Association between Circulating MicroRNAs (miR-21-5p, miR-20a-5p, miR-29b-3p, miR-126-3p and miR-101-3p) and Chronic Allograft Dysfunction in Renal Transplant Recipients
Abstract
1. Introduction
2. Results
3. Discussion
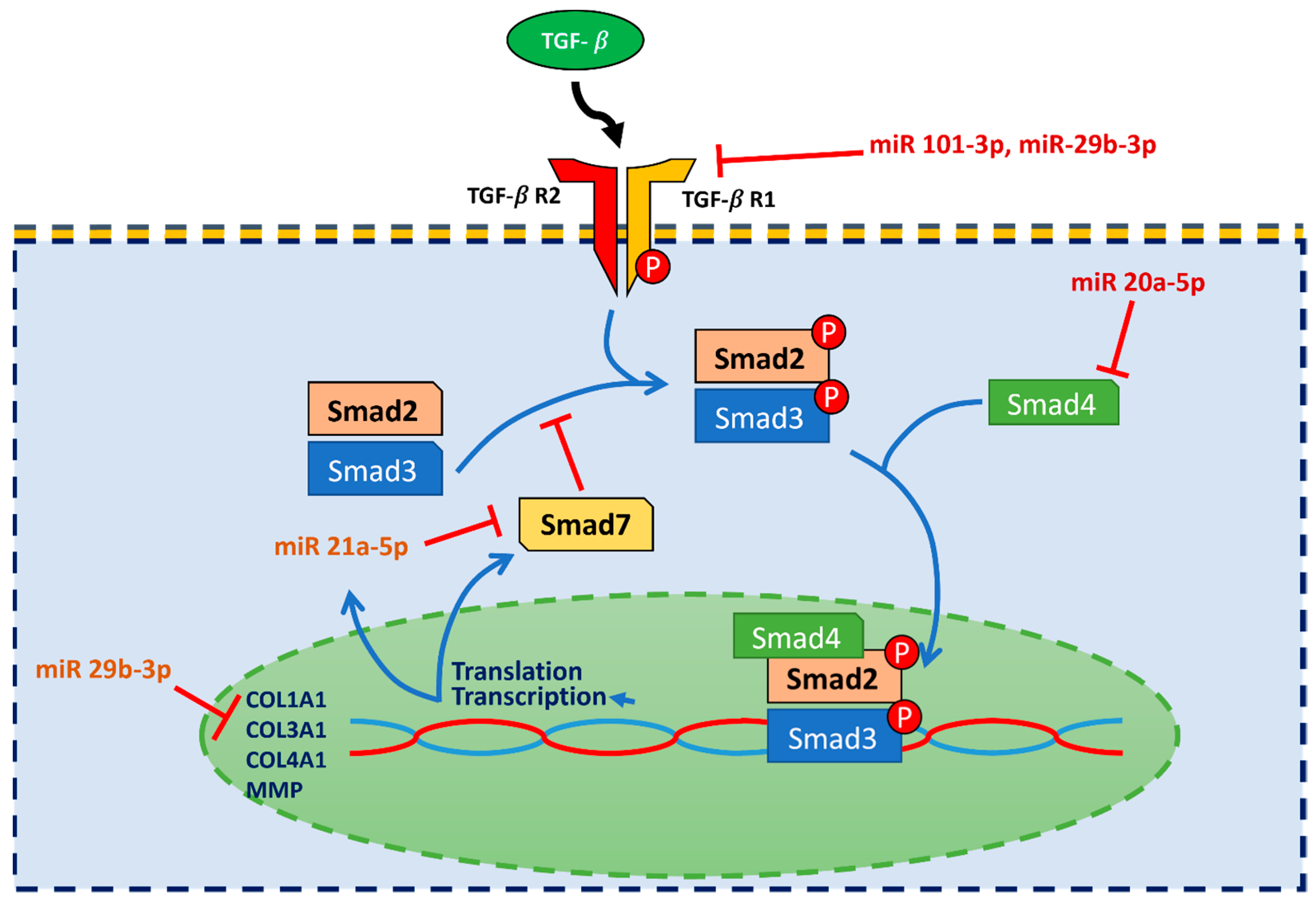
4. Materials and Methods
4.1. MicroRNA Extraction
4.2. cDNA Synthesis & qPCR Analysis
4.3. The miRNA-Target Functional Analysis
4.4. Pathway and Gene Ontology Analysis
4.5. PCA Analysis
4.6. Clustering Analysis
4.7. Statistics
4.8. Patients
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, S.-F.; Lin, M.-H.; Hsu, C.-C.; Wu, M.-J.; Wang, I.-K.; Chen, C.-H. Trends of kidney transplantation from the 2020 annual report on kidney disease in Taiwan. J. Formos. Med. Assoc. 2022, 121 (Suppl. 1), S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-Term Survival after Kidney Transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef]
- Rocchi, A.; Chiti, E.; Maiese, A.; Turillazzi, E.; Spinetti, I. MicroRNAs: An Update of Applications in Forensic Science. Diagnostics 2020, 11, 32. [Google Scholar] [CrossRef]
- Maiese, A.; Scatena, A.; Costantino, A.; Di Paolo, M.; La Russa, R.; Turillazzi, E.; Frati, P.; Fineschi, V. microRNAs as Useful Tools to Estimate Time Since Death. A Systematic Review of Current Literature. Diagnostics 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Ben-Dov, I.; Muthukumar, T.; Morozov, P.; Mueller, F.B.; Tuschl, T.; Suthanthiran, M. MicroRNA Sequence Profiles of Human Kidney Allografts with or Without Tubulointerstitial Fibrosis. Transplantation 2012, 94, 1086–1094. [Google Scholar] [CrossRef]
- Glowacki, F.; Savary, G.; Gnemmi, V.; Buob, D.; Van Der Hauwaert, C.; Lo-Guidice, J.-M.; Bouyé, S.; Hazzan, M.; Pottier, N.; Perrais, M.; et al. Increased Circulating miR-21 Levels Are Associated with Kidney Fibrosis. PLoS ONE 2013, 8, e58014. [Google Scholar] [CrossRef]
- Saejong, S.; Townamchai, N.; Somparn, P.; Tangtanatakul, P.; Ondee, T.; Hirankarn, N.; Leelahavanichkul, A. MicroRNA-21 in plasma exosome, but not from whole plasma, as a biomarker for the severe interstitial fibrosis and tubular atrophy (IF/TA) in post-renal transplantation. Asian Pac. J. Allergy Immunol. 2022, 40, 94–102. [Google Scholar]
- Vahed, S.Z.; Zonouzi, A.P.; Ghanbarian, H.; Ghojazadeh, M.; Samadi, N.; Omidi, Y.; Ardalan, M. Differential expression of circulating miR-21, miR-142-3p and miR-155 in renal transplant recipients with impaired graft function. Int. Urol. Nephrol. 2017, 49, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kwan, B.C.-H.; Lai, F.M.-M.; Chow, K.-M.; Li, P.K.-T.; Szeto, C.-C. Urinary miR-21, miR-29, and miR-93: Novel Biomarkers of Fibrosis. Am. J. Nephrol. 2012, 36, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Gniewkiewicz, M.S.; Paszkowska, I.; Gozdowska, J.; Czerwinska, K.; Sadowska-Jakubowicz, A.; Deborska-Materkowska, D.; Perkowska-Ptasinska, A.; Kosieradzki, M.; Durlik, M. Urinary MicroRNA-21-5p as Potential Biomarker of Interstitial Fibrosis and Tubular Atrophy (IFTA) in Kidney Transplant Recipients. Diagnostics 2020, 10, 113. [Google Scholar] [CrossRef]
- Vahed, S.Z.; Omidi, Y.; Ardalan, M.; Samadi, N. Dysregulation of urinary miR-21 and miR-200b associated with interstitial fibrosis and tubular atrophy (IFTA) in renal transplant recipients. Clin. Biochem. 2017, 50, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Kim, D.R. pcr: An R package for quality assessment, analysis and testing of qPCR data. PeerJ 2018, 6, e4473. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafè, M. miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef]
- Ghorbanmehr, N.; Gharbi, S.; Korsching, E.; Tavallaei, M.; Einollahi, B.; Mowla, S.J. miR-21-5p, miR-141-3p, and miR-205-5p levels in urine-promising biomarkers for the identification of prostate and bladder cancer. Prostate 2019, 79, 88–95. [Google Scholar] [CrossRef]
- Peters, L.; Floege, J.; Biessen, E.; Jankowski, J.; Van Der Vorst, E. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. Int. J. Mol. Sci. 2020, 21, 6547. [Google Scholar] [CrossRef]
- Hennino, M.-F.; Buob, D.; Van Der Hauwaert, C.; Gnemmi, V.; Jomaa, Z.; Pottier, N.; Savary, G.; Drumez, E.; Noël, C.; Cauffiez, C.; et al. miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci. Rep. 2016, 6, 27209. [Google Scholar] [CrossRef]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediat. Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef]
- Meng, X.-M.; Huang, X.R.; Xiao, J.; Chung, A.C.; Qin, W.; Chen, H.-Y.; Lan, H.Y. Disruption of Smad4 impairs TGF-β/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int. 2012, 81, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Maxwell, A.P.; Simpson, D.A.; McKay, G.J. Differential Expression of Urinary Exosomal MicroRNAs miR-21-5p and miR-30b-5p in Individuals with Diabetic Kidney Disease. Sci. Rep. 2019, 9, 10900. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Cheng, Y. MiR-126-3p inhibits ovarian cancer proliferation and invasion via targeting PLXNB2. Reprod. Biol. 2018, 18, 218–224. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Fujita, K.; Tomiyama, E.; Hatano, K.; Hayashi, Y.; Wang, C.; Ishizuya, Y.; Yamamoto, Y.; Hayashi, T.; Kato, T.; et al. MiR-30b-3p and miR-126-3p of urinary extracellular vesicles could be new biomarkers for prostate cancer. Transl. Androl. Urol. 2021, 10, 1918–1927. [Google Scholar] [CrossRef]
- Manganelli, M.; Grossi, I.; Ferracin, M.; Guerriero, P.; Negrini, M.; Ghidini, M.; Senti, C.; Ratti, M.; Pizzo, C.; Passalacqua, R.; et al. Longitudinal Circulating Levels of miR-23b-3p, miR-126-3p and lncRNA GAS5 in HCC Patients Treated with Sorafenib. Biomedicines 2021, 9, 813. [Google Scholar] [CrossRef]
- Li, J.; Ping, J.L.; Ma, B.; Chen, Y.R.; Li, L.Q. Deregulation of miR-126-3p in basal-like breast cancers stroma and its clinical significance. Pathol.-Res. Pr. 2017, 213, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.-S.; Zhang, S.; Cheng, Z.-M.; Yu, J.-L.; Zhou, S.; Song, J. MiR-126-3p suppresses the growth, migration and invasion of NSCLC via targeting CCR1. Eur. Rev. Med Pharmacol. Sci. 2019, 23, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.E.-S.; Abdelaleem, A.H.; Alhanafy, A.M.; Ibrahem, R.A.L.; Elhaded, A.S.A.; Assar, M.F.A. Circulating miR-21-5p and miR-126-3p: Diagnostic, prognostic value, and multivariate analysis in non-small-cell lung cancer. Mol. Biol. Rep. 2021, 48, 2543–2552. [Google Scholar] [CrossRef]
- Chen, J.; Ding, C.; Yang, X.; Zhao, J. BMSCs-Derived Exosomal MiR-126-3p Inhibits the Viability of NSCLC Cells by Targeting PTPN9. J. BU ON. Off. J. Balk. Union Oncol. 2021, 26, 1832–1841. [Google Scholar]
- Sibilano, M.; Tullio, V.; Adorno, G.; Savini, I.; Gasperi, V.; Catani, M.V. Platelet-Derived miR-126-3p Directly Targets AKT2 and Exerts Anti-Tumor Effects in Breast Cancer Cells: Further Insights in Platelet-Cancer Interplay. Int. J. Mol. Sci. 2022, 23, 5484. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.P.; Tingle, S.J.; Shuttleworth, V.G.; Cooke, K.; Redgrave, R.E.; Singh, E.; Glover, E.K.; Tajuddin, H.B.A.; Kirby, J.A.; Arthur, H.M.; et al. MiR-126-3p Is Dynamically Regulated in Endothelial-to-Mesenchymal Transition during Fibrosis. Int. J. Mol. Sci. 2021, 22, 8629. [Google Scholar] [CrossRef]
- Motshwari, D.D.; George, C.; Matshazi, D.M.; Weale, C.J.; Davids, S.F.G.; Zemlin, A.E.; Erasmus, R.T.; Kengne, A.P.; Matsha, T.E. Expression of whole blood miR-126-3p, -30a-5p, -1299, -182-5p and -30e-3p in chronic kidney disease in a South African community-based sample. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.-K.; Kiss, A.; Oszwald, A.; Nagel, F.; Acar, E.; Aliabadi-Zuckermann, A.; Hackl, M.; Zuckermann, A.; Kain, R.; Jakubowski, A.; et al. Single Donor Infusion of S-Nitroso-Human-Serum-Albumin Attenuates Cardiac Isograft Fibrosis and Preserves Myocardial Micro-RNA-126-3p in a Murine Heterotopic Heart Transplant Model. Transpl. Int. 2022, 35, 10057. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, Y.; Xie, H.; Liu, C.; Liu, P. MicroRNA-101 inhibits renal tub-ular epithelial-to-mesenchymal transition by targeting TGF-β1 type I receptor. Int. J. Mol. Med. 2021, 47, 119. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Wang, X.-L.; Yang, Y.-C.; Zhang, B.; Wu, Y.-B. Upregulated miR-101 inhibits acute kidney injury–chronic kidney disease transition by regulating epithelial–mesenchymal transition. Hum. Exp. Toxicol. 2020, 39, 1628–1638. [Google Scholar] [CrossRef]
- Ding, H.; Xu, Y.; Jiang, N. Upregulation of miR-101a Suppresses Chronic Renal Fibrosis by Regulating KDM3A via Blockade of the YAP-TGF-β-Smad Signaling Pathway. Mol. Ther. Nucleic Acids 2020, 19, 1276–1289. [Google Scholar] [CrossRef]
- Luo, W.; Li, G.; Yi, Z.; Nie, Q.; Zhang, X. E2F1-miR-20a-5p/20b-5p auto-regulatory feedback loop involved in myoblast proliferation and differentiation. Sci. Rep. 2016, 6, 27904. [Google Scholar] [CrossRef]
- Godwin, J.G.; Ge, X.; Stephan, K.; Jurisch, A.; Tullius, S.G.; Iacomini, J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc. Natl. Acad. Sci. USA 2010, 107, 14339–14344. [Google Scholar] [CrossRef]
- Ye, M.; Wang, S.; Sun, P.; Qie, J. Integrated MicroRNA Expression Profile Reveals Dysregulated miR-20a-5p and miR-200a-3p in Liver Fibrosis. BioMed Res. Int. 2021, 2021, 9583932. [Google Scholar] [CrossRef]
- Du, X.; Pan, Z.; Li, Q.; Liu, H.; Li, Q. SMAD4 feedback regulates the canonical TGF-β signaling pathway to control granulosa cell apoptosis. Cell Death Dis. 2018, 9, 151. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, S.; Tang, H.; Zhang, D.; Sun, H.; Yu, F.; Jiang, W.; Yue, B.; Wang, J.; Zhang, M.; et al. MicroRNA-20a-5p promotes colorectal cancer invasion and metastasis by downregulating Smad4. Oncotarget 2016, 7, 45199–45213. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Jin, Y.; Yang, Y.; Shi, J.; Chen, F.; Han, S.; Chu, P.; Lu, J.; Wang, H.; et al. MiR-20a-5p suppresses tumor proliferation by targeting autophagy-related gene 7 in neuroblastoma. Cancer Cell Int. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Mishra, L.; Deng, C.-X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018, 14, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Dardare, J.; Witz, A.; Merlin, J.-L.; Gilson, P.; Harlé, A. SMAD4 and the TGFβ Pathway in Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 3534. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 Expression by TGF-β1 Promotes Collagen Expression and Renal Fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef]
- Bowen, T.; Jenkins, R.H.; Fraser, D.J. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J. Pathol. 2013, 229, 274–285. [Google Scholar] [CrossRef]
- Qin, W.; Chung, A.C.; Huang, X.R.; Meng, X.-M.; Hui, D.; Yu, C.-M.; Sung, J.J.Y.; Lan, H.Y. TGF-β/Smad3 Signaling Promotes Renal Fibrosis by Inhibiting miR-29. J. Am. Soc. Nephrol. 2011, 22, 1462–1474. [Google Scholar] [CrossRef]
- Van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Hedayat, A.F.; Kanasaki, K.; Goodwin, J.E. microRNA Crosstalk Influences Epithelial-to-Mesenchymal, Endothelial-to-Mesenchymal, and Macrophage-to-Mesenchymal Transitions in the Kidney. Front. Pharmacol. 2019, 10, 904. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Gao, J.; Wang, M.; Li, X.; Cui, Z.; Fu, G. Long Noncoding RNA Tug1 Promotes Angiotensin II–Induced Renal Fibrosis by Binding to Mineralocorticoid Receptor and Negatively Regulating MicroR-29b-3p. Hypertension 2021, 78, 693–705. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Li, J.; Ye, Y.; Yu, W.-Q. rAAV9-mediated supplementation of miR-29b improve angiotensin-II induced renal fibrosis in mice. Mol. Med. 2021, 27, 89. [Google Scholar] [CrossRef] [PubMed]
- Gondaliya, P.; Dasare, A.P.; Jash, K.; Tekade, R.K.; Srivastava, A.; Kalia, K. miR-29b attenuates histone deacetylase-4 mediated podocyte dysfunction and renal fibrosis in diabetic nephropathy. J. Diabetes Metab. Disord. 2020, 19, 13–27. [Google Scholar] [CrossRef]
- Rubiś, P.; Totoń-Żurańska, J.; Kołton-Wróż, M.; Wołkow, P.; Pitera, E.; Wiśniowska-Śmiałek, S.; Dziewięcka, E.; Garlitski, A.; Podolec, P. Twelve-month kinetics of circulating fibrosis-linked microRNAs (miR-21, miR-29, miR-30, and miR-133a) and the relationship with extracellular matrix fibrosis in dilated cardiomyopathy. Arch. Med Sci. 2022, 15, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; He, Q.; Chen, Y.; Yu, D.; Sun, L.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. miR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J. Cell. Physiol. 2019, 234, 9746–9755. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Ruan, Z.-B.; Song, G.-X.; Chen, G.-C.; Wang, F.; Wang, M.-X.; Yuan, M.-K.; Zhu, L. miR-15a-5p regulates myocardial fibrosis in atrial fibrillation by targeting Smad7. PeerJ 2021, 9, e12686. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, X.; Du, G.; Zhang, Z.; Zhai, Y.; Xiong, X.; Luo, X. MicroRNA-122-5p Inhibition Improves Inflammation and Oxidative Stress Damage in Dietary-Induced Non-alcoholic Fatty Liver Disease Through Targeting FOXO3. Front. Physiol. 2022, 13, 803445. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z.; Huang, Z.; Zou, D.; Li, J.; Feng, P. MiR-122-5p promotes peritoneal fibrosis in a rat model of peritoneal dialysis by targeting Smad5 to activate Wnt/β-catenin pathway. Ren. Fail. 2022, 44, 191–203. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Z.-J.; Song, J.-W.; Liang, L.-R.; Sun, L.-L.; Liu, X.-Y.; Miao, R.; Xu, Y.-L.; Li, X.-T.; Zhang, M.-W.; et al. MicroRNA-122–5p promotes renal fibrosis and injury in spontaneously hypertensive rats by targeting FOXO3. Exp. Cell Res. 2022, 411, 113017. [Google Scholar] [CrossRef]
- Dieter, C.; Assmann, T.S.; Costa, A.R.; Canani, L.H.; De Souza, B.M.; Bauer, A.C.; Crispim, D. MiR-30e-5p and MiR-15a-5p Expressions in Plasma and Urine of Type 1 Diabetic Patients with Diabetic Kidney Disease. Front. Genet. 2019, 10, 563. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, C.; Ye, C.; Zhu, S.; Chen, X.; Wu, W.K.; Qian, W. miR-10a-5p, miR-99a-5p and miR-21-5p are steroid-responsive circulating microRNAs. Am. J. Transl. Res. 2018, 10, 1490–1497. [Google Scholar]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. Cardiovascular Disease-Associated MicroRNAs as Novel Biomarkers of First-Trimester Screening for Gestational Diabetes Mellitus in the Absence of Other Pregnancy-Related Complications. Int. J. Mol. Sci. 2022, 23, 10635. [Google Scholar] [CrossRef] [PubMed]
| Variable | CAD n = 20 | Control n = 20 | p-Value |
|---|---|---|---|
| Age (year) | 53 (10.7) | 55.3 (8.3) | 0.399 |
| Gender (Male), % | 8 (40%) | 15 (75%) | 0.025 |
| Donor, % | 1.000 | ||
| Cadaveric KT | 15 (75%) | 16 (80%) | |
| Living KT | 5 (25%) | 4 (20%) | |
| Graft Survival (year) | 17.5 ± 4.4 | 17 ± 3.8 | 0.754 |
| eGFR (ml/min) | 43.9 ± 9.1 | 74.4 ± 7.3 | 0.0 |
| Creatinine (mg/dL) | 1.6 ± 0.3 | 1 ± 0.1 | 0.0 |
| UPCR (unit) | 685.8 ± 1646.7 | 127.2 ± 75 | 0.161 |
| Tacrolimus (mg/day) | 5.7 ± 2.9 | 3.6 ± 1.8 | 0.026 |
| FK-506 level (mg/dL) | 5.7 ± 0.9 | 5.1 ± 1.2 | 0.829 |
| Cyclosporin (mg/day) | (n = 1, 100 mg/day) | 150 ± 35.35 | 0.206 * |
| CSA level (mg/dL) | (n = 1, 77.4 mg/dL) | 84.6 ± 23.69 | 0.480 * |
| Prednisolone (mg/day) | 5.6 ± 3.4 | 3.9 ± 2.2 | 0.150 |
| Malignancy (Positive), % | 2 (10%) | 5 (25%) | 0.407 |
| ESRD causes | |||
| IgA nephropathy | 1 | 4 | <0.05 |
| Herb nephropathy | 0 | 3 | NS |
| FSGS | 3 | 1 | NS |
| ADPKD | 0 | 3 | NS |
| Lupus Nephropathy | 3 | 0 | NS |
| NSAID Nephropathy | 1 | 1 | NS |
| Hypertensive Nephropathy | 2 | 0 | NS |
| Undiagnosed GN | 9 | 8 | NS |
| MPGN | 2 | 0 | NS |
| DM | 1 | 0 | NS |
| VUR | 1 | 0 | NS |
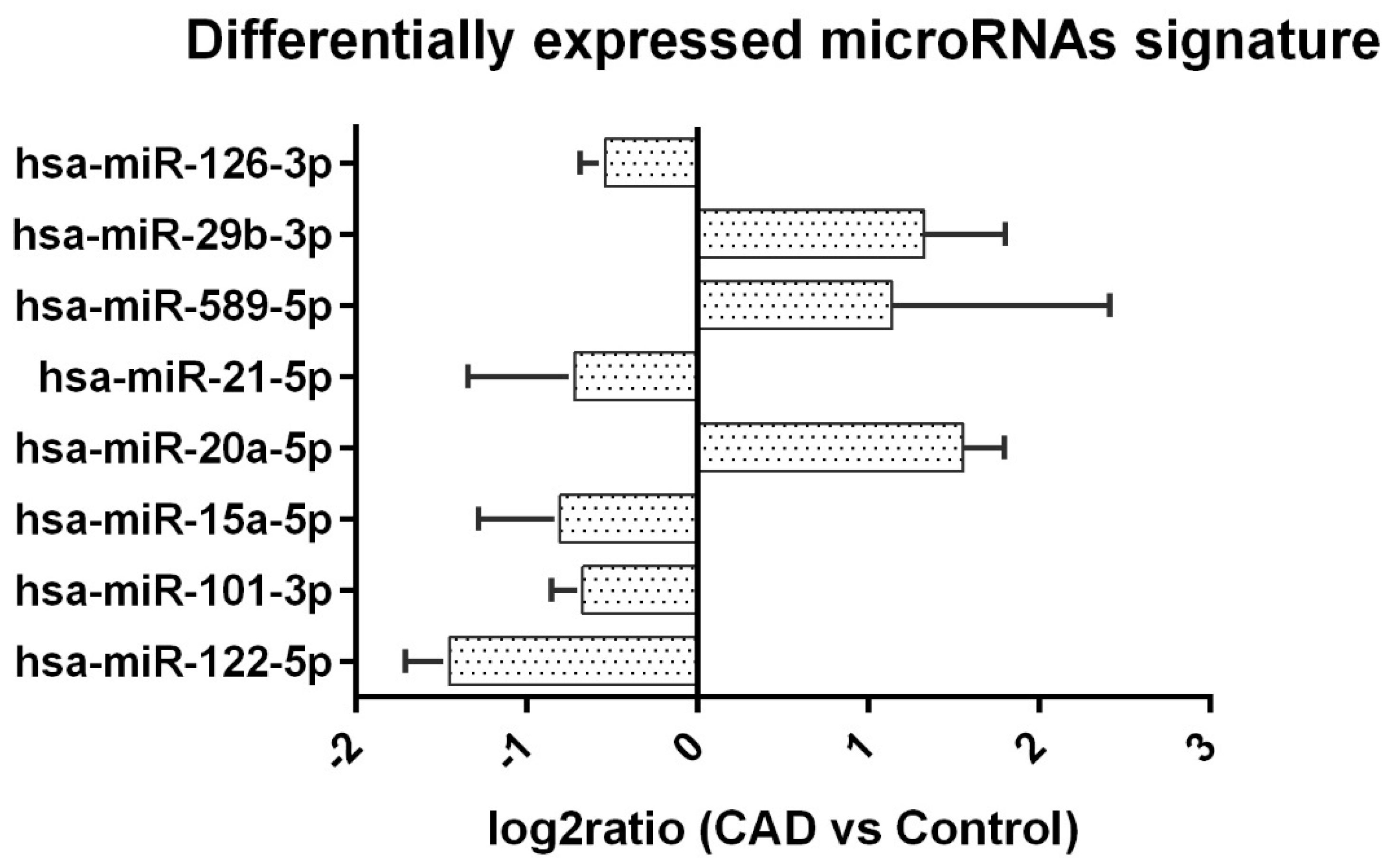
| Variate | miRNA Level (Mean Ct) | log2Ratio | p Value | |||
|---|---|---|---|---|---|---|
| CAD | N | Control (n = 20) | N | |||
| hsa-miR-122-5p | 28.80 ± 1.19 | 19 | 27.34 ± 1.96 | 19 | −1.45 ± 0.26 | 0.0097 |
| hsa-miR-101-3p | 26.87 ± 1.13 | 20 | 26.20 ± 0.66 | 20 | −0.67 ± 0.18 | 0.0288 |
| hsa-miR-15a-5p | 28.09 ± 1.38 | 20 | 27.28 ± 1.60 | 20 | −0.80 ± 0.47 | 0.0246 |
| hsa-miR-20a-5p | 28.73 ± 1.18 | 15 | 30.29 ± 1.05 | 7 | 1.55 ± 0.24 | 0.0132 |
| hsa-miR-21-5p | 26.92 ± 0.64 | 20 | 26.20 ± 0.42 | 20 | −0.72 ± 0.62 | 0.0002 |
| hsa-miR-589-5p | 28.55 ± 0.41 | 9 | 29.68 ± 0.90 | 5 | 1.13 ± 1.27 | 0.0077 |
| hsa-miR-29b-3p | 29.42 ± 1.39 | 16 | 30.75 ± 1.72 | 12 | 1.32 ± 0.47 | 0.0329 |
| hsa-miR-126-3p | 27.89 ± 0.90 | 20 | 27.35 ± 0.59 | 20 | −0.54 ± 0.14 | 0.0317 |
| GO Term | Gene Ratio | Bg Ratio | p Value |
|---|---|---|---|
| Response to abiotic stimulus | 108/386 | 441/2689 | 3.00 × 10−10 |
| Extracellular matrix organization | 49/386 | 142/2689 | 4.04 × 10−10 |
| Extracellular structure organization | 49/386 | 142/2689 | 4.04 × 10−10 |
| External encapsulating structure organization | 49/386 | 142/2689 | 4.04 × 10−10 |
| Response to lipid | 94/386 | 367/2689 | 4.87 × 10−10 |
| Muscle cell proliferation | 43/386 | 120/2689 | 1.39 × 10−9 |
| Regulation of cellular component movement | 105/386 | 440/2689 | 3.06 × 10−9 |
| Tube development | 112/386 | 482/2689 | 4.06 × 10−9 |
| Negative regulation of signal transduction | 109/386 | 477/2689 | 2.05 × 10−8 |
| Neuron death | 52/386 | 178/2689 | 8.26 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Hsu, C.-T.; Tsai, S.-F.; Chen, C.-H. Association between Circulating MicroRNAs (miR-21-5p, miR-20a-5p, miR-29b-3p, miR-126-3p and miR-101-3p) and Chronic Allograft Dysfunction in Renal Transplant Recipients. Int. J. Mol. Sci. 2022, 23, 12253. https://doi.org/10.3390/ijms232012253
Chen Y-J, Hsu C-T, Tsai S-F, Chen C-H. Association between Circulating MicroRNAs (miR-21-5p, miR-20a-5p, miR-29b-3p, miR-126-3p and miR-101-3p) and Chronic Allograft Dysfunction in Renal Transplant Recipients. International Journal of Molecular Sciences. 2022; 23(20):12253. https://doi.org/10.3390/ijms232012253
Chicago/Turabian StyleChen, Yu-Jen, Chia-Tien Hsu, Shang-Feng Tsai, and Cheng-Hsu Chen. 2022. "Association between Circulating MicroRNAs (miR-21-5p, miR-20a-5p, miR-29b-3p, miR-126-3p and miR-101-3p) and Chronic Allograft Dysfunction in Renal Transplant Recipients" International Journal of Molecular Sciences 23, no. 20: 12253. https://doi.org/10.3390/ijms232012253
APA StyleChen, Y.-J., Hsu, C.-T., Tsai, S.-F., & Chen, C.-H. (2022). Association between Circulating MicroRNAs (miR-21-5p, miR-20a-5p, miR-29b-3p, miR-126-3p and miR-101-3p) and Chronic Allograft Dysfunction in Renal Transplant Recipients. International Journal of Molecular Sciences, 23(20), 12253. https://doi.org/10.3390/ijms232012253







