Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis
Abstract
:1. Introduction
2. Results
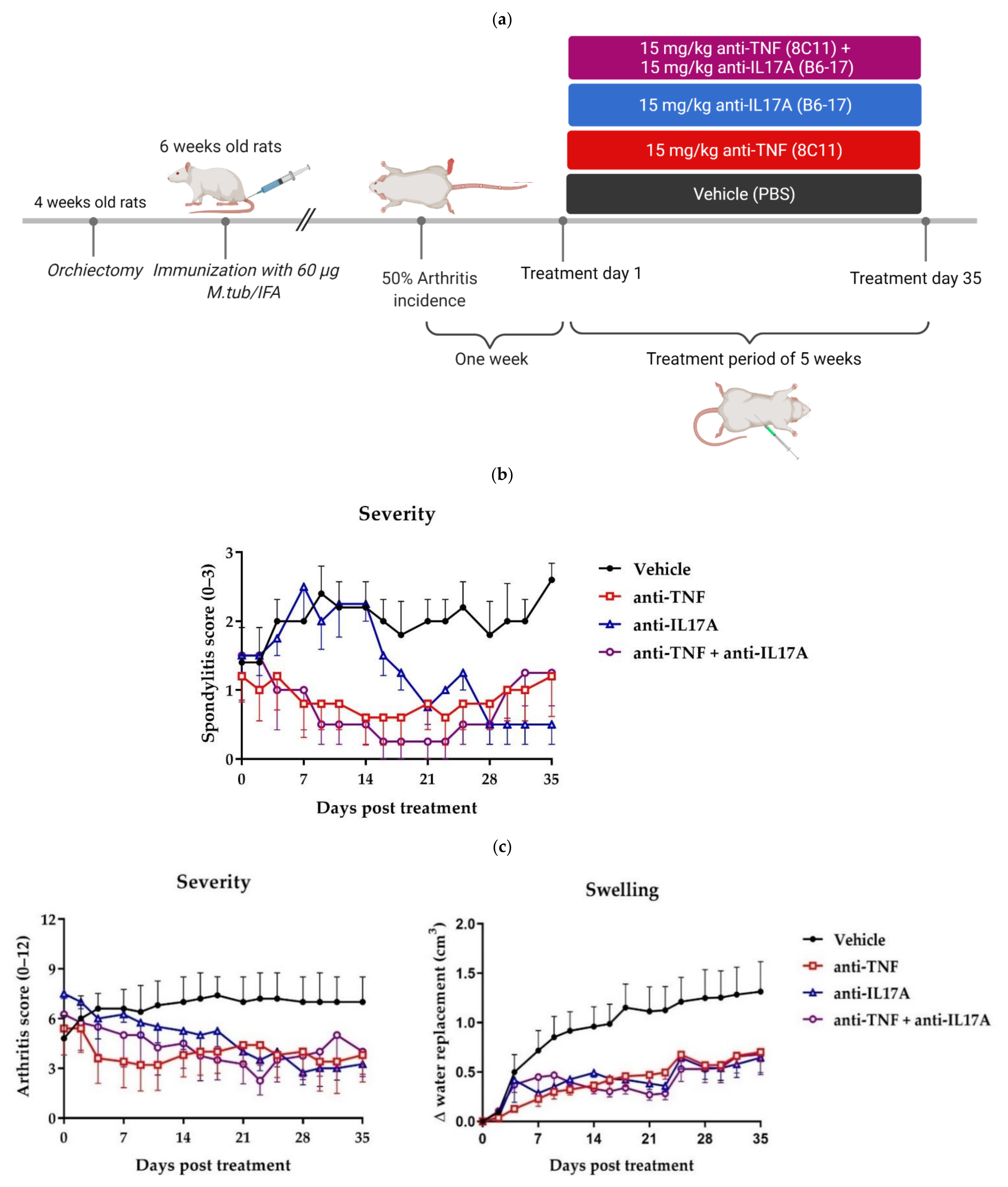
2.1. Dual Tumor Necrosis Factor (TNF) and Interleukin-17A (IL-17A) Blockade Reduces Clinical Spondylitis and Peripheral Arthritis
2.2. TNF and IL-17A Dual Blockade Therapy Reduces Inflammation and New Bone Formation in the Axial and Peripheral Joints
2.3. Micro-Computed Tomography (CT) Imaging Further Confirms the Efficacy of TNF and IL-17A Dual Blockade Therapy in Reducing Structural Damage in Axial and Peripheral Disease
3. Discussion
4. Materials and Methods
4.1. Rats
4.2. Orchiectomy and Immunization
4.3. Clinical Scoring
4.4. Treatment
4.5. Ex Vivo Micro-Computed Tomography (Micro-CT) Analysis
4.6. Histopathology
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieper, J.; Braun, J.; Dougados, M.; Baeten, D. Axial spondyloarthritis. Nat. Rev. Dis. Primers 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- van Echteld, I.; Cieza, A.; Boonen, A.; Stucki, G.; Zochling, J.; Braun, J.; van der Heijde, D. Identification of the most common problems by patients with ankylosing spondylitis using the international classification of functioning, disability and health. J. Rheumatol. 2006, 33, 2475–2483. [Google Scholar] [PubMed]
- Gravallese, E.M.; Schett, G. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat. Rev. Rheumatol. 2018, 14, 631–640. [Google Scholar] [CrossRef]
- Yeremenko, N.; Paramarta, J.E.; Baeten, D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr. Opin. Rheumatol. 2014, 26, 361–370. [Google Scholar] [CrossRef]
- Chen, S.; Noordenbos, T.; Blijdorp, I.; van Mens, L.; Ambarus, C.A.; Vogels, E.; Te Velde, A.; Alsina, M.; Cañete, J.D.; Yeremenko, N. Histologic evidence that mast cells contribute to local tissue inflammation in peripheral spondyloarthritis by regulating interleukin-17A content. Rheumatology 2019, 58, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Heijde, D.v.d.; Landewé, R.; Baraliakos, X.; Houben, H.; Tubergen, A.v.; Williamson, P.; Xu, W.; Baker, D.; Goldstein, N.; Braun, J. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008, 58, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- van Mens, L.J.J.; van de Sande, M.G.H.; Menegatti, S.; Chen, S.; Blijdorp, I.C.J.; de Jong, H.M.; Fluri, I.A.; Latuhihin, T.E.; van Kuijk, A.W.R.; Rogge, L.; et al. Brief Report: Interleukin-17 Blockade with Secukinumab in Peripheral Spondyloarthritis Impacts Synovial Immunopathology Without Compromising Systemic Immune Responses. Arthritis Rheumatol. 2018, 70, 1994–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Mens, L.J.; de Jong, H.M.; Fluri, I.; Nurmohamed, M.T.; van de Sande, M.G.; Kok, M.; van Kuijk, A.W.; Baeten, D. Achieving remission in psoriatic arthritis by early initiation of TNF inhibition: A double-blind, randomised, placebo-controlled trial of golimumab plus methotrexate versus placebo plus methotrexate. Ann. Rheum. Dis. 2019, 78, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Baeten, D.; Kruithof, E.; Van den Bosch, F.; Demetter, P.; Van Damme, N.; Cuvelier, C.; De Vos, M.; Mielants, H.; Veys, E.M.; De Keyser, F. Immunomodulatory effects of anti–tumor necrosis factor α therapy on synovium in spondylarthropathy: Histologic findings in eight patients from an open-label pilot study. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2001, 44, 186–195. [Google Scholar] [CrossRef]
- Paramarta, J.E.; De Rycke, L.; Heijda, T.F.; Ambarus, C.A.; Vos, K.; Dinant, H.J.; Tak, P.P.; Baeten, D.L. Efficacy and safety of adalimumab for the treatment of peripheral arthritis in spondyloarthritis patients without ankylosing spondylitis or psoriatic arthritis. Ann. Rheum. Dis. 2013, 72, 1793–1799. [Google Scholar] [CrossRef]
- van der Heijde, D.; Kivitz, A.; Schiff, M.H.; Sieper, J.; Dijkmans, B.A.; Braun, J.; Dougados, M.; Reveille, J.D.; Wong, R.L.; Kupper, H.; et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006, 54, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; van der Heijde, D.; Dougados, M.; Mease, P.J.; Maksymowych, W.P.; Brown, M.A.; Arora, V.; Pangan, A.L. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: Results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 2013, 72, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieper, J.; Lenaerts, J.; Wollenhaupt, J.; Rudwaleit, M.; Mazurov, V.I.; Myasoutova, L.; Park, S.; Song, Y.; Yao, R.; Chitkara, D.; et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: Results from the double-blind, placebo-controlled INFAST study, Part 1. Ann. Rheum. Dis. 2014, 73, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef] [Green Version]
- Pavelka, K.; Kivitz, A.; Dokoupilova, E.; Blanco, R.; Maradiaga, M.; Tahir, H.; Pricop, L.; Andersson, M.; Readie, A.; Porter, B. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: A randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res. Ther. 2017, 19, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poddubnyy, D.; Sieper, J. What is the best treatment target in axial spondyloarthritis: Tumour necrosis factor α, interleukin 17, or both? Rheumatology 2018, 57, 1145–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzo-Ortega, H.; Sieper, J.; Kivitz, A.; Blanco, R.; Cohen, M.; Martin, R.; Readie, A.; Richards, H.; Porter, B.; Group, M.S. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: Results from a phase III study. Arthritis Care Res. 2017, 69, 1020–1029. [Google Scholar] [CrossRef] [Green Version]
- Shimabuco, A.Y.; Gonçalves, C.R.; Moraes, J.C.; Waisberg, M.G.; Ribeiro, A.C.d.M.; Sampaio-Barros, P.D.; Goldenstein-Schainberg, C.; Bonfa, E.; Saad, C.G. Factors associated with ASDAS remission in a long-term study of ankylosing spondylitis patients under tumor necrosis factor inhibitors. Adv. Rheumatol. 2019, 58. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Baeten, D.; Sieper, J.; Emery, P.; Readie, A.; Martin, R.; Mpofu, S.; Richards, H.B.; et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann. Rheum. Dis. 2017, 76, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Poddubnyy, D.; Emery, P.; Delicha, E.M.; Talloczy, Z.; Porter, B. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology 2019, 58, 859–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Tok, M.N.; Na, S.; Lao, C.R.; Alvi, M.; Pots, D.; van de Sande, M.G.H.; Taurog, J.D.; Sedgwick, J.D.; Baeten, D.L.; van Duivenvoorde, L.M. The Initiation, but Not the Persistence, of Experimental Spondyloarthritis Is Dependent on Interleukin-23 Signaling. Front. Immunol. 2018, 9, 1550. [Google Scholar] [CrossRef]
- van Tok, M.N.; van Duivenvoorde, L.M.; Kramer, I.; Ingold, P.; Pfister, S.; Roth, L.; Blijdorp, I.C.; van de Sande, M.G.H.; Taurog, J.D.; Kolbinger, F.; et al. Interleukin-17A Inhibition Diminishes Inflammation and New Bone Formation in Experimental Spondyloarthritis. Arthritis Rheumatol. 2019, 71, 612–625. [Google Scholar] [CrossRef]
- Milanez, F.M.; Saad, C.G.; Viana, V.T.; Moraes, J.C.; Perico, G.V.; Sampaio-Barros, P.D.; Goncalves, C.R.; Bonfa, E. IL-23/Th17 axis is not influenced by TNF-blocking agents in ankylosing spondylitis patients. Arthritis Res. Ther. 2016, 18, 52. [Google Scholar] [CrossRef] [Green Version]
- Sikorska, D.; Rutkowski, R.; Łuczak, J.; Samborski, W.; Witowski, J. No effect of anti-TNF-α treatment on serum IL-17 in patients with rheumatoid arthritis. Cent. -Eur. J. Immunol. 2018, 43, 270. [Google Scholar] [CrossRef]
- Fiechter, R.H.; de Jong, H.M.; van Mens, L.J.; Fluri, I.A.; Tas, S.W.; Baeten, D.L.; Yeremenko, N.G.; van de Sande, M.G. IL-12p40/IL-23p40 blockade with ustekinumab decreases the synovial inflammatory infiltrate through modulation of multiple signaling pathways including MAPK-ERK and Wnt. Front. Immunol. 2021, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijde, D.; Landewe, R.; Einstein, S.; Ory, P.; Vosse, D.; Ni, L.; Lin, S.L.; Tsuji, W.; Davis, J., Jr. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008, 58, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Paramarta, J.E.; Baeten, D.; De Rycke, L. Suppl 1: Synovial Tissue Response to Treatment with TNF Blockers in Peripheral Spondyloarthritis. Open Rheumatol. J. 2011, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; Van Der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebihara, S.; Date, F.; Dong, Y.; Ono, M. Interleukin-17 is a critical target for the treatment of ankylosing enthesitis and psoriasis-like dermatitis in mice. Autoimmunity 2015, 48, 259–266. [Google Scholar] [CrossRef]
- Koenders, M.I.; Marijnissen, R.J.; Devesa, I.; Lubberts, E.; Joosten, L.A.; Roth, J.; van Lent, P.L.; van de Loo, F.A.; van den Berg, W.B. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1beta, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: Rationale for combination treatment during arthritis. Arthritis Rheum. 2011, 63, 2329–2339. [Google Scholar] [CrossRef]
- Fischer, J.A.; Hueber, A.J.; Wilson, S.; Galm, M.; Baum, W.; Kitson, C.; Auer, J.; Lorenz, S.H.; Moelleken, J.; Bader, M.; et al. Combined inhibition of tumor necrosis factor alpha and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: Development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 2015, 67, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Weinblatt, M.E.; Aelion, J.A.; Mansikka, H.T.; Peloso, P.M.; Chen, K.; Li, Y.; Othman, A.A.; Khatri, A.; Khan, N.S.; et al. ABT-122, a Bispecific Dual Variable Domain Immunoglobulin Targeting Tumor Necrosis Factor and Interleukin-17A, in Patients with Rheumatoid Arthritis with an Inadequate Response to Methotrexate: A Randomized, Double-Blind Study. Arthritis Rheumatol. 2018, 70, 1710–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mease, P.J.; Genovese, M.C.; Weinblatt, M.E.; Peloso, P.M.; Chen, K.; Othman, A.A.; Li, Y.; Mansikka, H.T.; Khatri, A.; Wishart, N.; et al. Phase II Study of ABT-122, a Tumor Necrosis Factor- and Interleukin-17A-Targeted Dual Variable Domain Immunoglobulin, in Patients with Psoriatic Arthritis with an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2018, 70, 1778–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatt, S.; Taylor, P.C.; McInnes, I.B.; Schett, G.; Landewe, R.; Baeten, D.; Ionescu, L.; Strimenopoulou, F.; Watling, M.I.L.; Shaw, S. Efficacy and safety of bimekizumab as add-on therapy for rheumatoid arthritis in patients with inadequate response to certolizumab pegol: A proof-of-concept study. Ann. Rheum. Dis. 2019, 78, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- van Tok, M.N.; Satumtira, N.; Dorris, M.; Pots, D.; Slobodin, G.; van de Sande, M.G.; Taurog, J.D.; Baeten, D.L.; van Duivenvoorde, L.M. Innate Immune Activation Can Trigger Experimental Spondyloarthritis in HLA-B27/Hubeta2m Transgenic Rats. Front. Immunol. 2017, 8, 920. [Google Scholar] [CrossRef] [Green Version]
- Schett, G.; Lories, R.J.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef]
- Kaaij, M.H.; van Tok, M.N.; Blijdorp, I.C.; Ambarus, C.A.; Stock, M.; Pots, D.; Knaup, V.L.; Armaka, M.; Christodoulou-Vafeiadou, E.; van Melsen, T.K.; et al. Transmembrane TNF drives osteoproliferative joint inflammation reminiscent of human spondyloarthritis. J. Exp. Med. 2020, 217, e20200288. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou-Vafeiadou, E.; Geka, C.; Ntari, L.; Kranidioti, K.; Argyropoulou, E.; Meier, F.; Armaka, M.; Mourouzis, I.; Pantos, C.; Rouchota, M. Ectopic bone formation and systemic bone loss in a transmembrane TNF-driven model of human spondyloarthritis. Arthritis Res. Ther. 2020, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Landewé, R.; Hermann, K.G.A.; Han, J.; Yan, S.; Williamson, P.; van der Heijde, D. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: Results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2006, 54, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.G.; Salonen, D.; Rahman, P.; Inman, R.D.; Wong, R.L.; Einstein, S.G.; Thomson, G.T.; Beaulieu, A.; Choquette, D.; Maksymowych, W.P. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2007, 56, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Baraliakos, X.; Brandt, J.; Listing, J.; Haibel, H.; Sörensen, H.; Rudwaleit, M.; Sieper, J.; Braun, J. Outcome of patients with active ankylosing spondylitis after two years of therapy with etanercept: Clinical and magnetic resonance imaging data. Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2005, 53, 856–863. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Salonen, D.; Weissman, B.N.; Landewé, R.; Maksymowych, W.P.; Kupper, H.; Ballal, S.; Gibson, E.; Wong, R. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res. Ther. 2009, 11, R127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmacharya, P.; Duarte-Garcia, A.; Dubreuil, M.; Murad, M.H.; Shahukhal, R.; Shrestha, P.; Myasoedova, E.; Crowson, C.S.; Wright, K.; Davis, J.M., III. Effect of therapy on radiographic progression in axial spondyloarthritis: A systematic review and meta-analysis. Arthritis Rheumatol. 2020, 72, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Boers, N.; Michielsens, C.A.; Van Der Heijde, D.; Den Broeder, A.A.; Welsing, P.M. The effect of tumour necrosis factor inhibitors on radiographic progression in axial spondyloarthritis: A systematic literature review. Rheumatology 2019, 58, 1907–1922. [Google Scholar] [CrossRef]
- Baraliakos, X.; Gensler, L.S.; D’Angelo, S.; Iannone, F.; Favalli, E.G.; de Peyrecave, N.; Auteri, S.E.; Caporali, R. Biologic therapy and spinal radiographic progression in patients with axial spondyloarthritis: A structured literature review. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720–20906040. [Google Scholar] [CrossRef] [Green Version]
- Molnar, C.; Scherer, A.; Baraliakos, X.; de Hooge, M.; Micheroli, R.; Exer, P.; Kissling, R.O.; Tamborrini, G.; Wildi, L.M.; Nissen, M.J. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: Results from the Swiss Clinical Quality Management cohort. Ann. Rheum. Dis. 2018, 77, 63–69. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Wei, J.C.-C.; Dougados, M.; Mease, P.; Deodhar, A.; Maksymowych, W.P.; Van den Bosch, F.; Sieper, J.; Tomita, T.; Landewé, R. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018, 392, 2441–2451. [Google Scholar]
- Deodhar, A.; van der Heijde, D.; Gensler, L.S.; Kim, T.-H.; Maksymowych, W.P.; Østergaard, M.; Poddubnyy, D.; Marzo-Ortega, H.; Bessette, L.; Tomita, T. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): A randomised, placebo-controlled trial. Lancet 2020, 395, 53–64. [Google Scholar] [CrossRef]
- Deodhar, A.; Poddubnyy, D.; Pacheco-Tena, C.; Salvarani, C.; Lespessailles, E.; Rahman, P.; Järvinen, P.; Sanchez-Burson, J.; Gaffney, K.; Lee, E.B. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: Sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 2019, 71, 599–611. [Google Scholar]
- Baraliakos, X.; Borah, B.; Braun, J.; Baeten, D.; Laurent, D.; Sieper, J.; Emery, P.; McInnes, I.B.; van Laar, J.M.; Wordsworth, P. Long-term effects of secukinumab on MRI findings in relation to clinical efficacy in subjects with active ankylosing spondylitis: An observational study. Ann. Rheum. Dis. 2016, 75, 408–412. [Google Scholar] [CrossRef]
- Tran, T.M.; Dorris, M.L.; Satumtira, N.; Richardson, J.A.; Hammer, R.E.; Shang, J.; Taurog, J.D. Additional human β2-microglobulin curbs HLA–B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA–B27–transgenic rats. Arthritis Rheum. 2006, 54, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Taurog, J.D.; Rival, C.; Van Duivenvoorde, L.M.; Satumtira, N.; Dorris, M.L.; Sun, M.; Shelton, J.M.; Richardson, J.A.; Hamra, F.K.; Hammer, R.E. Autoimmune epididymoorchitis is essential to the pathogenesis of male-specific spondylarthritis in HLA–B27–transgenic rats. Arthritis Rheum. 2012, 64, 2518–2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Duivenvoorde, L.M.; Dorris, M.L.; Satumtira, N.; Van Tok, M.N.; Redlich, K.; Tak, P.P.; Taurog, J.D.; Baeten, D.L. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA–B27/human β2-microglobulin–transgenic rat model of spondylarthritis. Arthritis Rheum. 2012, 64, 3210–3219. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoura, I.; Fiechter, R.H.; Bryant, S.H.; Westmoreland, S.; Kingsbury, G.; Waegell, W.; Tas, S.W.; Baeten, D.L.; van de Sande, M.G.H.; van Tok, M.N.; et al. Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis. Int. J. Mol. Sci. 2022, 23, 859. https://doi.org/10.3390/ijms23020859
Hammoura I, Fiechter RH, Bryant SH, Westmoreland S, Kingsbury G, Waegell W, Tas SW, Baeten DL, van de Sande MGH, van Tok MN, et al. Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis. International Journal of Molecular Sciences. 2022; 23(2):859. https://doi.org/10.3390/ijms23020859
Chicago/Turabian StyleHammoura, Ihsan, Renee H. Fiechter, Shaughn H. Bryant, Susan Westmoreland, Gillian Kingsbury, Wendy Waegell, Sander W. Tas, Dominique L. Baeten, Marleen G. H. van de Sande, Melissa N. van Tok, and et al. 2022. "Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis" International Journal of Molecular Sciences 23, no. 2: 859. https://doi.org/10.3390/ijms23020859
APA StyleHammoura, I., Fiechter, R. H., Bryant, S. H., Westmoreland, S., Kingsbury, G., Waegell, W., Tas, S. W., Baeten, D. L., van de Sande, M. G. H., van Tok, M. N., & van Duivenvoorde, L. M. (2022). Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis. International Journal of Molecular Sciences, 23(2), 859. https://doi.org/10.3390/ijms23020859





