Vitamin C Suppresses Pancreatic Carcinogenesis through the Inhibition of Both Glucose Metabolism and Wnt Signaling
Abstract
1. Introduction
2. Results
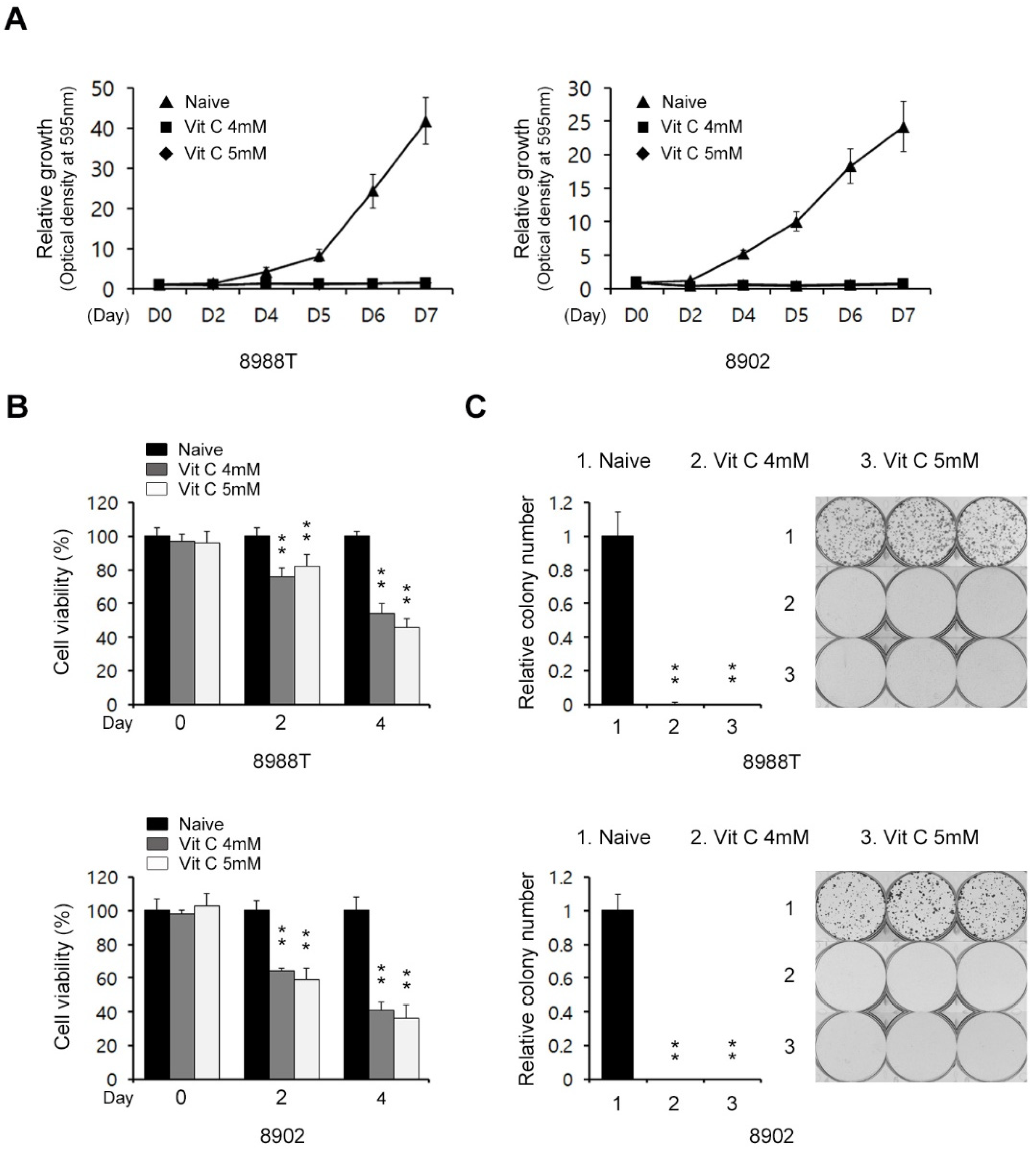
2.1. Vitamin C Impairs Pancreatic Cancer Growth
2.2. Vitamin C Induces Apoptotic Cell Death in a Caspase-Independent Manner
2.3. Vitamin C Exerts Antitumor Effects on PDAC Cells by Inhibiting Glucose Metabolism
2.4. Vitamin C Suppresses the Migration Ability of PDAC Cells
2.5. Vitamin C Inhibits the Migration Ability of PDAC Cells by Inhibiting Wnt/β-Catenin Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Growth and Cell Viability Assays
4.3. Colony Formation Assay
4.4. Annexin V/PI Assay
4.5. Western Blot
4.6. Metabolomics
4.7. Extracellular Acidification Rate Measurement
4.8. Quantification of Intracellular ATP
4.9. Transwell Assay for Invasion and Migration
4.10. TOF/FOP Promoter Assay
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Castellanos, G.; Masoud, R.; Carrier, A. Mitochondrial metabolism in PDAC: From better knowledge to new targeting strategies. Biomedicines 2020, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, Y.; Luo, Y.; Zhu, J.; Zheng, H.; Gao, B.; Guo, X.; Li, Z.; Chen, R.; Chen, C. Circnfib1 inhibits lymphangiogenesis and lymphatic metastasis via the miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol. Cancer 2020, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Dai, S.N.; Xu, D.L.; Hou, C.Q.; Liu, T.T.; Chen, Q.Y.; Wu, J.L.; Miao, Y. Efnb2 facilitates cell proliferation, migration, and invasion in pancreatic ductal adenocarcinoma via the p53/p21 pathway and emt. Biomed. Pharmacother. 2020, 125, 109972. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.A.; Neoptolemos, J.P. Understanding metastasis in pancreatic cancer: A call for new clinical approaches. Cell 2012, 148, 21–23. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, B.; Yang, G.; Wang, H.; Chen, G.; You, L.; Zhang, T.; Zhao, Y. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol. Life Sci. 2020, 77, 305–321. [Google Scholar] [CrossRef]
- Andersen, H.B.; Ialchina, R.; Pedersen, S.F.; Czaplinska, D. Metabolic reprogramming by driver mutation-tumor microenvironment interplay in pancreatic cancer: New therapeutic targets. Cancer Metastasis Rev. 2021, 40, 1093–1114. [Google Scholar] [CrossRef]
- Aiello, N.M.; Maddipati, R.; Norgard, R.J.; Balli, D.; Li, J.; Yuan, S.; Yamazoe, T.; Black, T.; Sahmoud, A.; Furth, E.E.; et al. Emt subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 2018, 45, 681–695.e4. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.J.; Cho, E.J.; Lee, Y.B.; Lee, J.H.; Yu, S.J.; Yoon, J.H.; Kim, Y.J. Inhibition of PI3K/Akt signaling suppresses epithelial-to-mesenchymal transition in hepatocellular carcinoma through the Snail/GSK-3/beta-catenin pathway. Clin. Mol. Hepatol. 2020, 26, 529–539. [Google Scholar] [CrossRef]
- Zeng, L.H.; Wang, Q.M.; Feng, L.Y.; Ke, Y.D.; Xu, Q.Z.; Wei, A.Y.; Zhang, C.; Ying, R.B. High-dose vitamin C suppresses the invasion and metastasis of breast cancer cells via inhibiting epithelial-mesenchymal transition. Onco Targets Ther. 2019, 12, 7405–7413. [Google Scholar] [CrossRef] [PubMed]
- Sajadian, S.O.; Tripura, C.; Samani, F.S.; Ruoss, M.; Dooley, S.; Baharvand, H.; Nussler, A.K. Vitamin C enhances epigenetic modifications induced by 5-azacytidine and cell cycle arrest in the hepatocellular carcinoma cell lines hle and huh7. Clin. Epigenetics 2016, 8, 46. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Marik, P.E. The antiviral properties of vitamin C. Expert Rev. Anti-Infect. Ther. 2020, 18, 99–101. [Google Scholar] [CrossRef]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; LaPrade, R.F. Efficacy of vitamin C supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries: A systematic review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, C.; Chen, X.; Fei, Y.; Jiang, L.; Wang, G. Vitamin C promotes apoptosis and cell cycle arrest in oral squamous cell carcinoma. Front. Oncol. 2020, 10, 976. [Google Scholar] [CrossRef]
- Aguilera, O.; Munoz-Sagastibelza, M.; Torrejon, B.; Borrero-Palacios, A.; Del Puerto-Nevado, L.; Martinez-Useros, J.; Rodriguez-Remirez, M.; Zazo, S.; Garcia, E.; Fraga, M.; et al. Vitamin C uncouples the warburg metabolic switch in KRAS mutant colon cancer. Oncotarget 2016, 7, 47954–47965. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeong, J.H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of high-dose vitamin C combined with anti-cancer treatment on breast cancer cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef]
- James Jamison, K.M. Vitamin C and K3 combination causes enhanced anticancer activity against rt-4 bladder cancer cells. J. Cancer Sci. Ther. 2013, 5, 325–333. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, K.; Barsotti, A.; Feng, X.J.; Momcilovic, M.; Liu, K.G.; Kim, J.I.; Morris, K.; Lamarque, C.; Gaffney, J.; Yu, X.; et al. Inhibition of glucose transport synergizes with chemical or genetic disruption of mitochondrial metabolism and suppresses TCA cycle-deficient tumors. Cell Chem. Biol. 2022, 29, 423–435.e10. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, R.; Furuichi, K.; Yamaguchi, M.; Miyazaki, N.; Aoki, H.; Chibana, H.; Ito, K.; Aoki, S. Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci. Rep. 2019, 9, 18699. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Quinonero, F.; Mesas, C.; Doello, K.; Cabeza, L.; Perazzoli, G.; Jimenez-Luna, C.; Rama, A.R.; Melguizo, C.; Prados, J. The challenge of drug resistance in pancreatic ductal adenocarcinoma: A current overview. Cancer Biol. Med. 2019, 16, 688–699. [Google Scholar] [CrossRef]
- Teeuwssen, M.; Fodde, R. Wnt signaling in ovarian cancer stemness, EMT, and therapy resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef]
- Tiwari, N.; Gheldof, A.; Tatari, M.; Christofori, G. EMT as the ultimate survival mechanism of cancer cells. Semin. Cancer Biol. 2012, 22, 194–207. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The role of snail in emt and tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Snail: More than emt. Cell Adh. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic potential of targeting the wnt/beta-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. (Landmark Ed.) 2013, 18, 1017–1029. [Google Scholar] [PubMed]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.; Bos, G.M.J. The effect of vitamin C (ascorbic acid) in the treatment of patients with cancer: A systematic review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, L.; Orskov, A.D.; Liu, M.; Harslof, L.B.S.; Jones, P.A.; Gronbaek, K. Vitamin C—A new player in regulation of the cancer epigenome. Semin. Cancer Biol. 2018, 51, 59–67. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. Cancer. Revisiting vitamin C and cancer. Science 2015, 350, 1317–1318. [Google Scholar] [CrossRef]
- Yue, X.; Rao, A. Tet family dioxygenases and the tet activator vitamin C in immune responses and cancer. Blood 2020, 136, 1394–1401. [Google Scholar] [CrossRef]
- Sant, D.W.; Mustafi, S.; Gustafson, C.B.; Chen, J.; Slingerland, J.M.; Wang, G. Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression. Sci. Rep. 2018, 8, 5306. [Google Scholar] [CrossRef]
- Cohen, R.; Neuzillet, C.; Tijeras-Raballand, A.; Faivre, S.; de Gramont, A.; Raymond, E. Targeting cancer cell metabolism in pancreatic adenocarcinoma. Oncotarget 2015, 6, 16832–16847. [Google Scholar] [CrossRef]
- Yan, L.; Raj, P.; Yao, W.; Ying, H. Glucose metabolism in pancreatic cancer. Cancers 2019, 11, 1460. [Google Scholar] [CrossRef]
- Park, S.; Ahn, S.; Shin, Y.; Yang, Y.; Yeom, C.H. Vitamin C in cancer: A metabolomics perspective. Front. Physiol. 2018, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, D.; Kim, B.R.; Jun, J.S.; Yeom, J.S.; Park, J.S.; Seo, J.H.; Park, C.H.; Woo, H.O.; Youn, H.S.; et al. Vitamin C induces apoptosis in AGS cells via production of ros of mitochondria. Oncol. Lett. 2016, 12, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Zhang, S.; Lu, X.; Wu, J.; Yu, K.; Ji, A.; Lu, W.; Wang, Z.; Wu, J.; et al. Iqgap1 promotes pancreatic cancer progression and epithelial-mesenchymal transition (EMT) through wnt/beta-catenin signaling. Sci. Rep. 2019, 9, 7539. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, H.; Liao, Y.; Wu, W.; Liu, L.; Liu, L.; Wu, Y.; Sun, F.; Lin, H.W. Inhibition of wnt/beta-catenin pathway reverses multi-drug resistance and EMT in Oct4(+)/Nanog(+) NSCLC cells. Biomed. Pharmacother. 2020, 127, 110225. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, T.; Huang, X.; Wu, W.; Li, J.; Wei, L.; Qian, Y.; Xu, H.; Wang, Q.; Wang, L. Depdc1b promotes migration and invasion in pancreatic ductal adenocarcinoma by activating the Akt/GSK3beta/Snail pathway. Oncol. Lett. 2020, 20, 146. [Google Scholar] [CrossRef]
- Liu, M.; Hancock, S.E.; Sultani, G.; Wilkins, B.P.; Ding, E.; Osborne, B.; Quek, L.E.; Turner, N. Snail-overexpression induces epithelial-mesenchymal transition and metabolic reprogramming in human pancreatic ductal adenocarcinoma and non-tumorigenic ductal cells. J. Clin. Med. 2019, 8, 822. [Google Scholar] [CrossRef]
- Yook, J.I.; Li, X.Y.; Ota, I.; Fearon, E.R.; Weiss, S.J. Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 2005, 280, 11740–11748. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeon, H.M.; Ju, M.K.; Kim, C.H.; Yoon, G.; Han, S.I.; Park, H.G.; Kang, H.S. Wnt/snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res. 2012, 72, 3607–3617. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Hwang, S.; Lee, J.-H.; Im, S.S.; Son, J. Vitamin C Suppresses Pancreatic Carcinogenesis through the Inhibition of Both Glucose Metabolism and Wnt Signaling. Int. J. Mol. Sci. 2022, 23, 12249. https://doi.org/10.3390/ijms232012249
Kim JH, Hwang S, Lee J-H, Im SS, Son J. Vitamin C Suppresses Pancreatic Carcinogenesis through the Inhibition of Both Glucose Metabolism and Wnt Signaling. International Journal of Molecular Sciences. 2022; 23(20):12249. https://doi.org/10.3390/ijms232012249
Chicago/Turabian StyleKim, Ji Hye, Sein Hwang, Ji-Hye Lee, Se Seul Im, and Jaekyoung Son. 2022. "Vitamin C Suppresses Pancreatic Carcinogenesis through the Inhibition of Both Glucose Metabolism and Wnt Signaling" International Journal of Molecular Sciences 23, no. 20: 12249. https://doi.org/10.3390/ijms232012249
APA StyleKim, J. H., Hwang, S., Lee, J.-H., Im, S. S., & Son, J. (2022). Vitamin C Suppresses Pancreatic Carcinogenesis through the Inhibition of Both Glucose Metabolism and Wnt Signaling. International Journal of Molecular Sciences, 23(20), 12249. https://doi.org/10.3390/ijms232012249




