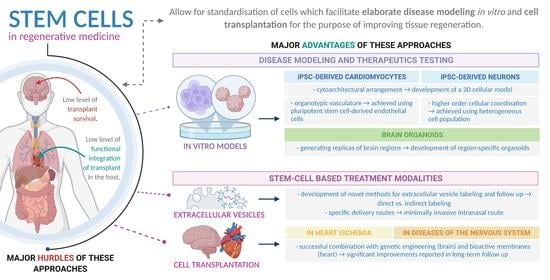
Regenerative Neurology and Regenerative Cardiology: Shared Hurdles and Achievements
Abstract
:1. Introduction
2. Induced Pluripotent Stem Cells–Flying Start to Boosting In Vitro Models of the Nervous System and the Heart
3. iPSC-Derived Cardiomyocytes
4. Specific Requirements for In Vitro Heart Muscle Model
5. iPSC-Derived Neurons
6. Specific Requirements for the In Vitro Nervous Tissue Model
7. Brain Organoids
8. Sources of Cells for Transplantation into Nervous and Heart Tissue
9. Extracellular Vesicles–Desired Cellular Product on a Way towards Clinical Application
10. Cell Transplantation for Heart Ischemia
11. Specific Requirements for Further Improvement of Cell-Based Therapy of Heart Diseases
12. Cell Transplantation for Diseases of the Nervous System
13. Specific Requirements for Further Improvement of Cell-Based Therapy of Brain Diseases
14. Conclusions Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Luo, L.; Tian, R.; Yu, C. A Review and Update for Registered Clinical Studies of Stem Cells for Non-Tumorous and Non-Hematological Diseases. Regen. Ther. 2021, 18, 355–362. [Google Scholar] [CrossRef]
- Borlongan, M.C.; Farooq, J.; Sadanandan, N.; Wang, Z.J.; Cozene, B.; Lee, J.Y.; Steinberg, G.K. Stem Cells for Aging-Related Disorders. Stem Cell Rev. Rep. 2021, 17, 2054–2058. [Google Scholar] [CrossRef]
- Ilic, D.; Ogilvie, C. Concise Review: Human Embryonic Stem Cells—What Have We Done? What Are We Doing? Where Are We Going? Stem Cells 2017, 35, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. 2021, 8, 2002944. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.; Archana, S.; Verma, R.S. Mesenchymal Stem Cells for Cardiac Regeneration: From Differentiation to Cell Delivery. Stem Cell Rev. Rep. 2021, 17, 1666–1694. [Google Scholar] [CrossRef]
- Monti, M.; Perotti, C.; del Fante, C.; Cervio, M.; Redi, C.A. Stem Cells: Sources and Therapies. Biol. Res. 2012, 45, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced Pluripotent Stem Cell Technology: A Decade of Progress. Nat. Rev. Drug Discov. 2016, 16, 115–130. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of IPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.G.; Daley, G.Q. Induced Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced Pluripotent Stem Cells: Applications in Regenerative Medicine, Disease Modeling, and Drug Discovery. Front. Cell Dev. Biol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Bekhite, M.M.; González Delgado, A.; Menz, F.; Kretzschmar, T.; Wu, J.M.F.; Bekfani, T.; Nietzsche, S.; Wartenberg, M.; Westermann, M.; Greber, B.; et al. Longitudinal Metabolic Profiling of Cardiomyocytes Derived from Human-Induced Pluripotent Stem Cells. Basic Res. Cardiol. 2020, 115, 1–15. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically Defined Generation of Human Cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Cyganek, L.; Tiburcy, M.; Sekeres, K.; Gerstenberg, K.; Bohnenberger, H.; Lenz, C.; Henze, S.; Stauske, M.; Salinas, G.; Zimmermann, W.-H.; et al. Deep Phenotyping of Human Induced Pluripotent Stem Cell–Derived Atrial and Ventricular Cardiomyocytes. JCI Insight 2018, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolanowski, T.J.; Antos, C.L.; Guan, K. Making Human Cardiomyocytes up to Date: Derivation, Maturation State and Perspectives. Int. J. Cardiol. 2017, 241, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of De Novo Cardiomyocytes: Human Pluripotent Stem Cell Differentiation and Direct Reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, R.; Xu, R.-H.; Xu, C. Efficient Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells with Growth Factors. Cardiomyocytes Methods Protoc. 2015, 115–131. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed Cardiomyocyte Differentiation from Human Pluripotent Stem Cells by Modulating Wnt/β-Catenin Signaling under Fully Defined Conditions. Nat. Protoc. 2012, 8, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-Specific Optimization of Activin/Nodal and BMP Signaling Promotes Cardiac Differentiation of Mouse and Human Pluripotent Stem Cell Lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Hamad, S.; Derichsweiler, D.; Papadopoulos, S.; Nguemo, F.; Šarić, T.; Sachinidis, A.; Brockmeier, K.; Hescheler, J.; Boukens, B.J.; Pfannkuche, K. Generation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in 2D Monolayer and Scalable 3D Suspension Bioreactor Cultures with Reduced Batch-to-Batch Variations. Theranostics 2019, 9, 7222–7238. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust Cardiomyocyte Differentiation from Human Pluripotent Stem Cells via Temporal Modulation of Canonical Wnt Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [Green Version]
- Weng, Z.; Kong, C.-W.; Ren, L.; Karakikes, I.; Geng, L.; Jiaozi, H.; Ying, C.Z.; Fai, M.; Keung, W.; Chow, H.; et al. A Simple, Cost-Effective but Highly Efficient System for Deriving Ventricular Cardiomyocytes from Human Pluripotent Stem Cells. Stem Cells Dev. 2014, 23, 1704–1716. [Google Scholar] [CrossRef] [Green Version]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Lopes, S.M.C.d.S.; Mummery, C.L.; et al. Atrial-like Cardiomyocytes from Human Pluripotent Stem Cells Are a Robust Preclinical Model for Assessing Atrial-Selective Pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating Ring-Shaped Engineered Heart Tissues from Ventricular and Atrial Human Pluripotent Stem Cell-Derived Cardiomyocytes. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tanwar, V.; Bylund, J.B.; Hu, J.; Yan, J.; Walthall, J.M.; Mukherjee, A.; Heaton, W.H.; Wang, W.-D.; Potet, F.; Rai, M.; et al. Gremlin 2 Promotes Differentiation of Embryonic Stem Cells to Atrial Fate by Activation of the JNK Signaling Pathway. Stem Cells 2014, 32, 1774–1788. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Jiang, J.; Han, P.; Yuan, Q.; Zhang, J.; Zhang, X.; Xu, Y.; Cao, H.; Meng, Q.; Chen, L.; et al. Direct Differentiation of Atrial and Ventricular Myocytes from Human Embryonic Stem Cells by Alternating Retinoid Signals. Cell Res. 2010, 21, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.-Z.; Xie, Y.; Moyes, K.W.; Gold, J.D.; Askari, B.; Laflamme, M.A. Neuregulin/ErbB Signaling Regulates Cardiac Subtype Specification in Differentiating Human Embryonic Stem Cells. Circ. Res. 2010, 107, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Han, P.; Kim, E.H.; Mak, J.; Zhang, R.; Torrente, A.G.; Goldhaber, J.I.; Marbán, E.; Cho, H.C. Canonical Wnt Signaling Promotes Pacemaker Cell Specification of Cardiac Mesodermal Cells Derived from Mouse and Human Embryonic Stem Cells. Stem Cells 2020, 38, 352–368. [Google Scholar] [CrossRef]
- Yechikov, S.; Kao, H.K.J.; Chang, C.W.; Pretto, D.; Zhang, X.D.; Sun, Y.H.; Smithers, R.; Sirish, P.; Nolta, J.A.; Chan, J.W.; et al. NODAL Inhibition Promotes Differentiation of Pacemaker-like Cardiomyocytes from Human Induced Pluripotent Stem Cells. Stem Cell Res. 2020, 49, 102043. [Google Scholar] [CrossRef]
- Scuderi, G.J.; Butcher, J. Naturally Engineered Maturation of Cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Rodriguez, M.L.; Leonard, A.; Sun, L.; Fischer, K.A.; Wang, Y.; Ritterhoff, J.; Zhao, L.; Kolwicz, S.C.; Pabon, L.; et al. Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 13, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Pianezzi, E.; Altomare, C.; Bolis, S.; Balbi, C.; Torre, T.; Rinaldi, A.; Camici, G.G.; Barile, L.; Vassalli, G. Role of Somatic Cell Sources in the Maturation Degree of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118538. [Google Scholar] [CrossRef]
- Sanchez-Freire, V.; Lee, A.S.; Hu, S.; Abilez, O.J.; Liang, P.; Lan, F.; Huber, B.C.; Ong, S.G.; Hong, W.X.; Huang, M.; et al. Effect of Human Donor Cell Source on Differentiation and Function of Cardiac Induced Pluripotent Stem Cells. J. Am. Coll. Cardiol. 2014, 64, 436–448. [Google Scholar] [CrossRef] [Green Version]
- Abulaiti, M.; Yalikun, Y.; Murata, K.; Sato, A.; Sami, M.M.; Sasaki, Y.; Fujiwara, Y.; Minatoya, K.; Shiba, Y.; Tanaka, Y.; et al. Establishment of a Heart-on-a-Chip Microdevice Based on Human IPS Cells for the Evaluation of Human Heart Tissue Function. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human Cardiac Organoids for the Modelling of Myocardial Infarction and Drug Cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. Organotypic Vasculature: From Descriptive Heterogeneity to Functional Pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [Green Version]
- Rafii, S.; Butler, J.M.; Ding, B.-S. Angiocrine Functions of Organ-Specific Endothelial Cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of Blood Vessel Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef]
- Tremblay, M.È. A Diversity of Cell Types, Subtypes and Phenotypes in the Central Nervous System: The Importance of Studying Their Complex Relationships. Front. Cell. Neurosci. 2020, 14, 424. [Google Scholar] [CrossRef]
- McKenzie, A.T.; Wang, M.; Hauberg, M.E.; Fullard, J.F.; Kozlenkov, A.; Keenan, A.; Hurd, Y.L.; Dracheva, S.; Casaccia, P.; Roussos, P.; et al. Brain Cell Type Specific Gene Expression and Co-Expression Network Architectures. Sci. Rep. 2018, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Zhang, S.C. Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell 2016, 19, 573–586. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Kirwan, P.; Livesey, F.J. Directed Differentiation of Human Pluripotent Stem Cells to Cerebral Cortex Neurons and Neural Networks. Nat. Protoc. 2012, 7, 1836–1846. [Google Scholar] [CrossRef]
- Bell, S.; Hettige, N.; Silveira, H.; Peng, H.; Wu, H.; Jefri, M.; Antonyan, L.; Zhang, Y.; Zhang, X.; Ernst, C. Differentiation of Human Induced Pluripotent Stem Cells (IPSCs) into an Effective Model of Forebrain Neural Progenitor Cells and Mature Neurons. Bio-Protocol 2019, 9, e3188. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Q.; Zhao, C.; Da, Y.; Zhang, H.-L.; Chen, Z. Differentiation of Glial Cells from HiPSCs: Potential Applications in Neurological Diseases and Cell Replacement Therapy. Front. Cell. Neurosci. 2018, 12, 239. [Google Scholar] [CrossRef]
- Yan, Y.; Shin, S.; Jha, B.S.; Liu, Q.; Sheng, J.; Li, F.; Zhan, M.; Davis, J.; Bharti, K.; Zeng, X.; et al. Efficient and Rapid Derivation of Primitive Neural Stem Cells and Generation of Brain Subtype Neurons from Human Pluripotent Stem Cells. Stem Cells Transl. Med. 2013, 2, 862–870. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Avci, H.X.; Ochalek, A.; Rösingh, L.N.; Molnár, K.; László, L.; Bellák, T.; Téglási, A.; Pesti, K.; Mike, A.; et al. Comparison of 2D and 3D Neural Induction Methods for the Generation of Neural Progenitor Cells from Human Induced Pluripotent Stem Cells. Stem Cell Res. 2017, 25, 139–151. [Google Scholar] [CrossRef]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in Vitro Modeling of the Central Nervous System. Prog. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Hofrichter, M.; Nimtz, L.; Tigges, J.; Kabiri, Y.; Schröter, F.; Royer-Pokora, B.; Hildebrandt, B.; Schmuck, M.; Epanchintsev, A.; Theiss, S.; et al. Comparative Performance Analysis of Human IPSC-Derived and Primary Neural Progenitor Cells (NPC) Grown as Neurospheres in Vitro. Stem Cell Res. 2017, 25, 72–82. [Google Scholar] [CrossRef]
- Gregory, P.M.; Brent, A.R.; Eric, D. Laywell. Using the Neurosphere Assay to Quantify Neural Stem Cells in Vivo. Curr. Pharm. Biotechnol. 2007, 8, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Brännvall, K.; Bergman, K.; Wallenquist, U.; Svahn, S.; Bowden, T.; Hilborn, J.; Forsberg-Nilsson, K. Enhanced Neuronal Differentiation in a Three-Dimensional Collagen-Hyaluronan Matrix. J. Neurosci. Res. 2007, 85, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Haynes, J.M.; Laslett, A.L.; Cameron, N.R.; O’Brien, C.M. Three-Dimensional Differentiation of Human Pluripotent Stem Cell-Derived Neural Precursor Cells Using Tailored Porous Polymer Scaffolds. Acta Biomater. 2020, 101, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, A.; Deiwick, A.; El-Tamer, A.; Koch, L.; Shi, Y.; Estévez-Priego, E.; Ludl, A.A.; Soriano, J.; Guseva, D.; Ponimaskin, E.; et al. In Vitro Development of Human IPSC-Derived Functional Neuronal Networks on Laser-Fabricated 3D Scaffolds. ACS Appl. Mater. Interfaces 2021, 13, 7839–7853. [Google Scholar] [CrossRef] [PubMed]
- Bolognin, S.; Fossépré, M.; Qing, X.; Jarazo, J.; Ščančar, J.; Moreno, E.L.; Nickels, S.L.; Wasner, K.; Ouzren, N.; Walter, J.; et al. 3D Cultures of Parkinson’s Disease-Specific Dopaminergic Neurons for High Content Phenotyping and Drug Testing. Adv. Sci. 2019, 6, 1800927. [Google Scholar] [CrossRef]
- Rouleau, N.; Cantley, W.L.; Liaudanskaya, V.; Berk, A.; Du, C.; Rusk, W.; Peirent, E.; Koester, C.; Nieland, T.J.F.; Kaplan, D.L. A Long-Living Bioengineered Neural Tissue Platform to Study Neurodegeneration. Macromol. Biosci. 2020, 20, e2000004. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Freitas, B.C.; Qian, H.; Lux, J.; Acab, A.; Trujillo, C.A.; Herai, R.H.; Huu, V.A.N.; Wen, J.H.; Joshi-Barr, S.; et al. Layered Hydrogels Accelerate IPSC-Derived Neuronal Maturation and Reveal Migration Defects Caused by MeCP2 Dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, 3185–3190. [Google Scholar] [CrossRef] [Green Version]
- Sutcliffe, M.; Lancaster, M.A. A Simple Method of Generating 3D Brain Organoids Using Standard Laboratory Equipment. Methods Mol. Biol. 2017, 1576, 1–12. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Velasco, S.; Paulsen, B.; Arlotta, P. 3D Brain Organoids: Studying Brain Development and Disease Outside the Embryo. Annu. Rev. Neurosci. 2020, 43, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Tomov, M.L.; O’Neil, A.; Abbasi, H.S.; Cimini, B.A.; Carpenter, A.E.; Rubin, L.L.; Bathe, M. Resolving Cell State in IPSC-Derived Human Neural Samples with Multiplexed Fluorescence Imaging. Commun. Biol. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Mohamed, A.; Chow, T.; Whiteley, J.; Fantin, A.; Sorra, K.; Hicks, R.; Rogers, I.M. Umbilical Cord Tissue as a Source of Young Cells for the Derivation of Induced Pluripotent Stem Cells Using Non-Integrating Episomal Vectors and Feeder-Free Conditions. Cells 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Joshi, J.M.; Sundaravadivelu, P.K.; Raina, K.; Lenka, N.; Kaveeshwar, V.; Thummer, R.P. An Overview on Promising Somatic Cell Sources Utilized for the Efficient Generation of Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2021, 17, 1954–1974. [Google Scholar] [CrossRef]
- Strässler, E.T.; Aalto-Setälä, K.; Kiamehr, M.; Landmesser, U.; Kränkel, N. Age Is Relative—Impact of Donor Age on Induced Pluripotent Stem Cell-Derived Cell Functionality. Front. Cardiovasc. Med. 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Rao, M.S. Can Cord Blood Banks Transform into Induced Pluripotent Stem Cell Banks? Cytotherapy 2015, 17, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Perrera, V.; Martello, G. How Does Reprogramming to Pluripotency Affect Genomic Imprinting? Front. Cell Dev. Biol. 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turinetto, V.; Orlando, L.; Giachino, C. Induced Pluripotent Stem Cells: Advances in the Quest for Genetic Stability during Reprogramming Process. Int. J. Mol. Sci. 2017, 18, 1952. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, M.; Hayashizaki, Y.; Murakawa, Y. Genomic Instability of IPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev. Rep. 2016, 13, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, O.; von Meyenn, F.; Hewitt, Z.; Alexander, J.; Wood, A.; Weightman, R.; Gregory, S.; Krueger, F.; Andrews, S.; Barbaric, I.; et al. Low Rates of Mutation in Clinical Grade Human Pluripotent Stem Cells under Different Culture Conditions. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Alajbeg, I.; Alić, I.; Andabak-Rogulj, A.; Brailo, V.; Mitrečić, D. Human- and Mouse-Derived Neurons Can Be Simultaneously Obtained by Co-Cultures of Human Oral Mucosal Stem Cells and Mouse Neural Stem Cells. Oral Dis. 2018, 24, 5–10. [Google Scholar] [CrossRef]
- Del Dosso, A.; Urenda, J.P.; Nguyen, T.; Quadrato, G. Upgrading the Physiological Relevance of Human Brain Organoids. Neuron 2020, 107, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Khakipoor, S.; Crouch, E.E.; Mayer, S. Human Organoids to Model the Developing Human Neocortex in Health and Disease. Brain Res. 2020, 1742, 146803. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Chen, J.; Velmeshev, D.; Mayer, A.; Eze, U.C.; Bhaduri, A.; Cunha, C.E.; Jung, D.; Arjun, A.; Li, E.; et al. Multimodal Single-Cell Analysis Reveals Physiological Maturation in the Developing Human Neocortex. Neuron 2019, 102, 143.e7–158.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, M.A.; Huch, M. Disease Modelling in Human Organoids. Dis. Model. Mech. 2019, 12, dmm039347. [Google Scholar] [CrossRef] [Green Version]
- Baldassari, S.; Musante, I.; Iacomino, M.; Zara, F.; Salpietro, V.; Scudieri, P. Brain Organoids as Model Systems for Genetic Neurodevelopmental Disorders. Front. Cell Dev. Biol. 2020, 8, 590119. [Google Scholar] [CrossRef]
- Stachowiak, E.K.; Benson, C.A.; Narla, S.T.; Dimitri, A.; Chuye, L.E.B.; Dhiman, S.; Harikrishnan, K.; Elahi, S.; Freedman, D.; Brennand, K.J.; et al. Cerebral Organoids Reveal Early Cortical Maldevelopment in Schizophrenia—Computational Anatomy and Genomics, Role of FGFR1. Transl. Psychiatry 2017, 7, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alić, I.; Goh, P.A.; Murray, A.; Portelius, E.; Gkanatsiou, E.; Gough, G.; Mok, K.Y.; Koschut, D.; Brunmeir, R.; Yeap, Y.J.; et al. Patient-Specific Alzheimer-like Pathology in Trisomy 21 Cerebral Organoids Reveals BACE2 as a Gene Dose-Sensitive AD Suppressor in Human Brain. Mol. Psychiatry 2020, 4, 1–23. [Google Scholar] [CrossRef]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s Disease in Midbrain-like Organoids. npj Park. Dis. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chlebanowska, P.; Tejchman, A.; Sułkowski, M.; Skrzypek, K.; Majka, M. Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allende, M.L.; Cook, E.K.; Larman, B.C.; Nugent, A.; Brady, J.M.; Golebiowski, D.; Sena-Esteves, M.; Tifft, C.J.; Proia, R.L. Cerebral Organoids Derived from Sandhoff Disease-Induced Pluripotent Stem Cells Exhibit Impaired Neurodifferentiation. J. Lipid Res. 2018, 59, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosi, N.; Alić, I.; Kolačević, M.; Vrsaljko, N.; Jovanov Milošević, N.; Sobol, M.; Philimonenko, A.; Hozák, P.; Gajović, S.; Pochet, R.; et al. Nop2 Is Expressed during Proliferation of Neural Stem Cells and in Adult Mouse and Human Brain. Brain Res. 2015, 1597, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Bueno, C.; Ramirez, C.; Rodríguez-Lozano, F.J.; Tabarés-Seisdedos, R.; Rodenas, M.; Moraleda, J.M.; Jones, J.R.; Martinez, S. Human Adult Periodontal Ligament-Derived Cells Integrate and Differentiate after Implantation into the Adult Mammalian Brain. Cell Transplant. 2013, 22, 2017–2028. [Google Scholar] [CrossRef] [Green Version]
- Bueno, C.; Martínez-Morga, M.; Martínez, S. Non-Proliferative Neurogenesis in Human Periodontal Ligament Stem Cells. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Bueno, C.; Martínez-Morga, M.; García-Bernal, D.; Moraleda, J.M.; Martínez, S. Differentiation of Human Adult-Derived Stem Cells towards a Neural Lineage Involves a Dedifferentiation Event Prior to Differentiation to Neural Phenotypes. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Simonović, J.; Toljić, B.; Rašković, B.; Jovanović, V.; Lazarević, M.; Milošević, M.; Nikolić, N.; Panajotović, R.; Milašin, J. Raman Microspectroscopy: Toward a Better Distinction and Profiling of Different Populations of Dental Stem Cells. Croat. Med. J. 2019, 60, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Simonovic, J.; Toljic, B.; Nikolic, N.; Peric, M.; Vujin, J.; Panajotovic, R.; Gajic, R.; Bekyarova, E.; Cataldi, A.; Parpura, V.; et al. Differentiation of Stem Cells from Apical Papilla into Neural Lineage Using Graphene Dispersion and Single Walled Carbon Nanotubes. J. Biomed. Mater. Res. A 2018, 106, 2653–2661. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, P.D.; Raposo, G. Exosomes and Extracellular Vesicles: The Path Forward. Essays Biochem. 2018, 62, 119–124. [Google Scholar] [CrossRef]
- Jarmalavičiūtė, A.; Pivoriūnas, A. Exosomes as a Potential Novel Therapeutic Tools against Neurodegenerative Diseases. Pharmacol. Res. 2016, 113 (Pt. B), 816–822. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular Vesicles as Modulators of Cell-to-Cell Communication in the Healthy and Diseased Brain. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stykel, M.G.; Humphries, K.M.; Kamski-Hennekam, E.; Buchner-Duby, B.; Porte-Trachsel, N.; Ryan, T.; Coackley, C.L.; Bamm, V.V.; Harauz, G.; Ryan, S.D. α-Synuclein Mutation Impairs Processing of Endomembrane Compartments and Promotes Exocytosis and Seeding of α-Synuclein Pathology. Cell Rep. 2021, 35, 109099. [Google Scholar] [CrossRef] [PubMed]
- Narbute, K.; Pilipenko, V.; Pupure, J.; Klinovičs, T.; Auders, J.; Jonavičė, U.; Kriaučiūnaitė, K.; Pivoriūnas, A.; Kluša, V. Time-Dependent Memory and Gait Improvement by Intranasally-Administered Extracellular Vesicles in Parkinson’s Disease Model Rats. Cell. Mol. Neurobiol. 2020, 41, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.L.; Escayg, A. Extracellular Vesicles in the Treatment of Neurological Disorders. Neurobiol. Dis. 2021, 157. [Google Scholar] [CrossRef] [PubMed]
- Andjus, P.; Kosanović, M.; Milićević, K.; Gautam, M.; Vainio, S.J.; Jagečić, D.; Kozlova, E.N.; Pivoriūnas, A.; Chachques, J.C.; Sakaj, M.; et al. Extracellular Vesicles as Innovative Tool for Diagnosis, Regeneration and Protection against Neurological Damage. Int. J. Mol. Sci. 2020, 21, 6859. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wu, J.Y.; Wang, J.M.; Hu, X.B.; Xiang, D.X. Emerging Strategies for Labeling and Tracking of Extracellular Vesicles. J. Control. Release 2020, 328, 141–159. [Google Scholar] [CrossRef]
- Gao, Y.; Chu, C.; Jablonska, A.; Bulte, J.W.M.; Walczak, P.; Janowski, M. Imaging as a Tool to Accelerate the Translation of Extracellular Vesicle-Based Therapies for Central Nervous System Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1688. [Google Scholar] [CrossRef]
- Choi, H.; Choi, Y.; Yim, H.Y.; Mirzaaghasi, A.; Yoo, J.-K.; Choi, C. Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Eng. Regen. Med. 2021, 18, 499–511. [Google Scholar] [CrossRef]
- Simonsen, J.B. Pitfalls Associated with Lipophilic Fluorophore Staining of Extracellular Vesicles for Uptake Studies. J. Extracell. Vesicles 2019, 8, 1582237. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, M.; Gulvin, S.M.; Flax, J.; Gaborski, T.R. Systematic Evaluation of PKH Labelling on Extracellular Vesicle Size by Nanoparticle Tracking Analysis. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Seino, R.; Umezaki, K.; Shimoda, A.; Ezoe, T.; Ishiyama, M.; Akiyoshi, K. New Lipophilic Fluorescent Dyes for Labeling Extracellular Vesicles: Characterization and Monitoring of Cellular Uptake. Bioconjugate Chem. 2021, 32, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Ender, F.; Zamzow, P.; von Bubnoff, N.; Gieseler, F. Detection and Quantification of Extracellular Vesicles via FACS: Membrane Labeling Matters! Int. J. Mol. Sci. 2020, 21, 291. [Google Scholar] [CrossRef] [Green Version]
- Van der Vlist, E.J.; Nolte-’t Hoen, E.N.M.; Stoorvogel, W.; Arkesteijn, G.J.A.; Wauben, M.H.M. Fluorescent Labeling of Nano-Sized Vesicles Released by Cells and Subsequent Quantitative and Qualitative Analysis by High-Resolution Flow Cytometry. Nat. Protoc. 2012, 7, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Zeng, E.; Zhang, P.; Liu, X.; Zhang, C.; Yang, J.; Liu, D. General Approach to Engineering Extracellular Vesicles for Biomedical Analysis. Anal. Chem. 2019, 91, 12752–12759. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Deng, Q.; Hu, D.-P.; Wang, Z.-Y.; Huang, B.-H.; Du, Z.-Y.; Fang, Y.-X.; Wong, W.-L.; Zhang, K.; Chow, C.-F. A Molecular Fluorescent Dye for Specific Staining and Imaging of RNA in Live Cells: A Novel Ligand Integration from Classical Thiazole Orange and Styryl Compounds. Chem. Commun. 2015, 51, 15241–15244. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in Vivo Tracking of the Exosomes of Murine Melanoma B16-BL6 Cells in Mice after Intravenous Injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Narbute, K.; Piļipenko, V.; Pupure, J.; Dzirkale, Z.; Jonavičė, U.; Tunaitis, V.; Kriaučiūnaitė, K.; Jarmalavičiūtė, A.; Jansone, B.; Kluša, V.; et al. Intranasal Administration of Extracellular Vesicles Derived from Human Teeth Stem Cells Improves Motor Symptoms and Normalizes Tyrosine Hydroxylase Expression in the Substantia Nigra and Striatum of the 6-Hydroxydopamine-Treated Rats. Stem Cells Transl. Med. 2019, 8, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, S.; Fishel, I.; Offen, D. Intranasal Delivery of Mesenchymal Stem Cells-Derived Extracellular Vesicles for the Treatment of Neurological Diseases. Stem Cells 2021, 39, 1589–1600. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perets, N.; Betzer, O.; Shapira, R.; Brenstein, S.; Angel, A.; Sadan, T.; Ashery, U.; Popovtzer, R.; Offen, D. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19, 3422–3431. [Google Scholar] [CrossRef]
- Chan, D.Z.L.; Kerr, A.J.; Doughty, R.N. Temporal Trends in the Burden of Heart Failure. Intern. Med. J. 2021, 51, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Kadota, S.; Tanaka, Y.; Shiba, Y. Heart Regeneration Using Pluripotent Stem Cells. J. Cardiol. 2020, 76, 459–463. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered from Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Fernandes, S.; Naumova, A.V.; Zhu, W.Z.; Laflamme, M.A.; Gold, J.; Murry, C.E. Human Embryonic Stem Cell-Derived Cardiomyocytes Engraft but Do Not Alter Cardiac Remodeling after Chronic Infarction in Rats. J. Mol. Cell. Cardiol. 2010, 49, 941–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima Correa, B.; El Harane, N.; Gomez, I.; Rachid Hocine, H.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M.; et al. Extracellular Vesicles from Human Cardiovascular Progenitors Trigger a Reparative Immune Response in Infarcted Hearts. Cardiovasc. Res. 2021, 117, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Hume, R.D.; Chong, J.J.H. The Cardiac Injury Immune Response as a Target for Regenerative and Cellular Therapies. Clin. Ther. 2020, 42, 1923–1943. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.N.S.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef]
- Cong, X.Q.; Li, Y.; Zhao, X.; Dai, Y.J.; Liu, Y. Short-Term Effect of Autologous Bone Marrow Stem Cells to Treat Acute Myocardial Infarction: A Meta-Analysis of Randomized Controlled Clinical Trials. J. Cardiovasc. Transl. Res. 2015, 8, 221–231. [Google Scholar] [CrossRef]
- Henry, T.D.; Pepine, C.J.; Lambert, C.R.; Traverse, J.H.; Schatz, R.; Costa, M.; Povsic, T.J.; David Anderson, R.; Willerson, J.T.; Kesten, S.; et al. The Athena Trials: Autologous Adipose-Derived Regenerative Cells for Refractory Chronic Myocardial Ischemia with Left Ventricular Dysfunction. Catheter. Cardiovasc. Interv. 2017, 89, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem Cell Paracrine Actions and Tissue Regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Zhang, J.; Deng, Z.; Liu, J.; Han, W.; Chen, G.; Si, Y.; Ye, P. Mesenchymal Stem Cells Ameliorate Myocardial Fibrosis in Diabetic Cardiomyopathy via the Secretion of Prostaglandin E2. Stem Cell Res. Ther. 2020, 11, 122. [Google Scholar] [CrossRef]
- Liu, M.; Chen, H.; Jiang, J.; Zhang, Z.; Wang, C.; Zhang, N.; Dong, L.; Hu, X.; Zhu, W.; Yu, H.; et al. Stem Cells and Diabetic Cardiomyopathy: From Pathology to Therapy. Heart Fail. Rev. 2016, 21, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.; Langford, S.; Giampietro, J.; Arancio, J.; Arabia, F. Total Artificial Heart Update. Surg. Technol. Int. 2021, 39, 545–557. [Google Scholar] [CrossRef]
- Baker, E.W.; Kinder, H.A.; West, F.D. Neural Stem Cell Therapy for Stroke: A Multimechanistic Approach to Restoring Neurological Function. Brain Behav. 2019, 9, e01214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, S.; Nito, C.; Yokobori, S.; Sakamoto, Y.; Nakajima, M.; Sowa, K.; Obinata, H.; Sasaki, K.; Savitz, S.I.; Kimura, K. Recent Advances in Cell-Based Therapies for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 6718. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Kawauchi, S.; Kin, K.; Morimoto, J.; Kameda, M.; Sasaki, T.; Bonsack, B.; Kingsbury, C.; Tajiri, N.; Borlongan, C.V.; et al. Cell Therapy for Central Nervous System Disorders: Current Obstacles to Progress. CNS Neurosci. Ther. 2020, 26, 595–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuazon, J.P.; Castelli, V.; Borlongan, C.V. Drug-like Delivery Methods of Stem Cells as Biologics for Stroke. Expert Opin. Drug Deliv. 2019, 16, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef] [PubMed]
- Mitrečić, D.; Nicaise, C.; Gajović, S.; Pochet, R. Distribution, Differentiation, and Survival of Intravenously Administered Neural Stem Cells in a Rat Model of Amyotrophic Lateral Sclerosis. Cell Transplant. 2010, 19, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boltze, J.; Jolkkonen, J. Safety Evaluation of Intra-Arterial Cell Delivery in Stroke Patients—a Framework for Future Trials. Ann. Transl. Med. 2019, 7, S271. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous Mesenchymal Stem Cell Transplantation in Stroke Patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and Efficacy of Multipotent Adult Progenitor Cells in Acute Ischaemic Stroke (MASTERS): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Dade Lunsford, L.; Kim, A.S.; Johnson, J.N.; Bates, D.; Poggio, G.; Case, C.; McGrogan, M.; et al. Two-Year Safety and Clinical Outcomes in Chronic Ischemic Stroke Patients after Implantation of Modified Bone Marrow-Derived Mesenchymal Stem Cells (SB623): A Phase 1/2a Study. J. Neurosurg. 2019, 131, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.W.; Noh, M.Y.; Kwon, M.S.; Kim, H.Y.; Oh, S.I.; Park, J.; Kim, H.J.; Ki, C.S.; Kim, S.H. Repeated Intrathecal Mesenchymal Stem Cells for Amyotrophic Lateral Sclerosis. Ann. Neurol. 2018, 84, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Blanquer, M.; Moraleda, J.M.; Iniesta, F.; Gómez-Espuch, J.; Meca-Lallana, J.; Villaverde, R.; Pérez-Espejo, M.Á.; Ruíz-López, F.J.; Santos, J.M.G.; Bleda, P.; et al. Neurotrophic Bone Marrow Cellular Nests Prevent Spinal Motoneuron Degeneration in Amyotrophic Lateral Sclerosis Patients: A Pilot Safety Study. Stem Cells 2012, 30, 1277–1285. [Google Scholar] [CrossRef]
- Shroff, G. A Review on Stem Cell Therapy for Multiple Sclerosis: Special Focus on Human Embryonic Stem Cells. Stem Cells Cloning 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Cheung, H.H. Stem Cell-Based Therapies for Parkinson Disease. Int. J. Mol. Sci. 2020, 21, 8060. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Tu, S.; Lu, J.; Zhang, J. Crosstalk between Stem Cell and Spinal Cord Injury: Pathophysiology and Treatment Strategies. Stem Cell Res. Ther. 2019, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Moron-Font, M.; Clos-Sansalvador, M.; Calle, A.; Gastelurrutia, P.; Cserkoova, A.; Morancho, A.; Ramírez, M.Á.; Rosell, A.; et al. Local Administration of Porcine Immunomodulatory, Chemotactic and Angiogenic Extracellular Vesicles Using Engineered Cardiac Scaffolds for Myocardial Infarction. Bioact. Mater. 2021, 6, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Chachques, J.C.; Gardin, C.; Lila, N.; Ferroni, L.; Migonney, V.; Falentin-Daudre, C.; Zanotti, F.; Trentini, M.; Brunello, G.; Rocca, T.; et al. Elastomeric Cardiowrap Scaffolds Functionalized with Mesenchymal Stem Cells-Derived Exosomes Induce a Positive Modulation in the Inflammatory and Wound Healing Response of Mesenchymal Stem Cell and Macrophage. Biomedicines 2021, 9, 824. [Google Scholar] [CrossRef]
- Chachques, J.C.; Lila, N.; Soler-Botija, C.; Martinez-Ramos, C.; Valles, A.; Autret, G.; Perier, M.C.; Mirochnik, N.; Monleon-Pradas, M.; Bayes-Genis, A.; et al. Elastomeric Cardiopatch Scaffold for Myocardial Repair and Ventricular Support. Eur. J. Cardio-Thoracic. Surg. 2020, 57, 545–555. [Google Scholar] [CrossRef]
- Bellin, G.; Gardin, C.; Ferroni, L.; Chachques, J.; Rogante, M.; Mitrečić, D.; Ferrari, R.; Zavan, B. Exosome in Cardiovascular Diseases: A Complex World Full of Hope. Cells 2019, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D Printed Scaffolds for Promoting Bone Regeneration. Biomaterials 2019, 190, 97–110. [Google Scholar] [CrossRef]
- Chachques, J.C.; Trainini, J.C.; Lago, N.; Masoli, O.H.; Barisani, J.L.; Cortes-Morichetti, M.; Schussler, O.; Carpentier, A. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM Clinical Trial): One Year Follow-Up. Cell Transplant. 2007, 16, 927–934. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, B. Sen. Key Immune Events of the Pathomechanisms of Early Cardioembolic Stroke: Multi-Database Mining and Systems Biology Approach. Int. J. Mol. Sci. 2016, 17, 305. [Google Scholar] [CrossRef] [Green Version]
- Tajiri, N.; Staples, M.; Acosta, S.; Pabon, M.; Dailey, T.; Kaneko, Y.; Borlongan, C.V. Stem Cell Therapies in Neurology. In Adult Stem Cell Therapies: Alternatives to Plasticity; Springer: Berlin/Heidelberg, Germany, 2014; pp. 117–136. [Google Scholar] [CrossRef]
- Schimmel, S.; Acosta, S.; Lozano, D. Neuroinflammation in Traumatic Brain Injury: A Chronic Response to an Acute Injury. Brain Circ. 2017, 3, 135. [Google Scholar] [CrossRef]
- Dailey, T.; Metcalf, C.; Mosley, Y.; Sullivan, R.; Shinozuka, K.; Tajiri, N.; Pabon, M.; Acosta, S.; Kaneko, Y.; Loveren, H.; et al. An Update on Translating Stem Cell Therapy for Stroke from Bench to Bedside. J. Clin. Med. 2013, 2, 220–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, L.; Zou, Z.; Tian, H.; Zhang, Y.; Zhou, H.; Liu, L. Stem Cell-Based Therapies for Ischemic Stroke. BioMed. Res. Int. 2014, 2014, 468748. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, A.; Yasuhara, T.; Date, I. Mesenchymal Stem Cell Therapy for Ischemic Stroke. Acta Med. Okayama 2017, 71, 263–268. [Google Scholar] [CrossRef]
- Kenmuir, C.L.; Wechsler, L.R. Update on Cell Therapy for Stroke. Stroke Vasc. Neurol. 2017, 2, 59–64. [Google Scholar] [CrossRef]
- Marcet, P.; Santos, N.; Borlongan, C.V. When Friend Turns Foe: Central and Peripheral Neuroinflammation in Central Nervous System Injury. Neuroimmunol. Neuroinflamm. 2017, 4, 82. [Google Scholar] [CrossRef] [Green Version]
- Hribljan, V.; Lisjak, D.; Petrović, D.J.; Mitrečić, D. Necroptosis Is One of the Modalities of Cell Death Accompanying Ischemic Brain Stroke: From Pathogenesis to Therapeutic Possibilities. Croat. Med. J. 2019, 60, 121–126. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, P.; Zhou, L.; Zhu, L.; Zhou, B.; Yu, Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur. Neurol. 2020, 83, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell-Based Therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from Mesenchymal Stem/Stromal Cells: A New Therapeutic Paradigm. Biomark. Res. 2019, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in Mesenchymal Stem Cell Clinical Trials 2004-2018: Is Efficacy Optimal in a Narrow Dose Range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Ritfeld, G.; Oudega, M. Bone Marrow-Derived Mesenchymal Stem Cell Transplant Survival in the Injured Rodent Spinal Cord. J Bone Marrow Res. 2014, 2, 2–9. [Google Scholar] [CrossRef]
- Shariati, A.; Nemati, R.; Sadeghipour, Y.; Yaghoubi, Y.; Baghbani, R.; Javidi, K.; Zamani, M.; Hassanzadeh, A. Mesenchymal Stromal Cells (MSCs) for Neurodegenerative Disease: A Promising Frontier. Eur. J. Cell Biol. 2020, 99, 151097. [Google Scholar] [CrossRef]
- Appelt, P.A.; Comella, K.; de Souza, L.A.P.S.; Luvizutto, G.J. Effect of Stem Cell Treatment on Functional Recovery of Spinocerebellar Ataxia: Systematic Review and Meta-Analysis. Cerebellum Ataxias 2021, 8, 8. [Google Scholar] [CrossRef]
- Mitrecic, D. Genetically Modified Stem Cells for the Treatment of Neurological Diseases. Front. Biosci. 2012, E4, 1170. [Google Scholar] [CrossRef]
- Mitrečić, D. Current Advances in Intravascular Administration of Stem Cells for Neurological Diseases: A New Dose of Rejuvenation Injected. Rejuvenation Res. 2011, 14, 553–555. [Google Scholar] [CrossRef]
- Morata-Tarifa, C.; Azkona, G.; Glass, J.; Mazzini, L.; Sanchez-Pernaute, R. Looking Backward to Move Forward: A Meta-Analysis of Stem Cell Therapy in Amyotrophic Lateral Sclerosis. npj Regen. Med. 2021, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.J.; Yanshree; Roy, J.; Tipoe, G.L.; Fung, M.L.; Lim, L.W. Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10151. [Google Scholar] [CrossRef] [PubMed]

| Diagnoses | Requirements from Cells |
|---|---|
| Ischemic heart disease | Reduces myocardial necrosis, promotes myogenesis [119,120,121,124] |
| Diabetic Cardiomyopathy | Prevents apoptosis Reduces myocardial fibrosis Improves overall cardiac function [125,126] |
| Cardiac Tissue Engineering | Stimulates cell attachment and migration Source of biochemical factors [127] |
| Stroke | Reduce damage, improve recovery [128,129,130,131,132,133,134,135,136,137] |
| Amyotrophic lateral sclerosis | Support survival of motoric neurons [138,139] |
| Multiple sclerosis | Immunomodulation and decrease in demyelination [140] |
| Parkinson disease | Production of dopamine, reduces symptoms [141] |
| Spinal cord damage | Opposes anti-regenerative action of glial scar and promotes axon growth [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrečić, D.; Hribljan, V.; Jagečić, D.; Isaković, J.; Lamberto, F.; Horánszky, A.; Zana, M.; Foldes, G.; Zavan, B.; Pivoriūnas, A.; et al. Regenerative Neurology and Regenerative Cardiology: Shared Hurdles and Achievements. Int. J. Mol. Sci. 2022, 23, 855. https://doi.org/10.3390/ijms23020855
Mitrečić D, Hribljan V, Jagečić D, Isaković J, Lamberto F, Horánszky A, Zana M, Foldes G, Zavan B, Pivoriūnas A, et al. Regenerative Neurology and Regenerative Cardiology: Shared Hurdles and Achievements. International Journal of Molecular Sciences. 2022; 23(2):855. https://doi.org/10.3390/ijms23020855
Chicago/Turabian StyleMitrečić, Dinko, Valentina Hribljan, Denis Jagečić, Jasmina Isaković, Federica Lamberto, Alex Horánszky, Melinda Zana, Gabor Foldes, Barbara Zavan, Augustas Pivoriūnas, and et al. 2022. "Regenerative Neurology and Regenerative Cardiology: Shared Hurdles and Achievements" International Journal of Molecular Sciences 23, no. 2: 855. https://doi.org/10.3390/ijms23020855
APA StyleMitrečić, D., Hribljan, V., Jagečić, D., Isaković, J., Lamberto, F., Horánszky, A., Zana, M., Foldes, G., Zavan, B., Pivoriūnas, A., Martinez, S., Mazzini, L., Radenovic, L., Milasin, J., Chachques, J. C., Buzanska, L., Song, M. S., & Dinnyés, A. (2022). Regenerative Neurology and Regenerative Cardiology: Shared Hurdles and Achievements. International Journal of Molecular Sciences, 23(2), 855. https://doi.org/10.3390/ijms23020855