Functions of the Zinc-Sensing Receptor GPR39 in Regulating Intestinal Health in Animals
Abstract
:1. Introduction
2. The Structure of GPR39
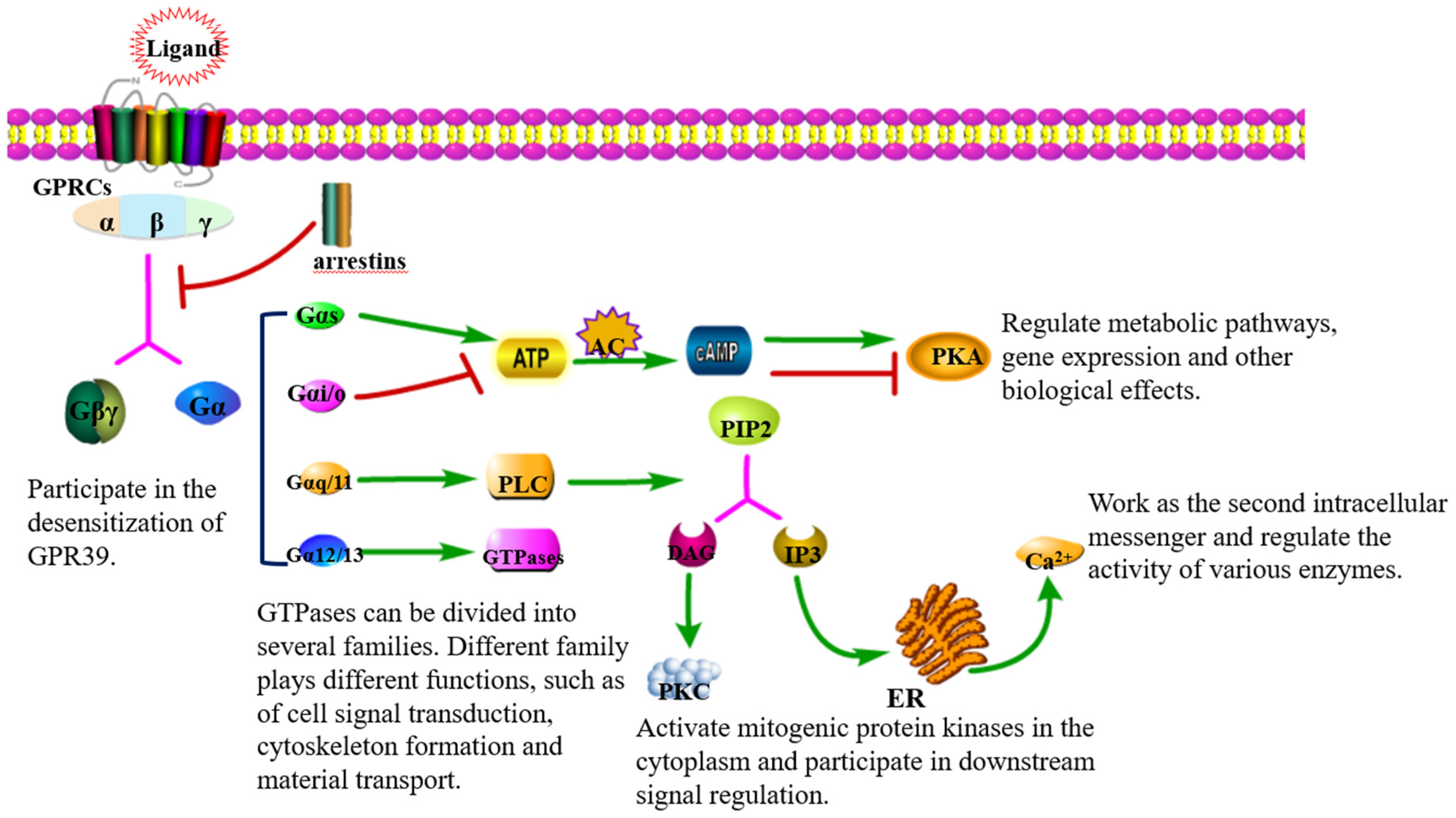
3. Signal Transduction via GPR39
4. The Zinc-Dependent Physiological Functions of GPR39
5. GPR39 Depends on Zn2+ to Regulate Animal Intestinal Health
5.1. GPR39 Regulates Intestinal Ion Transport in Animals
5.2. GPR39 Maintains Intestinal Homeostasis
5.3. GPR39 Affects the Expression of Tight Junction Proteins in the Intestine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Im, H.; Park, J.H.; Im, S.; Han, J.; Kim, K.; Lee, Y.H. Regulatory roles of G-protein coupled receptors in adipose tissue metabolism and their therapeutic potential. Arch. Pharm. Res. 2021, 44, 133–145. [Google Scholar] [CrossRef]
- Wang, P.; Huang, X.; Qiu, W.; Xiao, X. Identifying GPCR-drug interaction based on wordbook learning from sequences. BMC Bioinform. 2020, 21, 150. [Google Scholar] [CrossRef]
- Yang, Z.L.; Wu, B.L. Structural studies and drug discovery of G protein-coupled receptors. Chin. Sci. Bull. 2018, 63, 1362–1373. [Google Scholar] [CrossRef] [Green Version]
- McKee, K.K.; Tan, C.P.; Palyha, O.C.; Liu, J.; Feighner, S.D.; Hreniuk, D.L.; Smith, R.G.; Howard, A.D.; Van der Ploeg, L.H. Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 1997, 46, 426–434. [Google Scholar] [CrossRef]
- Feighner, S.D.; Tan, C.P.; McKee, K.K.; Palyha, O.C.; Hreniuk, D.L.; Pong, S.S.; Austin, C.P.; Figueroa, D.; MacNeil, D.; Cascieri, M.A.; et al. Receptor for motilin identified in the human gastrointestinal system. Science 1999, 284, 2184–2188. [Google Scholar] [CrossRef]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef] [Green Version]
- Lauwers, E.; Landuyt, B.; Arckens, L.; Schoofs, L.; Luyten, W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem. Biophys. Res. Commun. 2006, 351, 21–25. [Google Scholar] [CrossRef]
- Chartrel, N.; Alvear-Perez, R.; Leprince, J.; Iturrioz, X.; Reaux-Le Goazigo, A.; Audinot, V.; Chomarat, P.; Coge, F.; Nosjean, O.; Rodriguez, M.; et al. Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake”. Science 2007, 315, 766. [Google Scholar] [CrossRef] [Green Version]
- Storjohann, L.; Holst, B.; Schwartz, T.W. Molecular mechanism of Zn2+ agonism in the extracellular domain of GPR39. FEBS Lett. 2008, 582, 2583–2588. [Google Scholar] [CrossRef] [Green Version]
- Popovics, P.; Stewart, A.J. GPR39: A Zn2+-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell Mol. Life Sci. CMLS 2011, 68, 85–95. [Google Scholar] [CrossRef]
- Holst, B.; Egerod, K.L.; Schild, E.; Vickers, S.P.; Cheetham, S.; Gerlach, L.O.; Storjohann, L.; Stidsen, C.E.; Jones, R.; Beck-Sickinger, A.G.; et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 2007, 148, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Laitakari, A.; Liu, L.; Frimurer, T.M.; Holst, B. The zinc-sensing receptor GPR39 in physiology and as a pharmacological target. Int. J. Mol. Sci. 2021, 22, 3872. [Google Scholar] [CrossRef]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018, 49, 43. [Google Scholar] [CrossRef] [Green Version]
- Dierick, M.; Van der Weken, H.; Rybarczyk, J.; Vanrompay, D.; Devriendt, B.; Cox, E. Porcine and Bovine Forms of Lactoferrin Inhibit Growth of Porcine Enterotoxigenic Escherichia coli and Degrade Its Virulence Factors. Appl. Environ. Microb. 2020, 86, e0524-20. [Google Scholar] [CrossRef]
- Xia, P.; Lian, S.; Wu, Y.; Yan, L.; Quan, G.; Zhu, G. Zinc is an important inter-kingdom signal between the host and microbe. Vet. Res. 2021, 52, 39. [Google Scholar] [CrossRef]
- Sargeant, H.R.; Miller, H.M.; Shaw, M.A. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol. Immunol. 2011, 48, 2113–2121. [Google Scholar] [CrossRef]
- Hershfinkel, M. The Zinc Sensing Receptor, ZnR/GPR39, in Health and Disease. Int. J. Mol. Sci. 2018, 19, 439. [Google Scholar] [CrossRef] [Green Version]
- Azriel-Tamir, H.; Sharir, H.; Schwartz, B.; Hershfinkel, M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Bio. Chem. 2004, 279, 51804–51816. [Google Scholar] [CrossRef] [Green Version]
- Sunuwar, L.; Asraf, H.; Donowitz, M.; Sekler, I.; Hershfinkel, M. The Zn(2+)-sensing receptor, ZnR/GPR39, upregulates colonocytic Cl(−) absorption, via basolateral KCC1, and reduces fluid loss. BBA-Mol. Basis Dis. 2017, 1863, 947–960. [Google Scholar] [CrossRef]
- Wu, Y.P. The Effect of Zinc Sensitive Receptor GPR39 on Apoptosis of Intestinal in Piglets Induced by Enterotoxigenic Escherichia coli F4ac. Master’s Thesis, Yangzhou University, Yangzhou, China, 2022. [Google Scholar] [CrossRef]
- Storjohann, L.; Holst, B.; Schwartz, T.W. A second disulfide bridge from the N-terminal domain to extracellular loop 2 dampens receptor activity in GPR39. Biochemistry 2008, 47, 9198–9207. [Google Scholar] [CrossRef]
- Li, C.H.; Cui, S.Y.; Gu, Y.Y.; Xie, H.Q.; Tu, Z.L.; Shi, L.G. Research advances in G protein-coupled receptor. Bull. Seric. 2019, 50, 4–7. [Google Scholar]
- Nieto Gutierrez, A.; McDonald, P.H. GPCRs: Emerging anti-cancer drug targets. Cell Signal 2018, 41, 65–74. [Google Scholar] [CrossRef]
- Calebiro, D.; Koszegi, Z. The subcellular dynamics of GPCR signaling. Mol Cell Endocrinol. 2019, 483, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Qiao, Y.; Li, Z. New Insights into Modes of GPCR Activation. Trends Pharmacol. Sci. 2018, 39, 367–386. [Google Scholar] [CrossRef]
- Roux, B.T.; Cottrell, G.S. G protein-coupled receptors: What a difference a ‘partner’ makes. Int. J. Mol. Sci. 2014, 15, 1112–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, I.J.; Jenkins, M.L.; Tall, G.G.; Burke, J.E.; Smrcka, A.V. Activation of Phospholipase C β by Gβγ and Gα(q) Involves C-Terminal Rearrangement to Release Autoinhibition. Structure 2020, 28, 810–819.e815. [Google Scholar] [CrossRef]
- Dittmer, S.; Sahin, M.; Pantlen, A.; Saxena, A.; Toutzaris, D.; Pina, A.-L.; Geerts, A.; Golz, S.; Methner, A. The Constitutively Active Orphan G-protein-coupled Receptor GPR39 Protects from Cell Death by Increasing Secretion of Pigment Epithelium-derived Growth Factor. J. Bio. Chem. 2008, 283, 7074–7081. [Google Scholar] [CrossRef] [Green Version]
- Holliday, N.D.; Holst, B.; Rodionova, E.A.; Schwartz, T.W.; Cox, H.M. Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol. Endocrinol. 2007, 21, 3100–3112. [Google Scholar] [CrossRef] [Green Version]
- Asraf, H.; Salomon, S.; Nevo, A.; Sekler, I.; Mayer, D.; Hershfinkel, M. The ZnR/GPR39 interacts with the CaSR to enhance signaling in prostate and salivary epithelia. J. Cell Physiol. 2014, 229, 868–877. [Google Scholar] [CrossRef]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Bio. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef]
- Holst, B.; Holliday, N.D.; Bach, A.; Elling, C.E.; Cox, H.M.; Schwartz, T.W. Common structural basis for constitutive activity of the ghrelin receptor family. J. Bio. Chem. 2004, 279, 53806–53817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.N. Adaptive Evolution and Function of GPR39 in Vertebrates. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Zang, Z.; Tang, H.; Li, W.; Lai, S.; Deng, C. Adaptive evolution of GPR39 in diverse directions in vertebrates. Gen. Comp. Endocrinol. 2020, 299, 113610. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Laporte, S.A.; Holt, J.A.; Barak, L.S.; Caron, M.G. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis. J. Bio. Chem. 2001, 276, 19452–19460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellomo, E.; Massarotti, A.; Hogstrand, C.; Maret, W. Zinc ions modulate protein tyrosine phosphatase 1B activity. Met. Integr. Biometal Sci. 2014, 6, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Tie, Y.Q.; Wang, S.S. The role and mechanism of zinc-sensitive receptor GPR39 in disease occurrence and treatment. Chin. J. Cell Biol. 2022, 44, 520–528. [Google Scholar]
- Yasuda, S.; Ishida, J. GPR39-1b, the 5-transmembrane isoform of GPR39 interacts with neurotensin receptor NTSR1 and modifies its function. J. Recept. Signal Transduct Res. 2014, 34, 307–312. [Google Scholar] [CrossRef]
- Xu, Y.; Barnes, A.P.; Alkayed, N.J. Role of GPR39 in Neurovascular Homeostasis and Disease. Int. J. Mol. Sci. 2021, 22, 8200. [Google Scholar] [CrossRef]
- Mo, F.; Tang, Y.; Du, P.; Shen, Z.; Yang, J.; Cai, M.; Zhang, Y.; Li, H.; Shen, H. GPR39 protects against corticosterone-induced neuronal injury in hippocampal cells through the CREB-BDNF signaling pathway. J. Affect. Disord. 2020, 272, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jo, Y.; Lee, Y.H.; Park, K.; Park, H.K.; Choi, S.Y. Zn(2+) stimulates salivary secretions via metabotropic zinc receptor ZnR/GPR39 in human salivary gland cells. Sci. Rep. 2019, 9, 17648. [Google Scholar] [CrossRef] [Green Version]
- Petersen, P.S.; Jin, C.; Madsen, A.N.; Rasmussen, M.; Kuhre, R.; Egerod, K.L.; Nielsen, L.B.; Schwartz, T.W.; Holst, B. Deficiency of the GPR39 receptor is associated with obesity and altered adipocyte metabolism. FASEB J. 2011, 25, 3803–3814. [Google Scholar] [CrossRef]
- Holst, B.; Egerod, K.L.; Jin, C.; Petersen, P.S.; Østergaard, M.V.; Hald, J.; Sprinkel, A.M.; Størling, J.; Mandrup-Poulsen, T.; Holst, J.J.; et al. G protein-coupled receptor 39 deficiency is associated with pancreatic islet dysfunction. Endocrinology 2009, 150, 2577–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, B.M.; Miskelly, M.G.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; McKillop, A.M. Zinc-induced activation of GPR39 regulates glucose homeostasis through glucose-dependent insulinotropic polypeptide secretion from enteroendocrine K-cells. Biol. Chem. 2018, 400, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Gao, W.; Ma, J.; Xue, H.; Wang, Y.; Huang, D.; Yan, F.; Ye, Y. GPR39 promotes cardiac hypertrophy by regulating the AMPK-mTOR pathway and protein synthesis. Cell Biol. Int. 2021, 45, 1211–1219. [Google Scholar] [CrossRef]
- Chen, Z.; Gordillo-Martinez, F.; Jiang, L.; He, P.; Hong, W.; Wei, X.; Staines, K.A.; Macrae, V.E.; Zhang, C.; Yu, D.; et al. Zinc ameliorates human aortic valve calcification through GPR39 mediated ERK1/2 signalling pathway. Cardiovasc. Res. 2021, 117, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Zheng, Y.; Fu, B.; Tang, L.; Qin, Y.X. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am. J. Physiol. Cell Physiol. 2018, 314, c404–c414. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Hasegawa, A.; Yamasaki, S.; Uchida, R.; Ohashi, W.; Kurashima, Y.; Kunisawa, J.; Kimura, S.; Iwanaga, T.; Watarai, H.; et al. Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Sci. Rep. 2019, 9, 10842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Schmidt, F.N.; Guterman-Ram, G.; Khayyeri, H.; Hiram-Bab, S.; Orenbuch, A.; Katchkovsky, S.; Aflalo, A.; Isaksson, H.; Busse, B.; et al. Perturbed bone composition and integrity with disorganized osteoblast function in zinc receptor/Gpr39-deficient mice. FASEB J. 2018, 32, 2507–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrovanek, S.; DiGuilio, K.; Bailey, R.; Huntington, W.; Urbas, R.; Mayilvaganan, B.; Mercogliano, G.; Mullin, J.M. Zinc and gastrointestinal disease. World J. Gastrointest. Pathophysiol. 2014, 5, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Yadav, A. Zinc supplementation in childhood diarrhoea: An integral part of management. Paediatr. Int. Child Health 2020, 40, 211. [Google Scholar] [CrossRef]
- Wiegand, S.; Zakrzewski, S.S.; Eichner, M.; Schulz, E.; Günzel, D.; Pieper, R.; Rosenthal, R.; Barmeyer, C.; Bleich, A.; Dobrindt, U.; et al. Zinc treatment is efficient against Escherichia coli α-haemolysin-induced intestinal leakage in mice. Sci. Rep. 2017, 7, 45649. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Shao, Y.; Liu, D.; Yin, P.; Guo, Y.; Yuan, J. Zinc prevents Salmonella enterica serovar Typhimurium-induced loss of intestinal mucosal barrier function in broiler chickens. Avian Pathol. 2012, 41, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [Green Version]
- Hershfinkel, M.; Moran, A.; Grossman, N.; Sekler, I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA 2001, 98, 11749–11754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.L.; Black, R.E. Zinc for the treatment of diarrhoea: Effect on diarrhoea morbidity, mortality and incidence of future episodes. Int. J. Epidemiol. 2010, 39 (Suppl. 1), i63–i69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlowski, J.; Grinstein, S. Na+/H+ exchangers. Compr. Physiol. 2011, 1, 2083–2100. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Asraf, H.; Sekler, I.; Hershfinkel, M. Extracellular pH regulates zinc signaling via an Asp residue of the zinc-sensing receptor (ZnR/GPR39). J. Bio. Chem. 2012, 287, 33339–33350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moechars, D.; Depoortere, I.; Moreaux, B.; de Smet, B.; Goris, I.; Hoskens, L.; Daneels, G.; Kass, S.; Ver Donck, L.; Peeters, T.; et al. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology 2006, 131, 1131–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frimurer, T.M.; Mende, F.; Graae, A.S.; Engelstoft, M.S.; Egerod, K.L.; Nygaard, R.; Gerlach, L.O.; Hansen, J.B.; Schwartz, T.W.; Holst, B. Model-Based Discovery of Synthetic Agonists for the Zn(2+)-Sensing G-Protein-Coupled Receptor 39 (GPR39) Reveals Novel Biological Functions. J. Med. Chem. 2017, 60, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Azriel-Tamir, H.; Arotsker, N.; Sekler, I.; Hershfinkel, M. Zinc sensing receptor signaling, mediated by GPR39, reduces butyrate-induced cell death in HT29 colonocytes via upregulation of clusterin. PLoS ONE 2012, 7, e35482. [Google Scholar] [CrossRef] [PubMed]
- Sunuwar, L.; Medini, M.; Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, W.; Hara, T.; Takagishi, T.; Hase, K.; Fukada, T. Maintenance of Intestinal Epithelial Homeostasis by Zinc Transporters. Dig. Dis. Sci. 2019, 64, 2404–2415. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L. The Mechanism of ORP-1 in Improving Intestinal Mucosal Barrier Function. Master’s Thesis, Anhui University, Anhui, China, 2021. [Google Scholar] [CrossRef]
- Ohashi, W.; Kimura, S.; Iwanaga, T.; Furusawa, Y.; Irié, T.; Izumi, H.; Watanabe, T.; Hijikata, A.; Hara, T.; Ohara, O.; et al. Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress. PLoS Genet. 2016, 12, e1006349. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, W.; Fukada, T. Contribution of Zinc and Zinc Transporters in the Pathogenesis of Inflammatory Bowel Diseases. J. Immunol. Res. 2019, 2019, 8396878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.X.; Lei, Z.; Wolf, P.G.; Gao, Y.; Guo, Y.M.; Zhang, B.K. Zinc Supplementation, via GPR39, Upregulates PKCζ to Protect Intestinal Barrier Integrity in Caco-2 Cells Challenged by Salmonella enterica Serovar Typhimurium. J. Nutr. 2017, 147, 1282–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014, 5, e1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pongkorpsakol, P.; Buasakdi, C.; Chantivas, T.; Chatsudthipong, V.; Muanprasat, C. An agonist of a zinc-sensing receptor GPR39 enhances tight junction assembly in intestinal epithelial cells via an AMPK-dependent mechanism. Eur. J. Pharmacol. 2019, 842, 306–313. [Google Scholar] [CrossRef] [PubMed]
| Affected Parts | Physiological Function | References |
|---|---|---|
| Cerebrum | Protects neurons from cortisol injury, inhibits the expression of pro-apoptotic proteins, and upregulates the expression of anti-apoptotic proteins. | Mo et al. [40] |
| Submandibular | Regulates saliva secretion in human submandibular gland cells. | Kim et al. [41] |
| Adipose tissue | Regulates adipose tissue metabolism, especially lipolysis, and regulates the function of lipases, such as hormone-sensitive lipase and adipose triglyceride lipase. | Petersen et al. [42] |
| Pancreas | Controls the pancreatic duodenal homeobox-1 to regulate islet development and differentiation; regulates pancreatic secretion and glucose homeostasis. | Holst et al. [43], Moran et al. [44] |
| Heart | Regulates the Adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK)-mTOR-S6K1 signal pathway to promote myocardial hypertrophy (a target for the therapy of cardiac hypertrophy), and to improve human aortic valve calcification through the ERK1/2 signaling pathway. | Liao et al. [45], Chen et al. [46] |
| Vascular endothelium | Regulates vascular endothelial cell activity. | Zhu et al. [47] |
| Skin | Promotes the proliferation and differentiation of epithelial cells and is beneficial for wound healing. | Nishida et al. [48] |
| Reproductive system | Mediates acrosomal exocytosis of bovine spermatozoa and overactivation of human spermatozoa. | Allouche-Fitoussi et al. [49] |
| Skeleton | Regulates collagen synthesis and deposition in osteoblasts. | Jovanovic et al. [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, P.; Yan, L.; Ji, X.; Wu, Y.; Lian, S.; Zhu, G. Functions of the Zinc-Sensing Receptor GPR39 in Regulating Intestinal Health in Animals. Int. J. Mol. Sci. 2022, 23, 12133. https://doi.org/10.3390/ijms232012133
Xia P, Yan L, Ji X, Wu Y, Lian S, Zhu G. Functions of the Zinc-Sensing Receptor GPR39 in Regulating Intestinal Health in Animals. International Journal of Molecular Sciences. 2022; 23(20):12133. https://doi.org/10.3390/ijms232012133
Chicago/Turabian StyleXia, Pengpeng, Li Yan, Xingduo Ji, Yunping Wu, Siqi Lian, and Guoqiang Zhu. 2022. "Functions of the Zinc-Sensing Receptor GPR39 in Regulating Intestinal Health in Animals" International Journal of Molecular Sciences 23, no. 20: 12133. https://doi.org/10.3390/ijms232012133





