
- Agrawal, S.; Karcher, D.; Ruf, S.; Bock, R. The Functions of Chloroplast Glutamyl-tRNA in Translation and Tetrapyrrole Biosynthesis1 [OPEN]. Plant Physiol. 2020, 183, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Iida, K.; Kajiwara, M. Carbon Source Dependence of the Ratio of δ-Aminolevulinic Acid Biosynthesis via the C5 and Shemin Pathways in Euglena Gracilis (Euglenophyceae)1. J. Phycol. 2008, 44, 292–298. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Senge, M.O.; Ryan, A.A.; Letchford, K.A.; MacGowan, S.A.; Mielke, T. Chlorophylls, Symmetry, Chirality, and Photosynthesis. Symmetry 2014, 6, 781–843. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Zhang, J.; Zhou, J.; Qi, Q.; Du, G.; Chen, J. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12. Biotechnol. Adv. 2012, 30, 1533–1542. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Cheng, X.; Wang, J.; Wang, Q.; Qi, Q.; Liu, S.-J. A New Strategy for Production of 5-Aminolevulinic Acid in Recombinant Corynebacterium glutamicum with High Yield. Appl. Environ. Microbiol. 2016, 82, 2709–2717. [Google Scholar] [CrossRef] [Green Version]
- Noh, M.H.; Lim, H.G.; Park, S.; Seo, S.W.; Jung, G.Y. Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metab. Eng. 2017, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, J.; Feng, Y.; Le, K.; Zai, Y.; Tang, X.; Sun, Y.; Zeng, X.; Lin, L. Green and mild production of 5-aminolevulinic acid from algal biomass. Korean J. Chem. Eng. 2021, 38, 899–905. [Google Scholar] [CrossRef]
- Mohsin, T.; Adnan Noor, S. An insight into salt stress tolerance mechanisms of Chenopodium album. Environ. Sci. Pollut. Res. 2017, 24, 16531–16535. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Liyun, L.; Nguyen Tran, N.; Akihiro, U.; Hirofumi, S. Effects of 5-aminolevulinic acid on Swiss chard (Beta vulgaris L. subsp. cicla) seedling growth under saline conditions. Plant Growth Regul. 2014, 74, 219–228. [Google Scholar]
- Anirban, B. Strawberries under salt stress: ALA and ROS to the rescue. Physiol. Plant. 2019, 167, 2–4. [Google Scholar]
- Yue, W.; Na, L.; Linli, H.; Weibiao, L.; Zhongqi, T.; Xuemei, X.; Jian, L.; Jianming, X.; Alejandro, C.-U.; Jihua, Y. 5-Aminolevulinic Acid Improves Morphogenesis and Na+ Subcellular Distribution in the Apical Cells of Cucumis sativus L. under Salinity Stress. Front. Plant Sci. 2021, 12, 404. [Google Scholar]
- Jun-Lan, X.; Hang-Chao, W.; Xiao-Yu, T.; Chun-Lei, Z.; Muhammad Shahbaz, N. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol. Biochem. 2018, 124, 88–99. [Google Scholar]
- Nudrat Aisha, A.; Muhammad, A.; Al-Qurainy, F. Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci. Hortic-Amst. 2012, 142, 143–148. [Google Scholar]
- Jia, Y.; Qiang, C.; Ting, T.; Gui, W.; Feng, X. Promotive effects of 5-Aminolevulinic acid on growth, photosynthetic gas exchange, chlorophyll, and antioxidative enzymes under salinity stress in Prunnus persica (L.) Batseh Seedling. Emir. J. Food Agric. 2016, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, E.-E.; Ibrahim, A.; Abdulaziz, A.; Hayssam, A.; Aisha, A.; Jacques, W.; Margaret, A. Genetic Variation and Alleviation of Salinity Stress in Barley (Hordeum vulgare L.). Molecules 2018, 23, 2488. [Google Scholar]
- Yue, W.; Linli, H.; Weibiao, L.; Mohammed Mujitaba, D.; Jian, L.; Jianming, X.; Zhi, F.; Alejandro, C.-U.; Jihua, Y. Foliar application of 5-aminolevulinic acid (ALA) alleviates NaCl stress in cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci. Hortic-Amst. 2019, 257, 108761. [Google Scholar]
- Chun-Ping, Z.; Yi-Cun, L.; Feng-Gang, Y.; Shi-Jun, H.; Hai-Ying, L.; Ping, H. Role of 5-aminolevulinic acid in the salinity stress response of the seeds and seedlings of the medicinal plant Cassia obtusifolia L. Bot. Stud. 2013, 54, 1–13. [Google Scholar]
- Muhammad, S.N.; Hasitha, W.; Hongbo, L.; Dan, L.; Rashid, A.; Ejaz Ahmad, W.; Ling, X.; Weijun, Z. 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol. Biochem. 2012, 57, 84–92. [Google Scholar]
- Yue, W.; Xin, J.; Weibiao, L.; Linli, H.; Mohammed, M.D.; Xingjie, Z.; Zhongqi, T.; Tingyu, G.; Jihua, Y. 5-Aminolevulinic Acid (ALA) Alleviated Salinity Stress in Cucumber Seedlings by Enhancing Chlorophyll Synthesis Pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar]
- Chen, G.; Fan, P.S.; Feng, W.M.; Guan, A.Q.; Lu, Y.Y.; Wan, Y.L. Effects of 5-aminolevulinic acid on nitrogen metabolism and ion distribution of watermelon seedlings under salt stress. Russ. J. Plant Physiol. 2017, 64, 116–123. [Google Scholar] [CrossRef]
- Cengiz, K.; Muhammad, A. Nitric Oxide is Required for Aminolevulinic Acid-Induced Salt Tolerance by Lowering Oxidative Stress in Maize (Zea mays). J. Plant Growth Regul. 2020, 40, 617–627. [Google Scholar]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Yan, S.; Yang, T.; Zhang, S.; Chen, Y.-Q.; Liu, B. Small RNAs in regulating temperature stress response in plants. J. Integr. Plant Biol. 2017, 59, 774–791. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Low-temperature stress: Is phytohormones application a remedy? Environ. Sci. Pollut. Res. 2017, 24, 21574–21590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, D.M.; Gao, Y.; Yu, B.; Xia, C.X.; Bai, J.G. Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol. Plant. 2012, 56, 780–784. [Google Scholar] [CrossRef]
- Ahmet, K.; Yakup, K. Promotion by 5-aminolevulenic acid of pepper seed germination and seedling emergence under low-temperature stress. Sci. Hortic-Amst. 2009, 119, 98–102. [Google Scholar]
- Hotta, Y.; Tanaka, T.; Luo, B.; Takeuchi, Y.; Konnai, M. Improvement of Cold Resistance in Rice Seedlings by 5-Aminolevulinic Acid. J. Pestic. Sci. 1998, 23, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, J.; Gu, W.; Zhang, Q.; Tian, L.; Guo, S.; Wei, S. Exogenous application of 5-aminolevulinic acid improves low-temperature stress tolerance of maize seedlings. Crop Pasture Sci. 2018, 69, 587–593. [Google Scholar] [CrossRef]
- Ali, A.; Yan, Y.; Yumei, L.; Yansu, L.; Xianchang, Y. 5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar]
- Ali, A.; Jun, W.; Xianchang, Y.; Chaoxing, H.; Yansu, L. Substrate Application of 5-Aminolevulinic Acid Enhanced Low-temperature and Weak-light Stress Tolerance in Cucumber (Cucumis sativus L.). Agronomy 2020, 10, 472. [Google Scholar]
- Karina, B.B.; María, L.T.; Alcira, B.; Guillermo, O.N. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 2010, 71, 2038–2045. [Google Scholar]
- Ahmet, K.; Yakup, K.; Ali Rıza, D. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar]
- Juanjuan, F.; Yongfang, S.; Xitong, C.; Yuefei, X.; Tianming, H. Exogenous 5-aminolevulenic acid promotes seed germination in Elymus nutans against oxidative damage induced by cold stress. PLoS ONE 2014, 9, e107152. [Google Scholar]
- Liu, T.; Xu, J.; Zhang, J.; Li, J.; Hu, X. Exogenous 5-aminolevulinic acid pretreatment ameliorates oxidative stress triggered by low-temperature stress of Solanum lycopersicum. Acta Physiol Plant 2018, 40, 210. [Google Scholar] [CrossRef]
- Juanjuan, F.; Xitong, C.; Yongfang, S.; Yanjun, M.; Yuefei, X.; Tianming, H. Nitric Oxide Mediates 5-Aminolevulinic Acid-Induced Antioxidant Defense in Leaves of Elymus nutans Griseb. Exposed to Chilling Stress. PLoS ONE 2015, 10, e0130367. [Google Scholar]
- Tao, L.; Jiaojiao, X.; Jianming, L.; Xiaohui, H. NO is involved in JA- and H2O2-mediated ALA-induced oxidative stress tolerance at low temperatures in tomato. Environ. Exp. Bot. 2019, 161, 334–343. [Google Scholar]
- Fu, J.J.; Chu, X.T.; Sun, Y.F.; Xu, Y.F.; Hu, T.M. Involvement of nitric oxide in 5-aminolevulinic acid-induced antioxidant defense in roots of Elymus nutans exposed to cold stress. Biol. Plant. 2016, 60, 585–594. [Google Scholar] [CrossRef]
- Megha, S.; Basu, U.; Kav, N.N.V. Metabolic engineering of cold tolerance in plants. Biocatal. Agric. Biotechnol. 2014, 3, 88–95. [Google Scholar] [CrossRef]
- Yuan, S.; Cuiting, W.; Han, Y.H.C.; Honghua, R. Response of Plants to Water Stress: A Meta-Analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar]
- Kuiju, N.; Xiang, M.; Guoling, L.; Huiling, M.; Zhifeng, J.; Wenhui, L.; Qianqian, Y. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma 2017, 254, 2083–2094. [Google Scholar]
- Dan, L.; Lingtong, W.; Muhammad Shahbaz, N.; Hongbo, L.; Xiangqin, D.; Ling, X.; Fan, Z.; Weijun, Z. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant 2013, 35, 2747–2759. [Google Scholar]
- Al-Thabet, S.S. Promotive Effect of 5-amino Levulinic Acid on Growth and Yield of Wheat Grown under Dry Conditions. J. Agron. 2006, 5, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Nudrat Aisha, A.; Shamim, K.; Naila, F.; Muhammad, A.; Fahad, A.-Q. 5−Aminolevulinic Acid Induces Regulation in Growth, Yield and Physio-Biochemical Characteristics of Wheat under Water Stress. Sains Malays. 2018, 47, 661–670. [Google Scholar]
- Kosar, F.; Akram, N.A.; Ashraf, M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 2015, 96, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Anum Samreen, T.; Abdul, S.; Ahmad, N.; Abdul, Q.; Shabir, H.; Abdul, M. Foliage application of 5-aminolevulinic acid alleviates drought stress in sunflower (Helianthus annuus L.) through improving stay green and antioxidant enzymes activities. Acta Physiol. Plant 2021, 43, 1–7. [Google Scholar]
- Ostrowska, A.; Biesaga-Kościelniak, J.; Grzesiak, M.T.; Hura, T. Physiological responses of spring wheat to 5-aminolevulinic acid under water stress applied at seedling stage. Cereal Res. Commun. 2019, 47, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, R.; Humaira, Y.; Iqbal, H.; Muhammad, I.; Muhammad Arslan, A.; Abida, P. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol. Mol. Biol. Plants 2020, 26, 489–499. [Google Scholar]
- Liu, D.; Hu, L.Y.; Ali, B.; Yang, A.G.; Wan, G.L.; Xu, L.; Zhou, W.J. Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Sci. Plant Nutr. 2016, 62, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Yuyan, A.; Lin, Q.; Liangju, W. ALA Pretreatment Improves Waterlogging Tolerance of Fig Plants. PLoS ONE 2016, 11, e0147202. [Google Scholar]
- Dotto, M.; Casati, P. Developmental reprogramming by UV-B radiation in plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef]
- Shi, C.; Liu, H. How plants protect themselves from ultraviolet-B radiation stress. Plant Physiol. 2021, 187, 1096–1103. [Google Scholar] [CrossRef]
- Divya, G.; Sheo Mohan, P. 5-aminolevulinic acid (ALA) regulates photosynthetic performance and nitrogen metabolism status in UV-B challenged Cajanus cajan L. seedlings. J. Plant Biochem. Biot. 2021. [Google Scholar] [CrossRef]
- Ozkan, A.; Omer Faruk, A.; Feyza Icoglu, A.; Ferhunde, A. Exogenous 5-aminolevulinic acid alleviates the detrimental effects of UV-B stress on lettuce (Lactuca sativa L) seedlings. Acta Physiol Plant 2017, 39, 55. [Google Scholar]
- An, Y.Y.; Cheng, D.X.; Rao, Z.X.; Sun, Y.P.; Tang, Q.; Wang, L.J. 5-Aminolevulinic acid (ALA) promotes primary root elongation through modulation of auxin transport in Arabidopsis. Acta Physiol. Plant 2019, 41, 6. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Nishihara, E.; Watanabe, S.; Tanaka, T.; Takahashi, K.; Takeuchi, Y. Enhancement of growth and fruit maturity in 2-year-old grapevines cv. Delaware by 5-aminolevulinic acid. Plant Growth Regul. 2006, 49, 35–42. [Google Scholar] [CrossRef]
- Yonezawa, T.; Sunohara, Y.; Matsumoto, H. Involvement of heme synthesis in the growth stimulation of maize seedlings by 5-aminolevulinic acid. Weed Biol. Manag. 2015, 15, 53–60. [Google Scholar] [CrossRef]
- Tarek, Y.; Mohamed, A.A. Mechanisms of Enhancing Photosynthetic Gas Exchange in Date Palm Seedlings (Phoenix dactylifera L.) under Salinity Stress by a 5-Aminolevulinic Acid-based Fertilizer. J. Plant Growth Regul. 2007, 27, 1–9. [Google Scholar]
- Mohamed, A.A. Promotive effects of a 5-aminolevulinic acid-based fertilizer on growth of tissue culture-derived date palm plants (Phoenix dactylifera L.) during acclimatization. Sci. Hortic-Amst. 2008, 118, 48–52. [Google Scholar]
- Mahmoud, A.; Ali, A. Influence of Pentakeep-V on the nutrient interaction and availability for tomato production. Emir. J. Food Agric. 2010, 22, 174–188. [Google Scholar]
- Zheng, J.; An, Y.Y.; Feng, X.X.; Wang, L.J. Rhizospheric application with 5-aminolevulinic acid improves coloration and quality in ‘Fuji’ apples. Sci. Hortic-Amst. 2017, 224, 74–83. [Google Scholar] [CrossRef]
- Maodzeka, A.; Wang, Q.; Chen, X.Y.; Hussain, N.; Wu, D.Z.; Jiang, L. Effects of 5-aminolevulinic Acid on the Bioactive Compounds and Seedling Growth of Oilseed Rape (Brassica napus L.). J. Plant Biol. 2019, 62, 181–194. [Google Scholar] [CrossRef]
- Rebeiz, C.A.; Montazer-Zouhoor, A.; Hopen, H.J.; Wu, S.M. Photodynamic herbicides: 1. Concept and phenomenology. Enzym. Microb. Technol. 1984, 6, 390–396. [Google Scholar] [CrossRef]
- Phung, T.-H.; Jung, S. Perturbed porphyrin biosynthesis contributes to differential herbicidal symptoms in photodynamically stressed rice (Oryza sativa) treated with 5-aminolevulinic acid and oxyfluorfen. Pestic. Biochem. Physiol. 2014, 116, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hiroyuki, K.; Tatsuru, M.; Shooichi, M. Action mechanism of diphenyl ether herbicides; Stimulation of 5-aminolevulinic acid-synthesizing system activities. Pestic. Biochem. Physiol. 1989, 33, 230–238. [Google Scholar]
- Ling, X.; Wenfang, Z.; Basharat, A.; Faisal, I.; Jinwen, Z.; Weijun, Z. Synergism of herbicide toxicity by 5-aminolevulinic acid is related to physiological and ultra-structural disorders in crickweed (Malachium aquaticum L.). Pestic. Biochem. Physiol. 2015, 125, 53–61. [Google Scholar]
- Koizumi, N.; Harada, Y.; Minamikawa, T.; Tanaka, H.; Otsuji, E.; Takamatsu, T. Recent advances in photodynamic diagnosis of gastric cancer using 5-aminolevulinic acid. World J. Gastroentero. 2016, 22, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Keiji, I. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int. J. Urol. 2017, 24, 97–101. [Google Scholar]
- Peiru, W.; Guolong, Z.; Linglin, Z.; Zhongxia, Z.; Lei, S.; Qingyu, Z.; Lude, Z.; Xiuli, W. 5-Aminolevulinic acid photodynamic therapy for early-stage lip squamous cell carcinoma. Photodiagn. Photodyn. Ther. 2021, 35, 102321. [Google Scholar]
- Di Venosa, G.; Fukuda, H.; Batlle, A.; MacRobert, A.; Casas, A. Photodynamic therapy: Regulation of porphyrin synthesis and hydrolysis from ALA esters. J. Photochem. Photobiol. B Biol. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Chi, Z.; Ting, G.; Jiajia, W.; Adriana, C.; Jack, J.J. Topical Application of 5-Aminolevulinic Acid Is Sufficient for Photodynamic Therapy on Vocal Folds. Laryngoscope 2018, 129, E80–E86. [Google Scholar]
- Cappugi, P.; Campolmi, P.; Mavilia, L.; Prignano, F.; Rossi, R. Topical 5-aminolevulinic acid and photodynamic therapy in dermatology: A minireview. J. Chemother. 2002, 13, 494–502. [Google Scholar] [CrossRef]
- Amit, K.J.; Chang Hyun, L.; Harvinder, S.G. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J. Control. Release 2016, 239, 72–81. [Google Scholar]
- Wei, Z.; Xiao-Feng, S.; Chang-Liang, W.; Xin-Zhou, L.; Zhen, L.; Hai-Lu, X.; Zhong-Wei, L.; Rong-Tao, Z.; Jian-Ling, H.; Hong-Qing, T. Topical 5-aminolevulinic acid photodynamic therapy for intra anal-rectal warts. J. Dermatol. Treat. 2019, 31, 241–244. [Google Scholar]

| Type of Stress | Plant Species | Stress Concentration | Mode of ALA Application and ALA Level | Effects | References |
|---|---|---|---|---|---|
| Salt stress | Asparagus (Asparagus aethiopicus L.) | 2000 and 4000 ppm NaCl | Foliar application of 3, 5, and 10 ppm | An increase in plant biomass, leaf antioxidant activity, phenolic content, proline accumulation, and photosynthetic rate | Al-Ghamdi et al., 2018 |
| Barley (Hordeum vulgare L.) | 150 mM NaCl | Hydroponics of 10, 30, and 60 mg/L | Proline content increased and ROS content decreased | Averina et al., 2010 | |
| 100 mM NaCl | Foliar application of 7 ppm | Increased chlorophyll content, antioxidant enzyme activity, and stress responsive gene expression | El-Esawi et al., 2018 | ||
| Cassia seed (Cassia obtusifolia L.) | 100 mM NaCl | Seed soaking of 5, 10, 15, and 20 mg/L; root irrigation of 10, 25, 50, and 100 mg/L | Significantly increased chlorophyll content, total soluble sugars, free proline, and soluble protein content; increased photosynthesis and antioxidant enzyme activities | Zhang et al., 2013 | |
| Cucumber (Cucumis sativus L.) | 75 mM NaCl | Foliar application of 50 mg/L | ALA might delay and counteract the upregulated expression of cucumber PIP aquaporin gene (CsPIP1:1) and cucumber NIP aquaporin gene (CsNIP) genes in cucumber seedlings under NACL stress | Yan et al., 2014 | |
| 50 mM NaCl | Foliar application of 25 mg/L | Enhancement of ascorbate-glutathione cycle; increase in shoot and root growth | Wu et al., 2019 | ||
| 50 mM NaCl | Foliar application of 25 mg/L | Increased ROS production in roots, resulting in upregulation of ion trans-porters SOS1, NHX1, and HKT1 | Baral, 2019 | ||
| 50 mM NaCl | Foliar application of 25 mg/L | Improved plant growth; upregulation of Na+/H+ antiporter SOS1 and NHX1 at the plasma and vesicle membranes, thereby reducing ion toxicity | Wu et al., 2021 | ||
| 50 mM NaCl | Foliar application of 25 mg/L | Downregulation of ferrochelatase (HEMH) gene expression; increased in chlorophyll biosynthesis pathway | Wu et al., 2018 | ||
| Date Palm (Phoenix dactylifera L.) | Seawater treatments at 1, 15, and 30 mS cm–1 | Root irrigation of 0.08% ALA based fertilizer (PentaKeep-V) | Enhanced photosynthetic assimilation by increasing chlorophyll content and stomatal conductance | Tarek et al., 2007 | |
| Maize (Zea mays L.) | 100 mM NaCl | Foliar application and seed soaking of 20 mg/L | Improved plant growth; activated the synthesis and accumulation of endogenous NO, thereby increasing the antioxidant capacity of plants | Kaya et al., 2020 | |
| Oilseed rape (Brassica napus L.) | 100 and 200 mM NaCl | Foliar application of 30 mg/L | Increased plant growth and chloroplast photosynthetic efficiency; reduced Na+ uptake and oxidative stress | Naeem et al., 2012 | |
| 200 mM NaCl | Foliar application of 30 mg/L | Increased aboveground biomass and net photosynthetic rate; promoted chlorophyll accumulation by promoting increased intermediate levels of the tetrapyrrole synthesis pathway; upregulated the expression of genes P5CS and ProDH encoding proline metabolic enzymes | Xiong et al., 2018 | ||
| 100 and 200 mM NaCl | Foliar application of 30 mg/L | Improved root and shoot growth; Enhanced plant photosynthesis, chlorophyll content; regulated the uptake of Na+ and leaf water potential | Naeem et al., 2010 | ||
| Peach (Prunnus persica L.) | 100 mM NaCl | Foliar application of 200 mg/L | Exogenous ALA treatment could improve the growth and relieve the NACL stress injury of peach seedlings by increasing photochemical efficiency, osmotic content, and antioxidant enzyme activity | Ye et al., 2016 | |
| Radix Isatidis (Isatis indigotica Fort.) | 100 mM NaCl | Foliar application of 12.5, 16.7, 25.0, and 50.0 mg/L | Increased antioxidant enzyme activity, chlorophyll content, and net photo-synthetic rate | Tang et al., 2017 | |
| Sunflower (Helianthus annuus L.) | 150 mM NaCl | Foliar application of 20, 50, and 80 mg/L | Decreased leaf H2O2 content and increased SOD activity | Akram et al., 2012 | |
| Swiss chard (Beta vulgaris L.) | 40 and 80 mM saline (molar ratio NaCl/Na2SO4 = 9:1) | Foliar application of 60 and 120 μM | The ionic toxicity was reduced by decreasing the Na+ content and Na+/K+ ratio; increased the total nitrogen and GB content | Liu et al., 2014 | |
| Watermelon (Citrullus lanatus L.) | 100 mM NaCl | Foliar application of 1.25 mM | Regulated nitrogen metabolism, reduced ion toxicity caused by salt stress, and increased soluble protein and proline | Chen et al., 2017 | |
| Extreme Temperature | Cucumber (Cucumis sativus L.) | 42/38 °C (day/night) | Foliar application of 3 μM | Reduced ROS content and growth inhibition under heat stress; enhanced antioxidant enzyme (SOD, CAT, and GPX) activity and proline content | Zhang et al., 2012 |
| 12 °C/8 °C (day/night) | Foliar application of 15, 30, and 45 mg/L ALA | Nutrient contents (N, P, K, Mg, Ca, Cu, Fe, Mn, and Zn) and endogenous hormones (JA, IAA, BR, IPA, and ZR) were enhanced in roots and leaves; Increased chlorophyll content, photosynthetic capacity, and antioxidant enzymes (SOD, POD, CAT, APX, and GR); reduced growth inhibition of seedlings by cold stress | Anwar et al., 2018 | ||
| 16 °C/8 °C (day/night) | Add to the culture substrate of 10, 20, or 30 mg ALA·kg−1 (ALA were mixed with a constant weight of substrate (kg)) | Significantly reduced plant growth inhibition; increased chlorophyll content, antioxidant enzymes (SOD, CAT, and POD) activity; reduced accumulation of ROS and malondialdehyde in roots and leaves | Anwar et al., 2020 | ||
| Drooping wild ryegrass (Elymus nutans Griseb.) | 5 °C | Seed soaking of 0.1, 0.5, 1, 5, 10, and 25 mg/L | Significantly increased seed respiration rate and ATP synthesis and protected germinating seeds from cold stress; increased GSH, AsA, total glutathione, and total ascorbate concentrations, as well as SOD, CAT, APX, and GR activities | Fu et al., 2014 | |
| 5 °C | Foliar application of 0.5, 1, 5, 10, and 26 mg/L | NO might be a downstream signal that mediates ALA-induced cold tolerance, thereby enhancing antioxidant defense | Fu et al., 2015 | ||
| 5 °C | Root soaking of 0.5, 1, 5, 10, and 26 mg/L | NO might act as a downstream signal to mediate ALA-induced cold resistance by activating antioxidant defense and PM H+-ATPase and maintaining Na+ and K+ homeostasis | Fu et al., 2016 | ||
| Maize (Zea mays L.) | 14 °C/5 °C (day/night) | Foliar application of 0.15 mM | Increased proline accumulation, antioxidant enzymes (SOD and CAT) and RuBP carboxylase activity; prevented reductions in maize crop yield due to low-temperature stress | Wang et al., 2018 | |
| Red pepper (Capsicum annuum cv. Sena) | 4 °C | Seed soaking of 1, 10, 25, 50, and 100 ppm | Resulted in higher germination and seedling emergence percentages, as well as faster germination and seedling emergence | Korkmaz et al., 2009 | |
| 3 °C | Seed soaking, foliar spray and soil drench of 1, 10, 25, 50, and 100 ppm | Improved plant quality, chlorophyll content, sucrose, and proline content; enhanced SOD activity | Korkmaz et al., 2010 | ||
| Rice (Oryza sativa L.) | 3 °C, 5 °C | Root soaking of 0.001, 0.1, 1, and 5 ppm | Reduced cold injury-induced tissue electrolyte leakage | Hotta et al., 1998 | |
| 10 °C | Seed soaking of 8.5 mM | Increased antioxidant enzymes (SOD, POD, APX, and GPX) activity; increased relative gene expression of enzymes of PA biosynthesis | Sheteiwy et al., 2017 | ||
| Soybean (Glycine max L.) | 4 °C | Hydroponics of 5, 10, 15, 20, 30, and 40 μM | Increased chlorophyll content and relative water content of leaves; enhanced activity of antioxidant enzymes CAT and HO-1 | Balestrasse et al., 2010 | |
| Tomato (Solanum lycopersicum) | 15 °C/8 °C (day/night) | Foliar application of 5, 10, 25, 50, and 100 mg/L | ALA induced H2O2, which in turn increased the ratio of GSH and ASA, leading to enhanced antioxidant capacity; significantly increased the activities of SOD, CAT, APX, DHAR, and GSH | Liu et al., 2018 | |
| 15 °C/8 °C (day/night) | Foliar application of 5, 10, 25, 50, and 100 mg/L | ALA pretreatment-induced CAT and ASA-GSH reliably eliminated excess ROS under low temperature stress and maintained redox homeostasis | Liu et al., 2018 | ||
| 15 °C/8 °C (day/night) | Foliar application of 25 mg/L | ALA triggered NO production directly, or induced H2O2 and JA signals to trigger NO production, thus NO interacted with JA to regulate cold-induced oxidative stress | Liu et al., 2019 | ||
| Drought stress | Kentucky bluegrass (Poa pratensis L.) | 10% PEG 6000 | Foliar application of 10 mg/L | Improved turf quality and leaf relative water content; enhanced antioxidant enzymes (SOD, CAT, APX, GPX, DHAR, and GR), ASA, and GSH content, thus reducing oxidative damage | Niu et al., 2017 |
| Oilseed rape (Brassica napus L.) | Drought stress (40% of water-holding capacity) | Foliar application of 30 mg/L | Maintained relatively higher leaf water status; enhanced chlorophyll content and net photosynthetic rate; increased antioxidant enzyme (POD and CAT) activity | Liu et al., 2013 | |
| Drought stress (40% of water-holding capacity) | Foliar application of 30 mg/L | Expression of photosynthetic genes (RBCS, TPI, FBP, FBPA, and TKL) was upregulated; increased leaf hexose and sucrose accumulation and maintenance of starch content | Liu et al., 2016 | ||
| Sunflower (Helianthus annuus L.) | Water stress (70% field capacity) | Foliar application of 10, 20, and 30 mg/L | Reduced oxidative damage by lowering H2O2 and MDA contents | Rasheed et al., 2020 | |
| Drought stress (40% of water-holding capacity) | Foliar application of 25, 50, 75, and 100 mg/L | Enhancement of stay green and CAT, SOD, and APX activities, thus reducing drought-induced yield losses and improving oil contents | Sher et al., 2021 | ||
| Wheat (Triticum aestivum L.) | Irrigation interval of 7, 14, and 21 days | Foliar application of 25, 50, and 100 ppm | Increased grain yield | Al-Thabet et al., 2006 | |
| Water deficit (60% and 80% of field capacity) | Foliar application of 50, 100, and 150 mg/L | Improved leaf fluorescence (qN, NPQ, and Fv/Fm), shoot and root K+, root Ca2+, proline, and GB accumulation | Akram et al., 2018 | ||
| Water stress (30% maximum water capacity) | Foliar application of 30 mg/L | Increased plant growth, photosynthesis, and chlorophyll content; reduced the degree of damage to cell membranes during early nutritional development | Ostrowska et al., 2019 | ||
| 80% (mild drought stress), and 60% (high drought stress) | Foliar application of 50, 100, and 150 mg/L | Increased fresh and dry weight of shoots and roots, chlorophyll content, GB content, and N content in leaves and roots | Kosar et al., 2015 | ||
| UV-B stress | lettuce (Lactuca sativa L.) | 3.3 W m−2 UV-B | Foliar application of 10 and 25 ppm | ALA treatment resulted in a substantial increase in phenylalanine ammonia lyase (PAL) and γ-tocopherol methyltransferase (γ-TMT) gene expression, antioxidant enzyme activity, and chlorophyll a and b concentrations. | Aksakal et al., 2017 |
| Pigeon pea (Cajanus cajan L.) | enhanced UV-B (2.2 kJ m−2d−1) | Seed soaking of 25 and 100 μM | Reduced germination time and increased germination index; upregulated photosynthesis, antioxidant enzymes (CAT, SOD, and POD), total phenolic content, and total flavonoid content to balance ROS and reduce UV-B damage to plant productivity | Gupta et al., 2021 | |
| enhanced UV-B (2.2 kJ m−2d−1) | Seed soaking of 25 and 100 μM | Increased plant growth and growth regulating parameters; increased enzyme activity and non-enzymatic antioxidant content in the plant defense system and reduced oxidative stress in seedlings | Gupta et al., 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Cao, J.; Xia, X.; Li, Z. Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2022, 23, 702. https://doi.org/10.3390/ijms23020702
Tan S, Cao J, Xia X, Li Z. Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress. International Journal of Molecular Sciences. 2022; 23(2):702. https://doi.org/10.3390/ijms23020702
Chicago/Turabian StyleTan, Shuya, Jie Cao, Xinli Xia, and Zhonghai Li. 2022. "Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress" International Journal of Molecular Sciences 23, no. 2: 702. https://doi.org/10.3390/ijms23020702
APA StyleTan, S., Cao, J., Xia, X., & Li, Z. (2022). Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress. International Journal of Molecular Sciences, 23(2), 702. https://doi.org/10.3390/ijms23020702






