Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases
Abstract
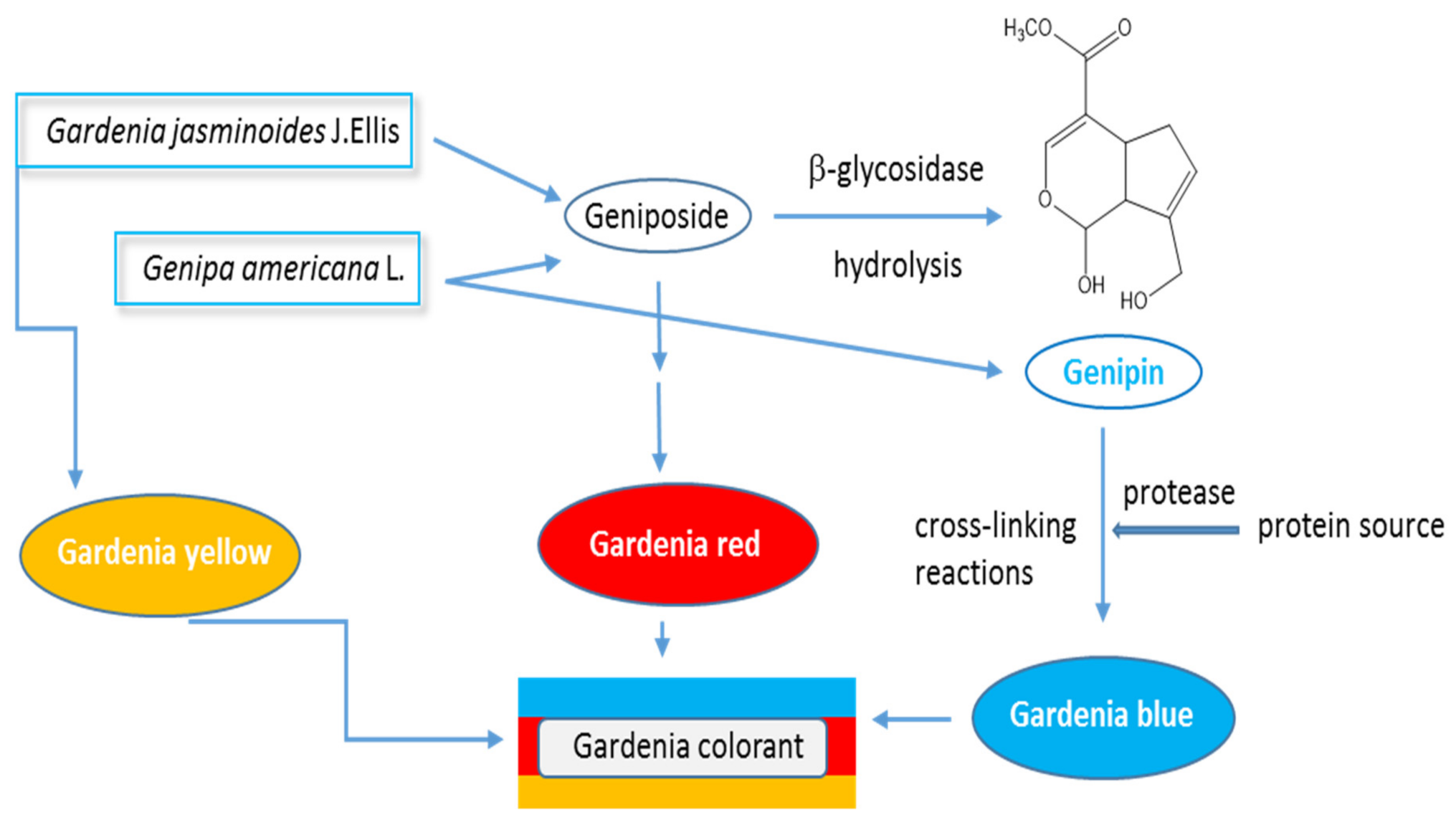
:1. Introduction
2. Genipin in Food Products
3. Hepatoprotective Properties
4. Neuroprotective Properties
5. The Molecular Basis of the Antitumor Activity of Genipin—An Update
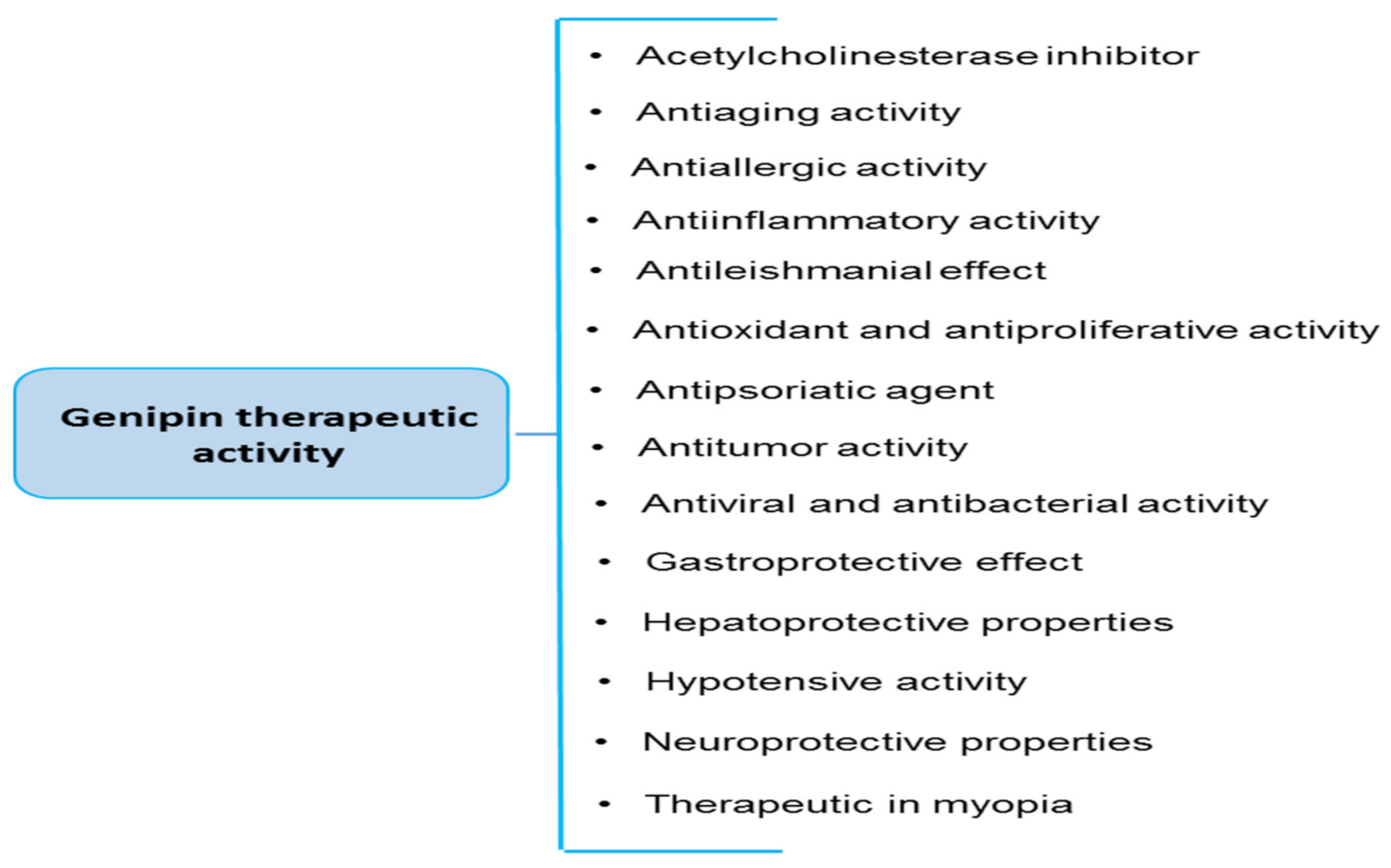
6. Other Biological Properties of Genipin
7. Genipin as a Crosslinking Compound
8. Toxicity of Genipin
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Hölscher, C. Therapeutic Potential of Genipin in Central Neurodegenerative Diseases. CNS Drugs 2016, 30, 889–970. [Google Scholar] [CrossRef] [Green Version]
- Surguchov, A.; Bernal, L.; Surguchev, A.A. Phytochemicals as Regulators of Genes Involved in Synucleinopathies. Biomolecules 2021, 11, 624. [Google Scholar] [CrossRef]
- Krępska, M.; Lasoń-Rydel, M.; Jagiełło, J. Charakterystyka, właściwości, perspektywy i trudności stosowania niebieskich barwników naturalnych do barwienia produktów spożywczych. Technol. Jakość. Wyr. 2016, 61, 63–68. [Google Scholar]
- Li, J.; Shi, J.; Li, P.; Guo, X.; Wang, T.; Liu, A. Genipin attenuates hyperoxia-induced lung injury and pulmonary hypertension via targeting glycogen synthase kinase-3 beta in neonatal rats. Nutrition 2019, 57, 237–244. [Google Scholar]
- Xiao, W.; Li, S.; Wang, S.; Ho, C.-T. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2016, 25, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Gardenia Blue. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/48413323 (accessed on 13 December 2021).
- Fan, X.; Lin, L.; Cui, B.; Zhao, T.; Mao, L.; Song, Y.; Wang, X.; Feng, H.; Qingxiang, Y.; Zhang, J.; et al. Therapeutic potential of genipin in various acute liver injury, fulminant hepatitis, NAFLD and other non-cancer liver diseases: More friend than foe. Pharmacol. Res. 2020, 159, 104945. [Google Scholar] [CrossRef]
- Manickam, B.; Sreedharan, R.; Elumalai, M. “Genipin”—The natural water soluble cross-linking agent and its importance in the modified drug delivery systems: An overview. Curr. Drug Deliv. 2014, 11, 139–145. [Google Scholar]
- Ramos-de-la-Peña, A.M.; Renard, C.M.G.C.; Montañez, J.; de la Luz Reyes-Vega, M.; Contreras-Esquivel, J.C. A review through recovery, purification and identification of genipin. Phytochem. Rev. 2014, 15, 37–49. [Google Scholar]
- Shu, P.; Yu, M.; Zhu, H.; Luo, Y.; Li, Y.; Li, N.; Zhang, H.; Zhang, J.; Liu, G.; Wei, X.; et al. Two new iridoid glycosides from Gardeniae Fructus. Carbohydr. Res. 2021, 501, 108259. [Google Scholar]
- Chestnut, C.; Subramaniam, D.; Dandawate, P.; Padhye, S.; Taylor, J., 3rd; Weir, S.; Anant, S. Targeting Major Signaling Pathways of Bladder Cancer with Phytochemicals: A Review. Nutr. Cancer 2020, 73, 1–23. [Google Scholar]
- Lv, S.; Ding, Y.; Zhao, H.; Liu, S.; Zhang, J.; Wang, J. Therapeutic Potential and Effective Components of the Chinese Herb Gardeniae Fructus in the Treatment of Senile Disease. Aging Dis. 2018, 9, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Neri-Numa, I.A.; Pessoa, M.G.; Paulino, B.N.; Pastore, G.M. Genipin: A natural blue pigment for food and health purposes. Trends Food Sci. Technol. 2017, 67, 271–279. [Google Scholar] [CrossRef]
- Olas, B.; Białecki, J.; Urbańska, K.; Bryś, M. The Effects of Natural and Synthetic Blue Dyes on Human Health: A Review of Current Knowledge and Therapeutic Perspectives. Adv. Nutr. 2021, 12, 2301–2311. [Google Scholar] [CrossRef]
- Xia, Z.-S.; Hao, E.-W.; Wei, Y.-T.; Hou, X.-T.; Chen, Z.-M.; Wei, M.; Du, Z.-C.; Deng, J.-G. Genipin induces developmental toxicity through oxidative stress and apoptosis in zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108951. [Google Scholar] [CrossRef]
- Jing-hua Peng, S.M. The Role of Genipin and Geniposide in Liver Diseases: A Review. Altern. Integr. Med. 2013, 2, 1–8. [Google Scholar]
- Okada, K.; Shoda, J.; Kano, M.; Suzuki, S.; Ohtake, N.; Yamamoto, M.; Takahashi, H.; Utsunomiya, H.; Oda, K.; Sato, K.; et al. Inchinkoto, a herbal medicine, and its ingredients dually exert Mrp2/MRP2-mediated choleresis and Nrf2-mediated antioxidative action in rat livers. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1450–G1463. [Google Scholar]
- Kim, S.-J.; Kim, J.-K.; Lee, D.-U.; Kwak, J.-H.; Lee, S.-M. Genipin protects lipopolysaccharide-induced apoptotic liver damage in d-galactosamine-sensitized mice. Eur. J. Pharmacol. 2010, 635, 188–193. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-Y.; Lee, S.-M. Protective Effects of Geniposide and Genipin against Hepatic Ischemia/Reperfusion Injury in Mice. Biomol. Ther. 2013, 21, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, T.; Deng, Y.; Hou, L.; Fan, X.; Lin, L.; Zhao, W.; Jiang, K.; Sun, C. Genipin Ameliorates Carbon Tetrachloride-Induced Liver Injury in Mice via the Concomitant Inhibition of Inflammation and Induction of Autophagy. Oxidative Med. Cell. Longev. 2019, 2019, 3729051. [Google Scholar] [CrossRef]
- Seo, M.-J.; Hong, J.-M.; Kim, S.-J.; Lee, S.-M. Genipin protects D-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the necroptosis-mediated inflammasome signaling. Eur. J. Pharmacol. 2017, 812, 128–137. [Google Scholar] [CrossRef]
- Zhong, H.; Chen, K.; Feng, M.; Shao, W.; Wu, J.; Chen, K.; Liang, T.; Liu, C. Genipin alleviates high-fat diet-induced hyperlipidemia and hepatic lipid accumulation in mice via miR-142a-5p/SREBP-1c axis. FEBS J. 2018, 285, 501–517. [Google Scholar]
- Sohn, Y.A.; Hwang, I.Y.; Lee, S.Y.; Cho, H.S.; Jeong, C.S. Protective Effects of Genipin on Gastrointestinal Disorders. Biol. Pharm. Bull. 2017, 40, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Surguchov, A. Biomarkers in Parkinson’s disease. Biomarkers 2021, 63, 155–180. [Google Scholar]
- Wang, J.; Chen, L.; Liang, Z.; Li, Y.; Yuan, F.; Liu, J.; Tian, Y.; Hao, Z.; Zhou, F.; Liu, X.; et al. Genipin Inhibits LPS-Induced Inflammatory Response in BV2 Microglial Cells. Neurochem. Res. 2017, 42, 2769–2776. [Google Scholar] [CrossRef]
- Lin, R.; Rao, S.; Li, Y.; Zhang, L.; Xu, L.; He, Y.; Liu, Z.; Chen, H. Conjugation of tacrine with genipin derivative not only enhances effects on AChE but also leads to autophagy against Alzheimer’s disease. Eur. J. Med. Chem. 2021, 211, 113067. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.T.; Porco, J.A., Jr.; Lowell, B.B. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006, 3, 417–427. [Google Scholar]
- Zhao, B. Genipin protects against H2O2-induced oxidative damagein retinal pigment epithelial cells by promoting Nrf2 signaling. Int. J. Mol. Med. 2019, 43, 936–944. [Google Scholar]
- Wang, Q.-S.; Tian, J.-S.; Cui, Y.-L.; Gao, S. Genipin is active via modulating monoaminergic transmission and levels of brain-derived neurotrophic factor (BDNF) in rat model of depression. Neuroscience 2014, 275, 365–373. [Google Scholar] [CrossRef]
- Chen, J.-L.; Shi, B.-Y.; Xiang, H.; Hou, W.-J.; Qin, X.-M.; Tian, J.-S.; Du, G.-H. 1H NMR-based metabolic profiling of liver in chronic unpredictable mild stress rats with genipin treatment. J. Pharm. Biomed. Anal. 2015, 115, 150–158. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. Plant-Derived Anticancer Agents: Lessons from the Pharmacology of Geniposide and Its Aglycone, Genipin. Biomedicines 2018, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Shen, H.; Tang, F.R.; Arfuso, F.; Rajesh, M.; Wang, L.; Kumar, A.P.; Bian, J.; Goh, B.C.; Bishayee, A.; et al. Potential role of genipin in cancer therapy. Pharmacol. Res. 2018, 133, 195–200. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.B.; Jia, D.H.; Sun, W.Q.; Wang, C.L.; Gu, A.Z.; Yang, X.M. Genipin inhibits the growth of human bladder cancer cells via inactivation of PI3K/Akt signaling. Oncol. Lett. 2017, 15, 2619–2624. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Wang, X.; Li, L. Medicinal supplement genipin induces p53 and Bax-dependent apoptosis in colon cancer cells. Oncol. Lett. 2018, 16, 2957–2964. [Google Scholar] [CrossRef]
- Wei, M.; Wu, Y.; Liu, H.; Xie, C. Genipin Induces Autophagy and Suppresses Cell Growth of Oral Squamous Cell Carcinoma via PI3K/AKT/MTOR Pathway. Drug Des. Dev. Ther. 2020, 14, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.J.; Jeong, S.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Na, Y.J.; Park, S.H.; Jeong, Y.A.; Kim, B.G.; et al. Genipin induces mitochondrial dysfunction and apoptosis via downregulation of Stat3/mcl-1 pathway in gastric cancer. BMC Cancer 2019, 19, 739. [Google Scholar]
- Hong, M.; Lee, S.; Clayton, J.; Yake, W.; Li, J. Genipin suppression of growth and metastasis in hepatocellular carcinoma through blocking activation of STAT-3. J. Exp. Clin. Cancer Res. 2020, 39, 146. [Google Scholar] [CrossRef]
- Tian, Y.-S.; Chen, K.-C.; Zulkefli, N.D.; Maner, R.S.; Hsieh, C.-L. Evaluation of the Inhibitory Effects of Genipin on the Fluoxetine-Induced Invasive and Metastatic Model in Human HepG2 Cells. Molecules 2018, 23, 3327. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.-M.; Xiong, J.; Qin, H.; Liu, W.; Chen, R.-N.; Shang, W.; Ning, R.; Hu, G.; Yang, J. Fluoxetine Induces Hepatic Lipid Accumulation Via Both Promotion of the SREBP1c-Related Lipogenesis and Reduction of Lipolysis in Primary Mouse Hepatocytes. CNS Neurosci. Ther. 2012, 18, 974–980. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, H.J.; Oh, S.C.; Lee, D.-H. Genipin inhibits the invasion and migration of colon cancer cells by the suppression of HIF-1α accumulation and VEGF expression. Food Chem. Toxicol. 2018, 116, 70–76. [Google Scholar] [CrossRef]
- Jin, C.E.; Lee, J.H.; Kim, G.J.; Lee, T.H. Genipin Inhibits Hypoxia-Induced Accumulation of HIF-1α and VEGF Expressions in Human Cervical Carcinoma Cells. Kosin Med. J. 2019, 34, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Cho, Y.S.; Jung, K.-H.; Park, J.W.; Lee, K.-H. Genipin enhances the antitumor effect of elesclomol in A549 lung cancer cells by blocking uncoupling protein-2 and stimulating reactive oxygen species production. Oncol. Lett. 2020, 20, 374. [Google Scholar] [CrossRef]
- Kreiter, J.; Rupprecht, A.; Zimmermann, L.; Moschinger, M.; Rokitskaya, T.; Antonenko, Y.N.; Gille, L.; Fedorova, M.; Pohl, E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019, 117, 1845–1857. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, F.A.; Vanni, S.; Graham, R.M. UCP2 as a Potential Biomarker for Adjunctive Metabolic Therapies in Tumor Management. Front. Oncol. 2021, 11, 640720. [Google Scholar] [CrossRef]
- Kim, B.R.; Jeong, Y.A.; Jo, M.J.; Park, S.H.; Na, Y.J.; Kim, J.L.; Jeong, S.; Yun, H.K.; Kang, S.H.; Lee, D.-H.; et al. Genipin Enhances the Therapeutic Effects of Oxaliplatin by Upregulating BIM in Colorectal Cancer. Mol. Cancer Ther. 2019, 18, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Roy, S.; Das, P.K. Antileishmanial effect of the natural immunomodulator genipin through suppression of host negative regulatory protein UCP2. J. Antimicrob. Chemother. 2020, 76, 135–145. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Nguyen, L.T.H.; Kim, B.-A.; Choi, M.-J.; Yang, I.-J.; Shin, H.-M. Natural Compound Mixture, Containing Emodin, Genipin, Chlorogenic Acid, Cimigenoside, and Ginsenoside Rb1, Ameliorates Psoriasis-Like Skin Lesions by Suppressing Inflammation and Proliferation in Keratinocytes. Evid.-Based Complement. Altern. Med. 2020, 2020, 9416962. [Google Scholar] [CrossRef]
- Kim, B.R.; Jeong, A.Y.; Kim, D.Y.; Kim, J.L.; Jeong, S.; Na, Y.J.; Yun, H.K.; Park, S.H.; Jo, M.J.; Ashktorab, H.; et al. Genipin increases oxaliplatin-induced cell death through autophagy in gastric cancer. J. Cancer 2020, 11, 460–467. [Google Scholar] [CrossRef]
- Huang, A.-G.; Tan, X.-P.; Qu, S.-Y.; Wang, G.-X.; Zhu, B. Evaluation on the antiviral activity of genipin against white spot syndrome virus in crayfish. Fish Shellfish Immunol. 2019, 93, 380–386. [Google Scholar] [CrossRef]
- Xia, Q.; Dong, J.; Li, L.; Wang, Q.; Liu, Y.; Wang, Q. Discovery of Glycosylated Genipin Derivatives as Novel Antiviral, Insecticidal, and Fungicidal Agents. J. Agric. Food Chem. 2018, 66, 1341–1348. [Google Scholar] [CrossRef]
- Ko, J.-W.; Shin, N.-R.; Park, S.-H.; Cho, Y.-K.; Kim, J.-C.; Seo, C.-S.; Shin, I.-S. Genipin inhibits allergic responses in ovalbumin-induced asthmatic mice. Int. Immunopharmacol. 2017, 53, 49–55. [Google Scholar] [CrossRef]
- Yu, D.; Shi, M.; Bao, J.; Yu, X.; Li, Y.; Liu, W. Genipin ameliorates hypertension-induced renal damage via the angiotensin II-TLR/MyD88/MAPK pathway. Fitoterapia 2016, 112, 244–253. [Google Scholar]
- Zhang, A.; Wang, S.; Zhang, J.; Wu, H. Genipin alleviates LPS-induced acute lung injury by inhibiting NF-kappaB and NLRP3 signaling pathways. Int. Immunopharmacol. 2016, 38, 115–119. [Google Scholar]
- Zhong, S.; Wu, B.; Wang, X.; Sun, D.; Liu, D.; Jiang, S.; Ge, J.; Zhang, Y.; Liu, X.; Zhou, X.; et al. Identification of driver genes and key pathways of prolactinoma predicts the therapeutic effect of genipin. Mol. Med. Rep. 2019, 20, 2712–2724. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Z.-K.; Liu, H.; Li, R.-Q.; Liu, Y.; Zhong, W.-J. Genipin inhibits the scleral expression of miR-29 and MMP2 and promotes COL1A1 expression in myopic eyes of guinea pigs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1031–1038. [Google Scholar] [CrossRef]
- Ahani, N.; Sangtarash, M.H.; Houshmand, M.; Eskandani, M.A. Genipin induces cell death via intrinsic apoptosis pathways in human glioblastoma cells. J. Cell. Biochem. 2018, 120, 2047–2057. [Google Scholar] [CrossRef]
- Chang, C.-H.; Wu, J.-B.; Yang, J.-S.; Lai, Y.-J.; Su, C.-H.; Lu, C.-C.; Hsu, Y.-M. The Suppressive Effects of Geniposide and Genipin on Helicobacter pylori Infections In Vitro and In Vivo. J. Food Sci. 2017, 82, 3021–3028. [Google Scholar] [CrossRef]
- Dando, I.; Pacchiana, R.; Pozza, E.D.; Cataldo, I.; Bruno, S.; Conti, P.; Cordani, M.; Grimaldi, A.; Butera, G.; Caraglia, M.; et al. UCP2 inhibition induces ROS/Akt/mTOR axis: Role of GAPDH nuclear translocation in genipin/everolimus anticancer synergism. Free Radic. Biol. Med. 2017, 113, 176–189. [Google Scholar] [CrossRef]
- Luo, R.; Lin, M.; Zhang, C.; Shi, J.; Zhang, S.; Chen, Q.; Hu, Y.; Zhang, M.; Zhang, J.; Gao, F. Genipin-crosslinked human serum albumin coating using a tannic acid layer for enhanced oral administration of curcumin in the treatment of ulcerative colitis. Food Chem. 2020, 330, 127241. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications. Catalysts 2019, 9, 1035. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef]
- Hobbs, C.A.; Koyanagi, M.; Swartz, C.; Davis, J.; Maronpot, R.; Recio, L.; Hayashi, S.-M. Genotoxicity evaluation of the naturally-derived food colorant, gardenia blue, and its precursor, genipin. Food Chem. Toxicol. 2018, 118, 695–708. [Google Scholar] [CrossRef]
- Kim, M.-H.; Lee, M.-H.; Hwang, Y.S. Natural Blue Pigment from Gardenia jasminoides Ellis (Rubiaceae) as a Dental Plaque Disclosant. J. Dent. Hyg. Sci. 2021, 21, 38–44. [Google Scholar] [CrossRef]
- Baell, J.B.; Nissink, J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017—Utility and Limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Neri-Numa, I.A.; Pessôa, M.G.; Arruda, H.S.; Pereira, G.A.; Paulino, B.N.; Angolini, C.F.F.; Ruiz, A.L.T.G.; Pastore, G.M. Genipap (Genipa americana L.) fruit extract as a source of antioxidant and antiproliferative iridoids. Food Res. Int. 2020, 134, 109252. [Google Scholar] [CrossRef]
- Ma, H.-F.; Meng, G.; Cui, B.; Si, J.; Dai, Y.-C. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: Laccase immobilization and characterization. Chem. Eng. Res. Des. 2018, 132, 664–676. [Google Scholar] [CrossRef]
- Shin, J.-K.; Lee, S.-M. Genipin protects the liver from ischemia/reperfusion injury by modulating mitochondrial quality control. Toxicol. Appl. Pharmacol. 2017, 328, 25–33. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, L.-K.; Jiang, X.; Zhang, Y.; Kang, J.; Meng, H.; Li, H.; Su, J. Genipin protects against cerebral ischemia-reperfusion injury by regulating the UCP2-SIRT3 signaling pathway. Eur. J. Pharmacol. 2019, 845, 56–64. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Cao, S.; Hu, R.; Liu, R.; Hua, K.; Guo, Z.; Di, H.-J.; Hu, Z. Genipin improves reproductive health problems caused by circadian disruption in male mice. Reprod. Biol. Endocrinol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, K.; Kim, W. Genipin inhibits rotavirus-induced diarrhea by suppressing viral replication and regulating inflammatory responses. Sci. Rep. 2020, 10, 15836. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Ahn, S.H.; Nguyen, U.T.; Yang, I.J.; Shin, H.M. Geniposide, a Principal Component of Gardeniae Fructus, Protects Skin from Diesel Exhaust Particulate Matter-Induced Oxidative Damage. Evid.-Based Complement. Altern. Med. 2021, 2021, 8847358. [Google Scholar] [CrossRef]
| Title | Publication Number and Date | Claim |
|---|---|---|
| Stable natural color process, products and use thereof. | USRE46695E (28 March 2008) | A method of preparing colored products from edible materials comprises processing Genipa americana fruit juice. |
| Stable natural color process and products. | WO2009120579 (6 March 2009) | Colorant for beverages, and dietary supplements. |
| Stable natural color comprising genipin and derivatives. | CA2718604C (15 September 2010) | A method of preparing colored products from edible materials comprises processing Genipa americana fruit juice. |
| Genipin-rich substances and uses thereof. | JP2017105851A (7 November 2011) | A method of producing a genipin rich colorant from Genipa americana. |
| A process for obtaining insoluble substances from genipap-extract precipitates, substances from genipap-extract precipitates and their uses. | EP2408319A1 (25 January 2012) | The precipitation of genipap extract (Genipa americana) for obtaining a substance insoluble in polar and/or non-polar media for applications in an food compositions. |
| Genipin-rich material and its use. | EP3238550A1 (7 November 2012) US8945640B2 (3 November 2015) USRE46314E (21 February 2017) | A method of preparing genipin-rich materials from the fruit of Genipa americana for their use as a cross-linking agent and as a raw material to produce colors is disclosed. |
| Colorant compounds derived from genipin or genipin containing materials. | US9376569 (28 June 2016) US10266698B2 (23 April 2019) | Colorant compounds and methods of its isolation from a reaction of genipin and an amine. |
| Compound and Its Concentration | Length of Study | Experimental Model | Biological Properties | References |
|---|---|---|---|---|
| Genipin (2.5–1000 µM) | - | In vitro—U87MG and A172 cell lines | Anticancer action | [57] |
| Geniposide and genipin (0.03–0.25 mM) Total of 31 and 62 mg/kg/day for geniposide, Total of 18 and 36 mg/kg/day for genipin | In vitro—3, 6 and 24 h In vivo—1 week | In vitro—AGS cells In vivo—C57BL/6 mice | Reducing H. pylori infections | [58] |
| Genipin (500 nM–200 µM) | 24 h | In vitro—pancreatic adenocarcinoma PaCa44, PaCa3, Panc1, MiaPaCa2 and T3M4 | Anticancer action | [59] |
| Genipin (10 and 75 µg/mL, 3–74 mg/kg bw/day) | In vitro—4 and 24 h In vivo—24 h | In vitro—human TP53 component human lymphoblast TK3 cells In vivo—B6C3F1 mice | Anticancer action | [60] |
| Genipin (50 mg/kg) | 24 h | In vivo—red swamp crayfish P. clarkii | Antiviral action | [50] |
| Genipin (20–50 µM) | 24 h | In vitro—human colorectal cancer cell lines—HCT16 and DLD-1 | Therapeutic potential with a minimal adverse effect of oxaliplatin | [46] |
| Genipin (50 µM) | 24 h | In vitro—human colon cancer lines: HCT116 and HT29, human breast cancer cell line—SKBR-3, human prostate cancer cell line—DU145 | Anticancer action | [41] |
| Genipin (10–200 µM, 20 and 50 mg/kg/three times/week) | In vitro—48 hIn vivo—4 weeks | In vitro—human bladder cancer cells: T24 and 5637 In vivo—BALB/c (nu/nu) mice | Anticancer action | [34] |
| Genipap fruit extract (60.77 mg/g fdw—concentration of genipini) | - | In vitro—the tumor cell lines U251 (glioma), MCF-7 (breast), NCI-ADR/RES (breast expressing the multiple drug resistance phenotype), 786–0 (renal), NCI-H460 (lung, non-small cells), PC-3 (prostate), HT-29 (colon) and K562 (leukaemia) | Antioxidant and antiproliferative effect | [14] |
| Genipin (25–100 mg/kg) | - | In vivo—ICR mice | Ameliorating LPS-induced hepatocellular damage | [22] |
| Genipin (100 mg/kg) | - | In vivo—C57BL/6 mice | Protecting the liver from ischemia/reperfusion injury | [61] |
| Genipin (5–20 µM, 1–5 mg/kg) | In vitro—24 hIn vivo—3 week | In vitro—BV2 microglia cells In vivo—ICR mice | Inhibiting LPS-induced inflammatory response | [6] |
| Genipin (1–400 µM, 30 mg/kg) | In vitro—72 hIn vivo—week | In vitro—human tongue squamous In vivo—BALB/c nude mice | Anticancer action | [36] |
| Genipin (50 and 100 µM, 50 and 100 mg/kg) | - | In vitro—AGS gastric cancer cells In vivo—Sprague–Dawley rats | Gastroprotective effect | [24] |
| Genipin (50 mg/kg) | 3 days | In vivo—C57BL/6 mice | Protecting against cerebral ischemia-reperfusion injury | [62] |
| Genipin (50 mg/kg) | - | In vivo—mice | Hepatoprotection against ischemia/reperfusion injury | [20] |
| Genipin (2.5 mg/kg) | Genipin 2 h before CCl4 | In vivo—rats | Hepatoprotective effect in the presence CCl4 | [21] |
| Genipin (5 and 20 mg/kg per day) | 9 weeks | In vivo—obese mice | Alleviating hepatic lipid accumulation | [23] |
| Genipin (25 mg/kg) | - | In vivo—male mice | Improving reproductive health problems | [63] |
| Genipin (5–100 µM) | - | In vitro—retinal pigment epithelial cells | Antioxidant activity | [29] |
| Genipin (100 µM–in vitro; 30 mg/kg/day—in vivo) | - | In vitro—macrophages In vivo—infected mice | Antileishmanial effect | [47] |
| Genipin (10–200 µM—in vitro; 100 mg/kg/day—in vivo) | - | In vitro—murine macrophages RAW264.7 cells In vivo—mice | Antiviral effect | [64] |
| Genipin (10 and 20 mg/kg) | - | In vivo—mice | Inhibiting allergic responses | [52] |
| Genipin (1%) | In vitro—myopic eyes of guinea pigs | Therapeutic potential in myopia | [56] | |
| Conjugate of genipin and tacrine | - | In vitro—SH-SY5Y cells | Inhibiting acetylcholinesterase (IC50 about 5.8 nM) | [27] |
| The mixture of herbal combinations, containing genipin (1%) | - | In vivo—C57BL/6 mice | Therapeutic potential in the treatment of psoriasis lesions | [65] |
| Genipin (50 mg/kg) and insulin (10 IU/kg) | - | In vivo—type 2 diabetic rats | Improving implant osseointegration | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryś, M.; Urbańska, K.; Olas, B. Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. Int. J. Mol. Sci. 2022, 23, 902. https://doi.org/10.3390/ijms23020902
Bryś M, Urbańska K, Olas B. Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. International Journal of Molecular Sciences. 2022; 23(2):902. https://doi.org/10.3390/ijms23020902
Chicago/Turabian StyleBryś, Magdalena, Karina Urbańska, and Beata Olas. 2022. "Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases" International Journal of Molecular Sciences 23, no. 2: 902. https://doi.org/10.3390/ijms23020902







