The Dynamics and Plasticity of Epigenetics in Diabetic Kidney Disease: Therapeutic Applications Vis-à-Vis
Abstract
1. Introduction
2. Epigenetic Machineries: An Introduction
2.1. DNA Methylation
2.2. Histone Modifications
2.3. Noncoding RNAs
3. DKD: An Overview
3.1. DNA Methylation in DKD
3.2. Histone Modifications in DKD
3.3. Noncoding RNAs in DKD
4. Kidney Cell-Centric View of Epigenetic Modifications
5. Epigenetic Modifications of Glomerular Endothelial Cells in DKD
6. Epigenetic Biomarkers
7. Epigenetic Machineries for Therapeutic Applications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- GBD 2017 DALYs and HALE Collaborators. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Wang, V.; Vilme, H.; Maciejewski, M.L.; Boulware, L.E. The Economic Burden of Chronic Kidney Disease and End-Stage Renal Disease. Semin. Nephrol. 2016, 36, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Data from: Global prevalence of chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Mauer, M. Histopathology of Diabetic Nephropathy. Semin. Nephrol. 2007, 27, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-T.; Chao, C.-T.; Lin, S.-H. Chronic Kidney Disease: Strategies to Retard Progression. Int. J. Mol. Sci. 2021, 22, 10084. [Google Scholar] [CrossRef]
- Gu, H.F. Genetic and Epigenetic Studies in Diabetic Kidney Disease. Front. Genet. 2019, 10, 507. [Google Scholar] [CrossRef]
- Tin, A.; Köttgen, A. Genome-Wide Association Studies of CKD and Related Traits. Clin. J. Am. Soc. Nephrol. 2020, 15, 1643–1656. [Google Scholar] [CrossRef]
- Wuttke, M.; Li, Y.; Li, M.; Sieber, K.B.; Feitosa, M.F.; Gorski, M.; Tin, A.; Wang, L.; Chu, A.Y.; Hoppmann, A.; et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 2019, 51, 957–972. [Google Scholar] [CrossRef]
- Teumer, A.; Li, Y.; Ghasemi, S.; Prins, B.P.; Wuttke, M.; Hermle, T.; Giri, A.; Sieber, K.B.; Qiu, C.; Kirsten, H.; et al. Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat. Commun. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Kato, M.; Natarajan, R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 2019, 15, 327–345. [Google Scholar] [CrossRef]
- Smyth, L.J.; McKay, G.J.; Maxwell, A.P.; McKnight, A.J. DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics 2013, 9, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.Y.C.; Short, A.K.; Bredy, T.W.; Hannan, A.J. Transgenerational paternal transmission of acquired traits: Stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr. Opin. Behav. Sci. 2017, 14, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Van Otterdijk, S.D.; Michels, K.B. Transgenerational epigenetic inheritance in mammals: How good is the evidence? FASEB J. 2016, 30, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Warych, A.; Szoszkiewicz, M. Novel Approaches to Epigenetic Therapies: From Drug Combinations to Epigenetic Editing. Genes 2021, 12, 208. [Google Scholar] [CrossRef]
- Leone, G.; D’Alo, F.; Zardo, G.; Voso, M.T.; Nervi, C. Epigenetic Treatment of Myelodysplastic Syndromes and Acute Myeloid Leukemias. Curr. Med. Chem. 2008, 15, 1274–1287. [Google Scholar] [CrossRef]
- Ehrlich, M.; Gama-Sosa, M.A.; Huang, L.-H.; Midgett, R.M.; Kuo, K.C.; McCune, R.A.; Gehrke, C. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues or cells. Nucleic Acids Res. 1982, 10, 2709–2721. [Google Scholar] [CrossRef]
- Deaton, A.M.; Bird, A. CpG islands and the regulation of transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef]
- Hermann, A.; Goyal, R.; Jeltsch, A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase Methylates DNA Processively with High Preference for Hemimethylated Target Sites. J. Biol. Chem. 2004, 279, 48350–48359. [Google Scholar] [CrossRef]
- Pradhan, S.; Bacolla, A.; Wells, R.D.; Roberts, R.J. Recombinant Human DNA (Cytosine-5) Methyltransferase: I. Expression, purification, and comparison of de novo and maintenance methylation. J. Biol. Chem. 1999, 274, 33002–33010. [Google Scholar] [CrossRef] [PubMed]
- Bestor, T.H. Activation of mammalian DNA methyltransferase by cleavage of a Zn binding regulatory domain. EMBO J. 1992, 11, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Hsieh, C.-L. In Vivo Activity of Murine De Novo Methyltransferases, Dnmt3a and Dnmt3b. Mol. Cell. Biol. 1999, 19, 8211–8218. [Google Scholar] [CrossRef]
- Okano, M.; Xie, S.; Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998, 19, 219–220. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Hamidi, T.; Singh, A.K.; Chen, T. Genetic alterations of DNA methylation machinery in human diseases. Epigenomics 2015, 7, 247–265. [Google Scholar] [CrossRef]
- Mariño-Ramírez, L.; Kann, M.G.; Shoemaker, B.A.; Landsman, D. Histone structure and nucleosome stability. Expert Rev. Proteom. 2005, 2, 719–729. [Google Scholar] [CrossRef]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef]
- Song, N.; Liu, J.; An, S.; Nishino, T.; Hishikawa, Y.; Koji, T. Immunohistochemical Analysis of Histone H3 Modifications in Germ Cells during Mouse Spermatogenesis. Acta Histochem. Cytochem. 2011, 44, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Zhou, V.W.; Goren, A.; Bernstein, B.E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 2011, 12, 7–18. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Yu, C.; Zhuang, S. Histone Methyltransferases as Therapeutic Targets for Kidney Diseases. Front. Pharmacol. 2019, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Carey, M.; Workman, J.L. The Role of Chromatin during Transcription. Cell 2007, 128, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.J., III; Nishioka, K.; Reinberg, D. Histone lysine methylation: A signature for chromatin function. Trends Genet. 2003, 19, 629–639. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Knapp, S. Targeting bromodomains: Epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 2014, 13, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Laganà, A.; Veneziano, D.; Russo, F.; Pulvirenti, A.; Giugno, R.; Croce, C.M.; Ferro, A. Computational Design of Artificial RNA Molecules for Gene Regulation. Methods Mol. Biol. 2015, 1269, 393–412. [Google Scholar] [PubMed]
- Siomi, M.C.; Sato, K.; Pezic, D.; Aravin, A.A. PIWI-interacting small RNAs: The vanguard of genome defence. Nat. Rev. Mol. Cell Biol. 2011, 12, 246–258. [Google Scholar] [CrossRef]
- Dorner, S.; Eulalio, A.; Huntzinger, E.; Izaurralde, E. Delving into the diversity of silencing pathways. In EMBO Reports 2007, Proceedings of the Symposium on MicroRNAs and siRNAs: Biological Functions and Mechanisms, Keystone, CO, USA, 28 January and 2 February 2007; European Molecular Biology Organization: Corvallis, OR, USA, 2017; Volume 8, pp. 723–729. [Google Scholar]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Tu, S.-T.; Sheu, W.H.-H. 2019 Diabetes Atlas: Achievements and challenges in diabetes care in Taiwan. J. Formos. Med. Assoc. 2019, 118, S130–S134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Kong, J.; Yun, K. Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J. Diabetes Res. 2020, 2020, 2315607. [Google Scholar] [CrossRef]
- Vasishta, S.; Umakanth, S.; Adiga, P.; Joshi, M.B. Extrinsic and intrinsic factors influencing metabolic memory in type 2 dia-betes. Vasc. Pharm. 2022, 142, 106933. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.G.; Teschendorff, A.E.; Rakyan, V.K.; Maxwell, A.P.; Beck, S.; Savage, D.A. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med. Genom. 2010, 3, 33. [Google Scholar] [CrossRef]
- Swan, E.J.; Maxwell, A.P.; McKnight, A.J. Distinct methylation patterns in genes that affect mitochondrial function are associated with kidney disease in blood-derived DNA from individuals with Type 1 diabetes. Diabet. Med. 2015, 32, 1110–1115. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, F.; Paterson, A.D.; Lachin, J.M.; Zhang, L.; Schones, D.E.; Wu, X.; Wang, J.; Tompkins, J.D.; Genuth, S.; et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc. Natl. Acad. Sci. USA 2016, 113, E3002–E3011. [Google Scholar] [CrossRef]
- Alhawiti, N.M.; Al Mahri, S.; Aziz, M.A.; Malik, S.S.; Mohammad, S. TXNIP in Metabolic Regulation: Physiological Role and Therapeutic Outlook. Curr. Drug Targets 2017, 18, 1095–1103. [Google Scholar] [CrossRef]
- VanderJagt, T.A.; Neugebauer, M.H.; Morgan, M.; Bowden, D.W.; Shah, V.O. Epigenetic profiles of pre-diabetes transitioning to type 2 diabetes and nephropathy. World J. Diabetes 2015, 6, 1113–1121. [Google Scholar] [CrossRef]
- Sheng, X.; Qiu, C.; Liu, H.; Gluck, C.; Hsu, J.Y.; He, J.; Hsu, C.-Y.; Sha, D.; Weir, M.R.; Isakova, T.; et al. Systematic integrated analysis of genetic and epigenetic variation in diabetic kidney disease. Proc. Natl. Acad. Sci. USA 2020, 117, 29013–29024. [Google Scholar] [CrossRef]
- Chu, A.Y.; Tin, A.; Schlosser, P.; Ko, Y.-A.; Qiu, C.; Yao, C.; Joehanes, R.; Grams, M.E.; Liang, L.; Gluck, C.A.; et al. Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat. Commun. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Marumo, T.; Yagi, S.; Kawarazaki, W.; Nishimoto, M.; Ayuzawa, N.; Watanabe, A.; Ueda, K.; Hirahashi, J.; Hishikawa, K.; Sakurai, H.; et al. Diabetes Induces Aberrant DNA Methylation in the Proximal Tubules of the Kidney. J. Am. Soc. Nephrol. 2015, 26, 2388–2397. [Google Scholar] [CrossRef]
- Chen, G.; Chen, H.; Ren, S.; Xia, M.; Zhu, J.; Liu, Y.; Zhang, L.; Tang, L.; Sun, L.; Liu, H.; et al. Aberrant DNA methylation of mTOR pathway genes promotes inflammatory activation of immune cells in diabetic kidney disease. Kidney Int. 2019, 96, 409–420. [Google Scholar] [CrossRef]
- Peng, R.; Liu, H.; Peng, H.; Zhou, J.; Zha, H.; Chen, X.; Zhang, L.; Sun, Y.; Yin, P.; Wen, L.; et al. Promoter hypermethylation of let-7a-3 is relevant to its down-expression in diabetic nephropathy by targeting UHRF1. Gene 2015, 570, 57–63. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Q.; Li, P.; Zhao, Q.; Zhang, J.; Li, J.; Koseki, H.; Wong, J. UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat. Commun. 2013, 4, 1563. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Zhou, X.; Ding, L.; Liu, D.; Xu, H. DNMT1-mediated PPARα methylation aggravates damage of retinal tissues in diabetic retinopathy mice. Biol. Res. 2021, 54, 25. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Yoshida, N.; Okumura, K.I.; Wakabayashi, S.; Matsutomo, R.; Takebayashi, K.; Inukai, T. Coagulation and inflammation in overt diabetic nephropathy: Association with hyperhomocysteinemia. Clin. Chim. Acta 2004, 348, 139–145. [Google Scholar] [CrossRef]
- Sayanthooran, S.; Magana-Arachchi, D.N.; Gunerathne, L.; Abeysekara, T. Potential diagnostic biomarkers for chronic kidney disease of unknown etiology (CKDu) in Sri Lanka: A pilot study. BMC Nephrol. 2017, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Aldemir, O.; Turgut, F.; Gokce, C. The association between methylation levels of targeted genes and albuminuria in patients with early diabetic kidney disease. Ren. Fail. 2017, 39, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-H.; Cao, R.-F.; Yu, Y.; Sui, M.; Zhang, T.; Xu, J.-Y.; Wang, X.-M. A study on the correlation between MTHFR promoter methylation and diabetic nephropathy. Am. J. Transl. Res. 2016, 8, 4960–4967. [Google Scholar]
- Gu, T.; Falhammar, H.; Gu, H.F.; Brismar, K. Epigenetic analyses of the insulin-like growth factor binding protein 1 gene in type 1 diabetes and diabetic nephropathy. Clin. Epigenetics 2014, 6, 10. [Google Scholar] [CrossRef]
- Yokoi, H.; Mukoyama, M.; Mori, K.; Kasahara, M.; Suganami, T.; Sawai, K.; Yoshioka, T.; Saito, Y.; Ogawa, Y.; Kuwabara, T.; et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008, 73, 446–455. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, X.; Yi, B.; Huang, J.; Wang, J.; Sun, J. Correlation of CTGF gene promoter methylation with CTGF expression in type 2 diabetes mellitus with or without nephropathy. Mol. Med. Rep. 2014, 9, 2138–2144. [Google Scholar] [CrossRef]
- Yi, B.; Zhang, H.; Zhou, H.; Cai, X.; Sun, J.; Liu, Y. High glucose induce the demethylation of CTGF promoter and gene ex-pression. Chin. J. Cell. Mol. Immunol. 2011, 27, 747–750. [Google Scholar]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Müller, G.A.; Kalbacher, H.; Salant, D.J.; Müller, C.A.; Kalluri, R.; Zeisberg, M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Tampe, B.; Tampe, D.; Müller, C.A.; Sugimoto, H.; LeBleu, V.; Xu, X.; Müller, G.A.; Zeisberg, E.M.; Kalluri, R.; Zeisberg, M. Tet3-Mediated Hydroxymethylation of Epigenetically Silenced Genes Contributes to Bone Morphogenic Protein 7-Induced Reversal of Kidney Fibrosis. J. Am. Soc. Nephrol. 2014, 25, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Chen, Z.; Genuth, S.; Paterson, A.; Zhang, L.; Wu, X.; Li, S.M.; Cleary, P.; Riggs, A.; Harlan, D.M.; et al. Evaluating the Role of Epigenetic Histone Modifications in the Metabolic Memory of Type 1 Diabetes. Diabetes 2014, 63, 1748–1762. [Google Scholar] [CrossRef]
- Yuan, H.; Reddy, M.A.; Sun, G.; Lanting, L.; Wang, M.; Kato, M.; Natarajan, R. Involvement of p300/CBP and epigenetic histone acetylation in TGF-β1-mediated gene transcription in mesangial cells. Am. J. Physiol. Ren. Physiol. 2013, 304, F601–F613. [Google Scholar] [CrossRef]
- Kato, M.; Dang, V.; Wang, M.; Park, J.T.; Deshpande, S.; Kadam, S.; Mardiros, A.; Zhan, Y.; Oettgen, P.; Putta, S.; et al. TGF-β induces acetylation of chromatin and of Ets-1 to alleviate repression of miR-192 in diabetic nephropathy. Sci. Signal. 2013, 6, ra43. [Google Scholar] [CrossRef]
- Dong, Z. Acetylation of Ets-1 Is the Key to Chromatin Remodeling for miR-192 Expression. Sci. Signal. 2013, 6, pe21. [Google Scholar] [CrossRef]
- Kato, M.; Zhang, J.; Wang, M.; Lanting, L.; Yuan, H.; Rossi, J.J.; Natarajan, R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. USA 2007, 104, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- Rerolle, J.-P.; Hertig, A.; Nguyen, G.; Sraer, J.-D.; Rondeau, E.P. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000, 58, 1841–1850. [Google Scholar] [CrossRef][Green Version]
- Kuan, C.J.; Al-Douahji, M.; Shankland, S.J. The cyclin kinase inhibitor p21WAF1, CIP1 is increased in experimental diabetic nephropathy: Potential role in glomerular hypertrophy. J. Am. Soc. Nephrol. 1998, 9, 986–993. [Google Scholar] [CrossRef]
- Liu, F.; Zong, M.; Wen, X.; Li, X.; Wang, J.; Wang, Y.; Jiang, W.; Li, X.; Guo, Z.; Qi, H. Silencing of Histone Deacetylase 9 Expression in Podocytes Attenuates Kidney Injury in Diabetic Nephropathy. Sci. Rep. 2016, 6, 33676. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhen, J.; Zhang, C.; Wan, Q.; Liu, G.; Wei, X.; Zhang, Y.; Wang, Z.; Han, H.; et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014, 86, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Jiao, L.; Petersen, R.B.; Li, J.; Peng, A.; Zheng, L.; Huang, K. Apelin inhibits the development of diabetic nephropathy by regulating histone acetylation in Akita mouse. J. Physiol. 2013, 592, 505–521. [Google Scholar] [CrossRef]
- Sun, G.; Reddy, M.A.; Yuan, H.; Lanting, L.; Kato, M.; Natarajan, R. Epigenetic Histone Methylation Modulates Fibrotic Gene Expression. J. Am. Soc. Nephrol. 2010, 21, 2069–2080. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Zeng, W.; Huang, L.; Pang, Q.; Nie, L.; Mu, J.; Yuan, F.; Feng, B. ER stress triggers MCP-1 expression through SET7/9-induced histone methylation in the kidneys of db/db mice. Am. J. Physiol. Ren. Physiol. 2014, 306, F916–F925. [Google Scholar] [CrossRef]
- Lin, S.-H.; Ho, W.-T.; Wang, Y.-T.; Chuang, C.-T.; Chuang, L.-Y.; Guh, J.-Y. Histone methyltransferase Suv39h1 attenuates high glucose-induced fibronectin and p21(WAF1) in mesangial cells. Int. J. Biochem. Cell Biol. 2016, 78, 96–105. [Google Scholar] [CrossRef]
- Wang, J.; Yan, W.; Peng, X.; Jiang, Y.; He, L.; Peng, Y.; Chen, X.; Ye, M.; Zhuo, H. Functional Role of SUV39H1 in Human Renal Tubular Epithelial Cells Under High-glucose Ambiance. Inflammation 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Goru, S.K.; Kadakol, A.; Pandey, A.; Malek, V.; Sharma, N.; Gaikwad, A.B. Histone H2AK119 and H2BK120 mono-ubiquitination modulate SET7/9 and SUV39H1 in type 1 diabetes-induced renal fibrosis. Biochem. J. 2016, 473, 3937–3949. [Google Scholar] [CrossRef]
- Siddiqi, F.S.; Majumder, S.; Thai, K.; Abdalla, M.; Hu, P.; Advani, S.L.; White, K.E.; Bowskill, B.B.; Guarna, G.; dos Santos, C.C.; et al. The Histone Methyltransferase Enzyme Enhancer of Zeste Homolog 2 Protects against Podocyte Oxidative Stress and Renal Injury in Diabetes. J. Am. Soc. Nephrol. 2016, 27, 2021–2034. [Google Scholar] [CrossRef]
- Majumder, S.; Thieme, K.; Batchu, S.N.; Alghamdi, T.A.; Bowskill, B.B.; Kabir, M.G.; Liu, Y.; Advani, S.L.; White, K.E.; Geldenhuys, L.; et al. Shifts in podocyte histone H3K27me3 regulate mouse and human glomerular disease. J. Clin. Investig. 2017, 128, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Reddy, M.A.; Das, S.; Oh, H.J.; Abdollahi, M.; Yuan, H.; Zhang, E.; Lanting, L.; Wang, M.; Natarajan, R. Dysregulation of histone H3 lysine 27 trimethylation in transforming growth factor-β1-induced gene expression in mesangial cells and diabetic kidney. J. Biol. Chem. 2019, 294, 12695–12707. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, R.; Peng, H.; Liu, H.; Wen, L.; Wu, T.; Yi, H.; Li, A.; Zhang, Z. MiR-451 suppresses the NF-kappaB-mediated proinflammatory molecules expression through inhibiting LMP7 in diabetic nephropathy. Mol. Cell. Endocrinol. 2016, 433, 75–86. [Google Scholar] [CrossRef]
- Bhatt, K.; Lanting, L.L.; Jia, Y.; Yadav, S.; Reddy, M.A.; Magilnick, N.; Boldin, M.; Natarajan, R. Anti-Inflammatory Role of MicroRNA-146a in the Pathogenesis of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Khan, S.Q.; Khaliqdina, S.; Altintas, M.M.; Grahammer, F.; Zhao, J.L.; Koh, K.H.; Tardi, N.J.; Faridi, M.H.; Geraghty, T.; et al. Absence of miR-146a in Podocytes Increases Risk of Diabetic Glomerulopathy via Up-regulation of ErbB4 and Notch-1. J. Biol. Chem. 2017, 292, 732–747. [Google Scholar] [CrossRef]
- Deshpande, S.D.; Putta, S.; Wang, M.; Lai, J.Y.; Bitzer, M.; Nelson, R.G.; Lanting, L.L.; Kato, M.; Natarajan, R. Transforming growth factor-β-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes 2013, 62, 3151–3162. [Google Scholar] [CrossRef]
- Putta, S.; Lanting, L.; Sun, G.; Lawson, G.; Kato, M.; Natarajan, R. Inhibiting MicroRNA-192 Ameliorates Renal Fibrosis in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2012, 23, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Krupa, A.; Jenkins, R.; Luo, D.D.; Lewis, A.; Phillips, A.; Fraser, D. Loss of MicroRNA-192 Promotes Fibrogenesis in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2010, 21, 438–447. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lee, P.-H.; Hsu, Y.-C.; Lei, C.-C.; Ko, J.-Y.; Chuang, P.-C.; Huang, Y.-T.; Wang, S.-Y.; Wu, S.-L.; Chen, Y.-S.; et al. MicroRNA-29a Promotion of Nephrin Acetylation Ameliorates Hyperglycemia-Induced Podocyte Dysfunction. J. Am. Soc. Nephrol. 2014, 25, 1698–1709. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhong, X.; Huang, X.R.; Meng, X.-M.; You, Y.; Chung, A.C.; Lan, H.Y. MicroRNA-29b Inhibits Diabetic Nephropathy in db/db Mice. Mol. Ther. 2014, 22, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.F.; Tang, P.M.K.; Feng, M.; Xiao, J.; Huang, X.R.; Li, P.; Ma, R.C.W.; Lan, H.Y. Novel lncRNA Erbb4-IR Promotes Diabetic Kidney Injury in db/db Mice by Targeting miR-29b. Diabetes 2017, 67, 731–744. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.J.; Danesh, F.R. MicroRNA-29c is a signature microRNA under high glucose con-ditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J. Biol. Chem. 2011, 286, 11837–11848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, S.; Luo, H. Regulation of podocyte lesions in diabetic nephropathy via miR-34a in the Notch signaling pathway. Medicine 2016, 95, e5050. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-D.; Zhang, L.-Y.; Zhu, T.-C.; Zhang, R.-F.; Wang, S.-L.; Bao, Y. Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int. J. Clin. Exp. Pathol. 2015, 8, 4525–4534. [Google Scholar] [PubMed]
- Li, A.; Peng, R.; Sun, Y.; Liu, H.; Peng, H.; Zhang, Z. LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via miR-34a-5p/Sirt1/HIF-1α signaling. Cell Death Dis. 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, L.; Tian, L.; Liu, J.; Wang, S.; Gao, Y.; Yang, J. Serum miR-21 may be a Potential Diagnostic Biomarker for Diabetic Nephropathy. Exp. Clin. Endocrinol. Diabetes 2016, 124, 417–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McClelland, A.D.; Herman-Edelstein, M.; Komers, R.; Jha, J.C.; Winbanks, C.E.; Hagiwara, S.; Gregorevic, P.; Kantharidis, P.; Cooper, M.E. MiR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin. Sci. 2015, 129, 1237–1249. [Google Scholar] [CrossRef]
- Park, J.T.; Kato, M.; Yuan, H.; Castro, N.; Lanting, L.; Wang, M.; Natarajan, R. FOG2 protein down-regulation by transforming growth factor-β1-induced microRNA-200b/c leads to Akt kinase activation and glomerular mesangial hypertrophy related to diabetic nephropathy. J. Biol. Chem. 2013, 288, 22469–22480. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Peng, F.; Xia, X.; Zhao, C.; Luo, Q.; Guan, W.; Li, Z.; Yu, X.; Huang, F. MiR-135a promotes renal fibrosis in diabetic nephropathy by regulating TRPC1. Diabetologia 2014, 57, 1726–1736. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Minto, A.W.; Wang, J.; Shi, Q.; Li, X.; Quigg, R.J. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008, 22, 4126–4135. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Yokoi, H.; Mori, K.; Kasahara, M.; Kuwabara, T.; Imamaki, H.; Ishii, A.; Mori, K.P.; Kato, Y.; Ohno, S.; et al. MicroRNA-26a inhibits TGF-β-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia 2015, 58, 2169–2180. [Google Scholar] [CrossRef]
- Wang, B.; Jha, J.C.; Hagiwara, S.; McClelland, A.D.; Jandeleit-Dahm, K.; Thomas, M.C.; Cooper, M.E.; Kantharidis, P. Transforming growth factor-β1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b. Kidney Int. 2014, 85, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T.; Kato, M.; Lanting, L.; Castro, N.; Nam, B.Y.; Wang, M.; Kang, S.-W.; Natarajan, R. Repression of let-7 by trans-forming growth factor-β1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. Am. J. Physiol. Ren. Physiol. 2014, 307, F1390–F1403. [Google Scholar] [CrossRef]
- Bai, X.; Geng, J.; Zhou, Z.; Tian, J.; Li, X. MicroRNA-130b improves renal tubulointerstitial fibrosis via repression of Snail-induced epithelial-mesenchymal transition in diabetic nephropathy. Sci. Rep. 2016, 6, 20475. [Google Scholar] [CrossRef]
- Wang, B.; Koh, P.; Winbanks, C.; Coughlan, M.T.; McClelland, A.; Watson, A.; Jandeleit-Dahm, K.; Burns, W.C.; Thomas, M.C.; Cooper, M.E.; et al. MiR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 2011, 60, 280–287. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.; Wang, F.; Guo, C. LncRNA-NR_033515 promotes proliferation, fibrogenesis and epithelial-to-mesenchymal transition by targeting miR-743b-5p in diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 543–552. [Google Scholar] [CrossRef]
- Li, X.; Zeng, L.; Cao, C.; Lu, C.; Lian, W.; Han, J.; Zhang, X.; Zhang, J.; Tang, T.; Li, M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp. Cell Res. 2017, 350, 327–335. [Google Scholar] [CrossRef]
- Bai, X.; Geng, J.; Li, X.; Wan, J.; Liu, J.; Zhou, Z.; Liu, X. Long Noncoding RNA LINC01619 Regulates MicroRNA-27a/Forkhead Box Protein O1 and Endoplasmic Reticulum Stress-Mediated Podocyte Injury in Diabetic Nephropathy. Antioxid. Redox Signal. 2018, 29, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Wang, M.; Chen, Z.; Bhatt, K.; Oh, H.J.; Lanting, L.; Deshpande, S.; Jia, Y.; Lai, J.Y.C.; O’Connor, C.L.; et al. An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA mega-cluster induces early features of diabetic nephropathy. Nat. Commun. 2016, 7, 12864. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Khosroheidari, M.; Eddy, E.; Kiefer, J. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: Implications for diabetic nephropathy. PLoS ONE 2013, 8, e77468. [Google Scholar]
- Cao, X.; Fan, Q.-L. LncRNA MIR503HG Promotes High-Glucose-Induced Proximal Tubular Cell Apoptosis by Targeting miR-503-5p/Bcl-2 Pathway. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4507–4517. [Google Scholar] [CrossRef]
- Rössig, L.; Li, H.; Fisslthaler, B.; Urbich, C.; Fleming, I.; Förstermann, U.; Zeiher, A.M.; Dimmeler, S. Inhibitors of Histone Deacetylation Downregulate the Expression of Endothelial Nitric Oxide Synthase and Compromise Endothelial Cell Function in Vasorelaxation and Angiogenesis. Circ. Res. 2002, 91, 837–844. [Google Scholar]
- Takahashi, T.; Harris, R.C. Role of Endothelial Nitric Oxide Synthase in Diabetic Nephropathy: Lessons from Diabetic eNOS Knockout Mice. J. Diabetes Res. 2014, 2014, 590541. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wei, C.; Zhang, W.; Schlondorff, D.; Wu, J.; Cai, M.; He, W.; Baron, M.H.; Chuang, P.Y.; Liu, Z.; et al. Gene expression profiles of glomerular endothelial cells support their role in the glomerulopathy of diabetic mice. Kidney Int. 2018, 94, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, D.; Xu, G. Long noncoding RNA MALAT1 mediates high glucose-induced glomerular endothelial cell injury by epigenetically inhibiting klotho via methyltransferase G9a. IUBMB Life 2019, 71, 873–881. [Google Scholar] [CrossRef]
- Alghamdi, T.A.; Batchu, S.N.; Hadden, M.J.; Yerra, V.G.; Liu, Y.; Bowskill, B.B.; Advani, S.L.; Geldenhuys, L.; Siddiqi, F.S.; Majumder, S.; et al. Histone H3 Serine 10 Phosphorylation Facilitates Endothelial Activation in Diabetic Kidney Disease. Diabetes 2018, 67, 2668–2681. [Google Scholar] [CrossRef]
- Jia, Y.; Guan, M.; Zheng, Z.; Zhang, Q.; Tang, C.; Xu, W.; Xiao, Z.; Wang, L.; Xue, Y. MiRNAs in Urine Extracellular Vesicles as Predictors of Early-Stage Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 7932765. [Google Scholar] [CrossRef]
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Bruno, G.; Cimino, D.; Taverna, D.; Deregibus, M.C.; et al. Urinary Exosomal MicroRNAs in Incipient Diabetic Nephropathy. PLoS ONE 2013, 8, e73798. [Google Scholar] [CrossRef] [PubMed]
- Al-Kafaji, G.; Al-Mahroos, G.; Al-Muhtaresh, H.A.; Skrypnyk, C.; Sabry, M.A.; Ramadan, A.R. Decreased expression of circulating microRNA-126 in patients with type 2 diabetic nephropathy: A potential blood-based biomarker. Exp. Ther. Med. 2016, 12, 815–822. [Google Scholar] [CrossRef]
- Barutta, F.; Bruno, G.; Matullo, G.; Chaturvedi, N.; Grimaldi, S.; Schalkwijk, C.; Stehouwer, C.D.; Fuller, J.H.; Gruden, G. MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta Diabetol. 2017, 54, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Gonzalez, M.; Srivastava, A.; Pavkovic, M.; Bijol, V.; Rennke, H.G.; Stillman, I.E.; Zhang, X.; Parikh, S.; Rovin, B.H.; Afkarian, M.; et al. Identification, Confirmation, and Replication of Novel Urinary MicroRNA Biomarkers in Lupus Nephritis and Diabetic Nephropathy. Clin. Chem. 2017, 63, 1515–1526. [Google Scholar] [CrossRef]
- Jones, P.A.; Issa, J.-P.; Baylin, S. Targeting the cancer epigenome for therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Q.; Liu, S.; Chen, Y.; Li, R.; Lin, T.; Yu, C.; Zhang, H.; Huang, Z.; Zhao, X.; et al. DNA methyltransferase 1 may be a therapy target for attenuating diabetic nephropathy and podocyte injury. Kidney Int. 2017, 92, 140–153. [Google Scholar] [CrossRef]
- Liu, R.; Zhong, Y.; Li, X.; Chen, H.; Jim, B.; Zhou, M.-M.; Chuang, P.Y.; He, J.C. Role of Transcription Factor Acetylation in Diabetic Kidney Disease. Diabetes 2014, 63, 2440–2453. [Google Scholar] [CrossRef]
- Nunez, J.K.; Chen, J.; Pommier, G.C.; Cogan, J.Z.; Replogle, J.M.; Adriaens, C.; Ramadoss, G.N.; Shi, Q.; Hung, K.L.; Samelson, A.J.; et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 2021, 184, 2503–2519.e17. [Google Scholar] [CrossRef]
- Pulecio, J.; Verma, N.; Mejia-Ramirez, E.; Huangfu, D.; Raya, A. CRISPR/Cas9-Based Engineering of the Epigenome. Cell Stem Cell 2017, 21, 431–447. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; de Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
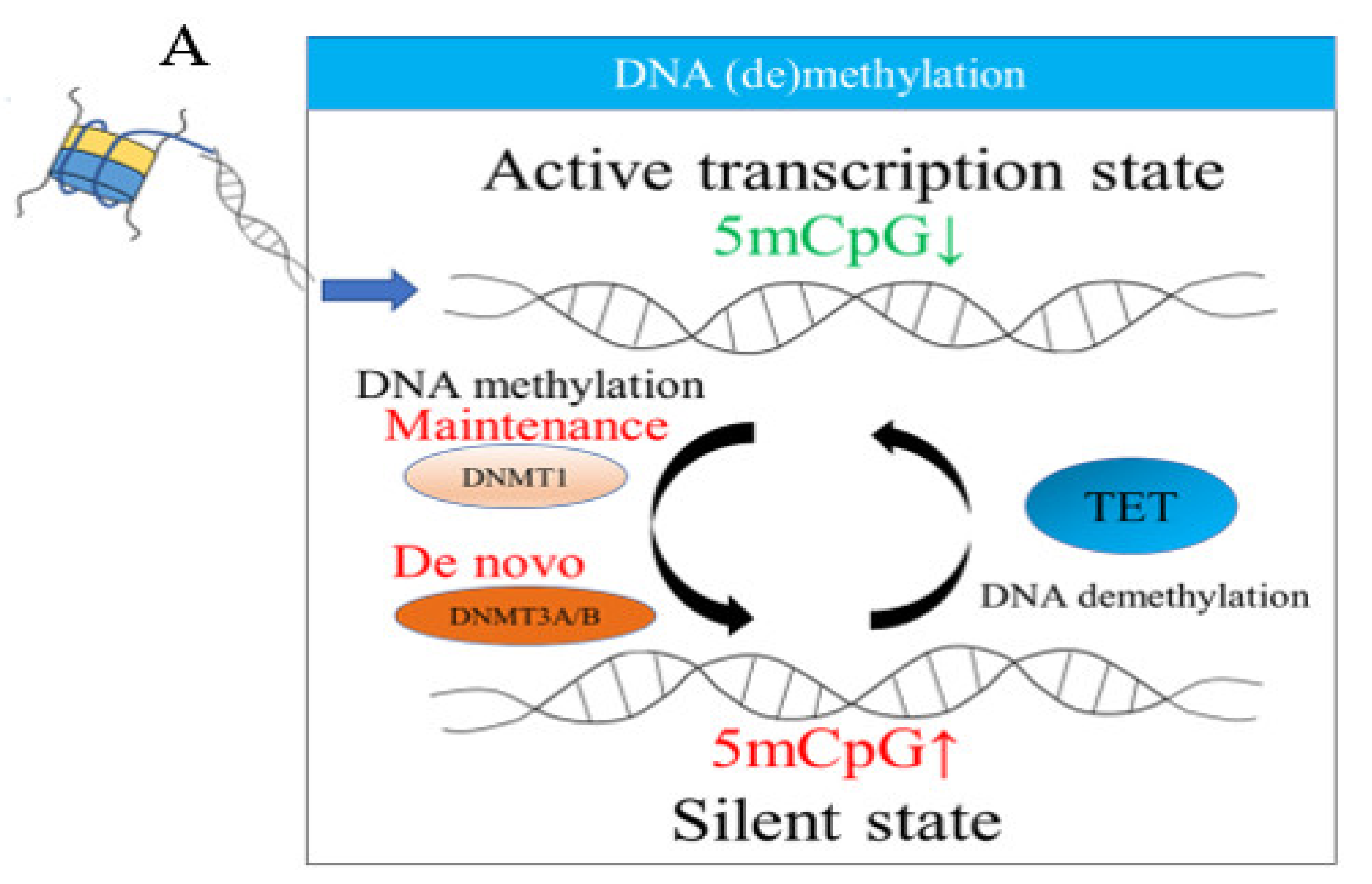
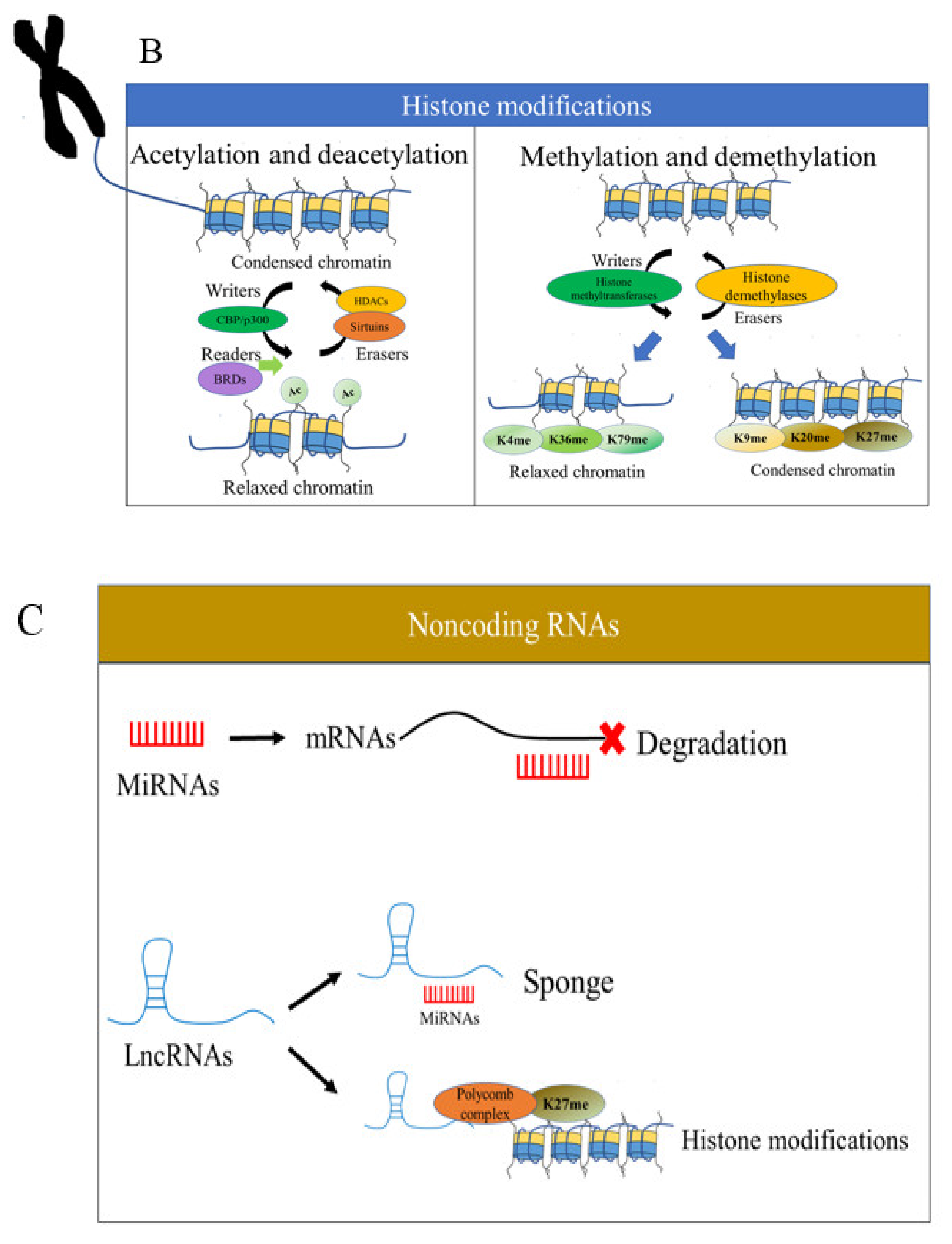
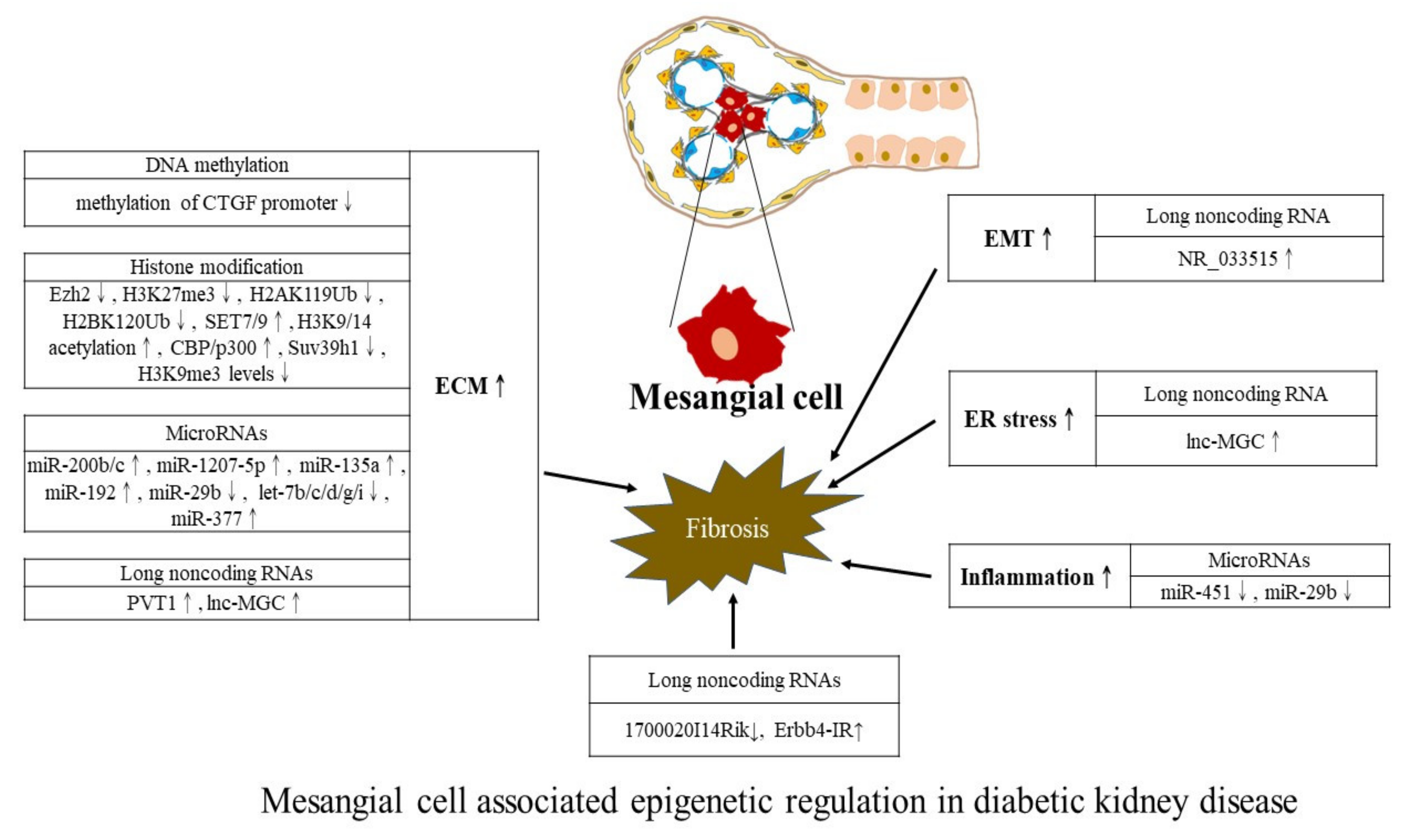
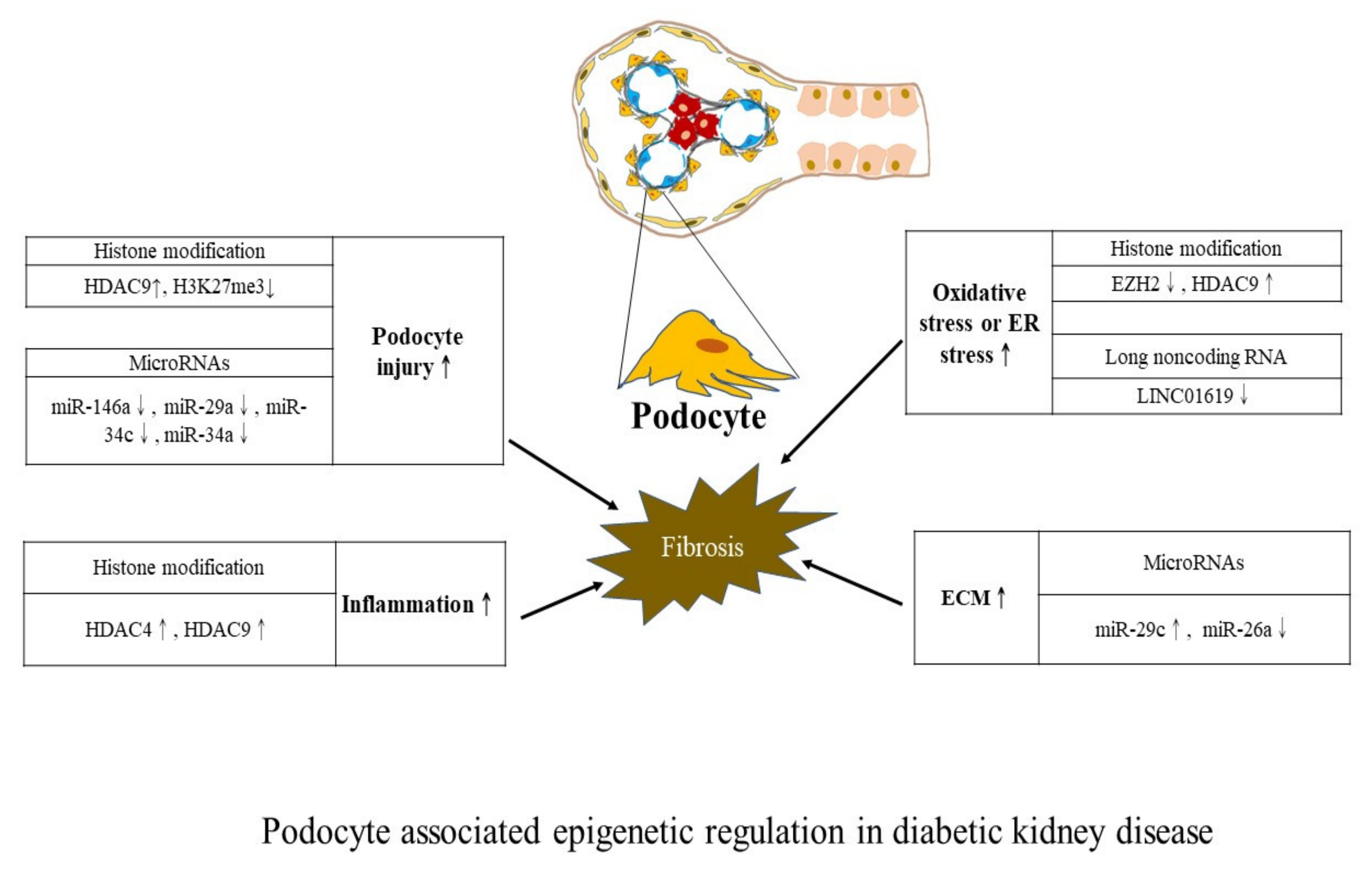

| Study Design (Reference) | Main Cells or Tissue Samples | Epigenetic Changes (Mechanisms Involved) |
|---|---|---|
| A case-control study of 192 Irish T1D patients. Cases had T1D and nephropathy whereas controls had T1D without renal disease [46] | Whole blood | Methylation state of 19 CpG sites associated with risk of diabetic kidney disease (EWAS, time to diabetic kidney disease) |
| A case-control association study (n = 196 T1DM and diabetic kidney disease vs. n = 246 without renal disease) [47] | Whole blood | PMPCB, TSFM, and AUH with differential methylation at multiple CpG sites (EWAS, mitochondria dysfunction) |
| DNA from Pre-DM (n = 11) at baseline and at their transition to T2DM [50] | Whole blood | 694 CpG sites hypomethylated and 174 CpG sites hypermethylated (EWAS, glucose/lipid metabolism, and inflammation) |
| Genome-wide methylome in 500 subjects with DKD from the Chronic Renal Insufficiency Cohort [51] | Whole blood | Prioritized 40 loci, methylation and gene-expression changes likely mediate the genotype effect on kidney disease development (EWAS, inflammation↑) |
| 60 individuals, with 20 cases in the control, DM and DKD groups respectively [55] | Whole blood | Higher methylation ratio of the let-7a-3 promoter (UHRF1↑and DNMT1↑) |
| Two groups of patients based on albumin excretion as patients with (n = 69) and without DKD (n = 27) [60] | Whole blood | Hypomethylation of TIMP-2 and AKR1B1 genes (albuminuria↑) |
| 24 cases of simple diabetes group; 34 cases of early DKD group; 27 cases of clinical DKD group; and 30 healthy controls [61] | Whole blood | Higher MTHFR promoter methylation in clinical diabetic kidney disease group (homocysteine↑) |
| 778 Swedish individuals, including T1D patients with or without DKD and subjects with normal glucose tolerance [62] | Whole blood | DNA methylation levels in the IGFBP1 gene↓ (circulating IGFBP-1↑) |
| Non-diabetes control (n = 29), diabetes without nephropathy (n = 37), and diabetes with nephropathy (n = 38) [64] | Whole blood | Lower CTGF methylation levels (ECM↑, albuminuria↑) |
| 32 cases (conventional therapy with retinopathy or albuminuria) vs. 31 subjects (intensive therapy without complication), human monocytes [48] | Whole blood isolated at EDIC Study year 10 and monocytes during year 16–17. | 12 differentially methylated loci were common in both whole blood and monocytes, including hypomethylation of TXNIP (EWAS, oxidative stress↑) |
| Mononuclear cells in DKD patients, diabetic mice, and cultured diabetic mononuclear cells [54] | Immune (mononuclear) cells | DNMT1↑ (inflammation↑) |
| Whole-blood DNA methylation of 2264 (586 DM) Atherosclerosis Risk in Communities and 2595 (394 DM) Framingham Heart Study participants [52] | Whole blood and renal biopsy | Lead CpGs at PTPN6/PHB2, ANKRD11, and TNRC18 map to active enhancers in kidney cortex (EWAS, fibrosis↑) |
| UUO and DKD kidney mice model, primary renal fibroblast [67] | Kidney of mice model, primary renal fibroblast | Hypermethylation of the Rasal1 promoter (fibrosis↑) |
| High glucose treated human glomerular mesangial cells [65] | Mesangial cell | Reduced methylation of CTGF promoter (ECM↑) |
| Proximal tubules of db/db mice [53] | Tubular epithelia | Aberrant hypomethylation of Agt, Abcc4, Cyp4a10, Glut5, and Met and hypermethylation of Kif20b, Cldn18, and Slco1a1 (mitochondria dysfunction) |
| Study Design (Reference) | Main Cells or Tissue Samples | Epigenetic Changes (Mechanisms Involved) |
|---|---|---|
| 30 DCCT conventional treatment subjects (cases: mean HbA1c level >9.1% with retinopathy or nephropathy by EDIC year 10 of follow-up) versus 30 intensive treatment subjects (controls: mean HbA1c level <7.3% without complications) [68] | Blood monocytes and lymphocytes | Promoter regions with enrichment H3K9Ac↑ (inflammation↑) |
| db/db mice [80] | Kidneys of db/db mice | SET7/9 and the recruitment to promoters↑, H3K4me1 recruitment at MCP-1 promoters↑ (ER stress↑) |
| Glomeruli from diabetic mice, TGF-β1 or high glucose treated rat mesangial cell [69] | Mesangial cell | H3K9/14 acetylation↑ and CBP/p300 occupancies↑ at the PAI-1 and P21 promoters (ECM↑) |
| Diabetic db/db mice, TGF-β treated mouse mesangial cell [70] | Mesangial cell | Akt and p300↑, acetylation of Ets-1 and histone H3↑ (ECM↑) |
| Type 1 diabetic model, high glucose- or sodium butyrate-treated mesangial cells in the presence or absence of apelin-13 [78] | Mesangial cell | Apelin-13 treatment inhibited histone hyperacetylation by upregulation of histone deacetylase (inflammation) |
| TGF-β1 treated rat mesangial cell under high or normal glucose [79] | Mesangial cell | SET7/9↑ at promoters of the ECM-associated genes (ECM↑) |
| High glucose treated mouse mesangial [81] | Mesangial cell | Suv39h1↓, H3K9me3 levels↓ at the promoters of fibronectin and p21(WAF1) genes (ECM↑) |
| Type 1 diabetic rat kidney [83] | Mesangial cell | H2AK119Ub↓ and H2BK120Ub↓ (ECM↑) |
| STZ-induced diabetic rats, TGF-β treated rat, mouse, and human mesangial cells [86] | Mesangial cell | miR-101b↑/Ezh2↓, Jmjd3 and Utx↑/H3K27me3↓ (mesangial dysfunction, ECM↑) |
| Kidney tissues from diabetic db/db mice and patients with DKD, high glucose-treated mouse podocytes [75] | Podocyte | HDAC9↑ (oxidative stress, apoptosis, and inflammation↑) |
| Human DKD renal biopsy, STZ-induced diabetic rats, diabetic db/db mice, glucose, or AGEs or TGF-β treated podocyte [76] | Podocyte | HDAC4↑ (inflammation↑) |
| Kidneys of diabetic rats, high glucose treated podocytes [84] | Podocyte | EZH2 expression↓ (oxidative stress↑) |
| Human FSGS or DKD renal biopsy, animal studies of adriamycin nephrotoxicity, subtotal nephrectomy and diabetic db/db mice, mouse and human podocytes [85] | Podocyte | H3K27me3↓ (podocyte dedifferentiation↑) |
| STZ-induced or obese-type (db/db) diabetic mice, high glucose treated human-derived renal epithelial cells [77] | Tubular epithelia | Sirt1↓ (podocyte foot process effacement↑) |
| Human DKD renal biopsy compared to non-DKD minimal change diseases, high glucose treated human proximal tubular epithelial cells [82] | Tubular epithelia | SUV39H1↑ (DM renal tubules), SUV39H1↓ (greater glucose and prolonged stimulation in cells) (inflammation↑) |
| Study Design (Reference) | Main Cells or Tissue Samples | Epigenetic Changes (Mechanisms Involved) |
|---|---|---|
| MicroRNAs | ||
| STZ-induced diabetes [88] | Kidney and macrophages of T1D mice | miR-146a↓ (inflammation↑) |
| Glomeruli of early DKD patients, renal cortex of diabetic (STZ-injected) mice [90] | Kidney of T1D mice | miR-192↑ (ECM, fibrosis↑) |
| PBMCs of DKD patients, kidneys of db/db mice, glucose treated mesangial cells [87] | Mesangial cell | miR-451↓ (inflammation↑) |
| STZ-injected diabetic mice, diabetic db/db mice, TGF-β treated mesangial cells [72] | Mesangial cell | miR-192↑ (ECM↑) |
| Glomeruli from diabetic (STZ-injected) mice, TGF-β treated glomerular mesangial cells [91] | Mesangial cell | miR-192↑ (ECM↑) |
| Type 2 diabetes in db/db mice, cultured mesangial cell [94] | Mesangial cell | miR-29b↓ (ECM, inflammation↑) |
| Glomeruli of diabetic mice, TGF-β treated mouse mesangial cells [102] | Mesangial cell | miR-200b/c↑ (mesangial hypertrophy, ECM↑) |
| Serum and kidney tissues of patients with DKD, db/db mice, cultured mesangial cells [103] | Mesangial cell | miR-135a↑ (ECM↑) |
| Mouse diabetic kidney disease models, high glucose or TGF-β treated human and mouse mesangial cells [104] | Mesangial cell | miR-377↑ (ECM↑) |
| Glomeruli of diabetic mice, TGF-β-treated mouse mesangial cells [107] | Mesangial cell | let-7 family members (let-7b/c/d/g/i) ↓ (ECM↑) |
| Glomeruli of diabetic patients, glomeruli of albuminuric BTBR ob/ob mice [89] | Podocyte | miR-146a↓ (podocyte injury↑) |
| Primary renal glomeruli from STZ-induced diabetic mice [93] | Podocyte | miR-29a↓ (podocyte injury↑) |
| Glomeruli of db/db mice, kidney microvascular endothelial cells and podocytes treated with high glucose [96] | Podocyte | miR-29c↑ (ECM↑) |
| High glucose treated mouse podocyte [97] | Podocyte | miR-34a↓ (podocyte apoptosis↑) |
| High glucose treated podocytes [98] | Podocyte | miR-34c↓ (podocyte apoptosis↑) |
| Humans and mouse (STZ-injected) models of DKD, cultured podocytes [105] | Podocyte | miR-26a↓ (ECM↑) |
| Renal biopsy from patients with diabetic kidney disease, high glucose treated proximal tubular cells [92] | Tubular epithelia | miR-192↓ (EMT↑) |
| Diabetic kidney disease animal models, cultured human tubular epithelial cells [100] | Tubular epithelia | miR-21↑ (ECM↑) |
| Human DKD, models of fibrotic renal disease and experimental DKD [101] | Tubular epithelia | miR-21↑ (ECM↑) |
| Mouse models of early and advanced DKD, TGF-β1 treated rat tubular cells [106] | Tubular epithelia | let-7b↓ (ECM↑) |
| Renal biopsies and plasma of DKD patients, STZ-induced diabetic rats, high glucose cultured rat proximal tubular cells [108] | Tubular epithelia | miR-130b↓ (EMT↑) |
| Early and advanced DKD mice models, TGF-β treated rat proximal-tubular cells [109] | Tubular epithelia | miR-141 and miR-200a ↓ (EMT↑) |
| Long noncoding RNAs | ||
| Renal tissues of db/db DKD mice, mouse mesangial cell [99] | Mesangial cell | 1700020I14Rik↓, miR-34a-5p↑(fibrosis↑) |
| Serum of DKD patients, mouse mesangial cells [110] | Mesangial cell | NR_033515↑, miR-743b-5p↓ (EMT↑) |
| Glomeruli of DKD mouse models, TGF-beta or high glucose treated mesangial cells [113] | Mesangial cell | lnc-MGC↑, a megacluster of microRNAs↑ (ER stress↑) |
| db/db mice, mouse mesangial cells and tubular epithelial cells [95] | Mesangial cell and tubular epithelia | Erbb4-IR↑, miR-29b↓(fibrosis↑) |
| Mesangial cells and human proximal tubular cells [114] | Mesangial cell and tubular epithelia | PVT1↑miR-1207-5p↑ (ECM↑) |
| Human DKD renal biopsy, diabetic rat, high glucose cultured podocyte [112] | Podocyte | LINC01619↓, miR-27a↑ (ER stress↑) |
| STZ-induced diabetic rats, high glucose treated renal tubular epithelial cell [111] | Tubular epithelia | MALAT1↑, miR-23c↓ (inflammation↑) |
| High glucose treated human proximal tubular cells [115] | Tubular epithelia | lncRNA MIR503HG↑, miR-503↑ (apoptosis↑) |
| Epigenetic Biomarkers | ||
|---|---|---|
| Status of DKD (Reference) | Samples | Epigenetic Biomarkers |
| DKD [118] | Whole blood | miR-126↓ |
| DM with micro-/macrovascular complication [119] | Serum | miR-126↓ |
| DM with microalbuminuria [116] | Urinary exosome | miR-192↑, miR-194↑, and miR-215↑ |
| Type 1 DM with incipient diabetic kidney disease [117] | Urinary exosome | miR-130a↑, miR-145↑, miR-155↓, miR-424↓ |
| DKD [120] | Urine | miR-2861↓, miR-1915-3p↓, miR-4532↓ |
| Agents to Modulate Epigenetics | ||
| Types of Nephropathy (Reference) | Agents to Modulate Epigenetics | Epigenetic Effects |
| DKD [122] | 5-azacytidine or 5-aza-2’-deoxycytidine | DNA demethylation |
| DKD or CKD [67] | BMP7 | Tet3↑ and normalization of Rasal1 promoter hypermethylation |
| DKD [78] | Apelin-13 | Histone deacetylation |
| DKD [123] | MS417 | Bromodomain inhibitor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, F.-C.; Chao, C.-T.; Lin, S.-H. The Dynamics and Plasticity of Epigenetics in Diabetic Kidney Disease: Therapeutic Applications Vis-à-Vis. Int. J. Mol. Sci. 2022, 23, 843. https://doi.org/10.3390/ijms23020843
Kuo F-C, Chao C-T, Lin S-H. The Dynamics and Plasticity of Epigenetics in Diabetic Kidney Disease: Therapeutic Applications Vis-à-Vis. International Journal of Molecular Sciences. 2022; 23(2):843. https://doi.org/10.3390/ijms23020843
Chicago/Turabian StyleKuo, Feng-Chih, Chia-Ter Chao, and Shih-Hua Lin. 2022. "The Dynamics and Plasticity of Epigenetics in Diabetic Kidney Disease: Therapeutic Applications Vis-à-Vis" International Journal of Molecular Sciences 23, no. 2: 843. https://doi.org/10.3390/ijms23020843
APA StyleKuo, F.-C., Chao, C.-T., & Lin, S.-H. (2022). The Dynamics and Plasticity of Epigenetics in Diabetic Kidney Disease: Therapeutic Applications Vis-à-Vis. International Journal of Molecular Sciences, 23(2), 843. https://doi.org/10.3390/ijms23020843






