Proteomics Unveils Post-Mortem Changes in Beef Muscle Proteins and Provides Insight into Variations in Meat Quality Traits of Crossbred Young Steers and Heifers Raised in Feedlot
Abstract
1. Introduction
2. Results
2.1. Carcass Traits, Chemical Composition and Meat Quality
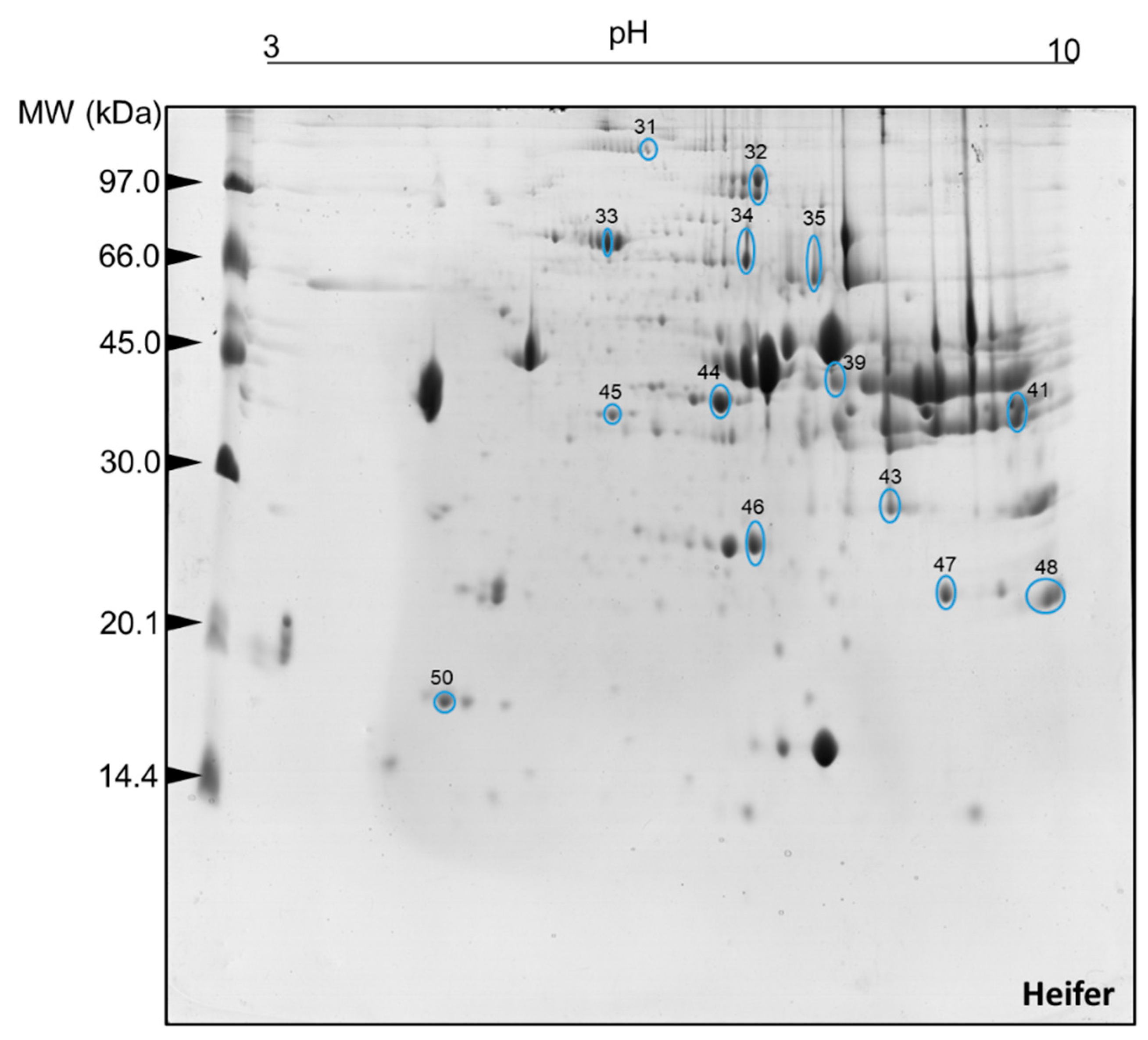
2.2. Muscle Tissue Proteome
| Spot ID | Gene Symbol | Full Protein Names | Uniprot ID | Mascot Score | Coverage (%) | pI/MW Experimental | pI/MW Theoretical | Expression 1 |
|---|---|---|---|---|---|---|---|---|
| Energy metabolism | ||||||||
| 16 | ENO1 | Phosphopyruvate hydratase | A0A3Q1MXQ0 | 5261.196 | 47.00 | 6.17/49,703 | 6.48/54,808 | 1.95 (UP in steers) |
| 18 | ENO3 | Beta-enolase | Q3ZC09 | 10,199.91 | 57.60 | 8.19/44,261 | 7.55/47,438 | 5.51 (UP in steers) |
| 32 | PYGM | Alpha 1–4 glucan phosphorylase | F1MJ28 | 7318.068 | 63.42 | 7.09/97,750 | 7.05/97,735 | 1.13 (UP in steers) |
| 34 | PGM1 | Phosphoglucomutase-1 | Q08DP0 | 9944.941 | 78.11 | 6.99/65,655 | 7.21/61,874 | 1.89 (UP in steers) |
| 46 | TPI1 | Triosephosphate isomerase | Q5E956 | 21,146.33 | 88.35 | 7.29/25,236 | 7.27/26,917 | 1.28 (UP in steers) |
| 47 | AK1 | Adenylate kinase isoenzyme 1 | P00570 | 12,755.28 | 58.76 | 8.71/22,375 | 8.35/21,778 | 1.73 (UP in steers) |
| 35 | PKM | Pyruvate kinase | A5D984 | 12,176.24 | 63.09 | 7.7/60,091 | 7.1/58,519 | 1.37 (UP in heifers) |
| 39 | ALDOA | Fructose-bisphosphate aldolase | A6QLL8 | 7590.983 | 70.33 | 7.73/42,863 | 7.94/39,949 | 2.20 (UP in heifers) |
| 41 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | P10096 | 30,163.61 | 47.75 | 9.19/34,988 | 8.97/36,096 | 4.01 (UP in heifers) |
| 19 | CKM | Creatine kinase M-type | Q9XSC6 | 16,598.07 | 70.60 | 7.02/43,080 | 6.8/43,217 | 1.89 (UP in heifers) |
| 14 | ATP5F1B | ATP synthase subunit beta, mitochondrial | P00829 | 12,307.51 | 56.44 | 5.05/51,780 | 5.04/56,283 | 2.07 (UP in steers) |
| Muscle structure | ||||||||
| 31 | MYBPC1 | Myosin binding protein C1 | A6QP89 | 1502.969 | 33.97 | 6.23/113,152 | 6.5/135,007 | 4.07 (UP in heifers) |
| 30 | TPM2 | Tropomyosin beta chain | Q5KR48 | 5317.557 | 44.01 | 4.33/40,213 | 4.55/32,950 | 1.44 (UP in steers) |
| 45 | TNNT1 | Troponin T, slow skeletal muscle | Q8MKH6 | 3035.667 | 19.39 | 5.88/37,343 | 5.67/31,284 | 2.49 (UP in heifers) |
| 48 | TNNI2 | Troponin I2, fast skeletal muscle | F6QIC1 | 3650.82 | 54.49 | 9.44/21,323 | 9.78/21,141 | 2.66 (UP in heifers) |
| 44 | TNNT3 | Troponin T, fast skeletal muscle | Q8MKI4 | 1410.723 | 36.9 | 6.84/39,524 | 6.85/32,124 | 1.37 (UP in steers) |
| 15 | ACTA1 | Actin, alpha skeletal muscle | P68138 | 29,409.83 | 81.96 | 5.28/42,998 | 5.23/42,393 | 2.50 (UP in steers) |
| Binding proteins | ||||||||
| 13 | TF | Serotransferrin | Q29443 | 270.5198 | 17.19 | 6.58/82,905 | 6.6/79,920 | 1.74 (UP in heifers) |
| 33 | ALB | Albumin | P02769 | 13261.69 | 64.91 | 5.97/69,477 | 5.87/71289 | 1.19 (UP in heifers) |
| 50 | MB | Myoglobin | A0A1K0FUF3 | 11,507.29 | 56.49 | 4.49/17,121 | 7.80/17077 | 3.65 (UP in heifers) |
| 28 | PEBP1 | Phosphatidylethanolamine binding protein 1 | P13696 | 6676.657 | 64.17 | 7.94/21,444 | 7.82/21099 | 2.08 (UP in steers) |
| 43 | CA3 | Carbonic Anhydrase 3 | Q3SZX4 | 10,936.17 | 60.00 | 8.37/27,806 | 8.1/29655 | 5.24 (UP in heifers) |
| Heat shock proteins | ||||||||
| 12 | HSPA8 | Heat shock cognate 71 kDa protein | P19120 | 19,088.86 | 44.92 | 5.46/73,533 | 5.25/71468 | 2.59 (UP in heifers) |

3. Discussion
3.1. Proteins with Possible Roles in Intramuscular Fat Content and Meat Quality
3.2. Key Roles of Oxidative Stress and Cell Defense
3.3. Muscle Structure, Contractile and Associated Proteins
3.4. Limitations
4. Materials and Methods
4.1. Animals, Carcass Traits and Muscle/Meat Sampling
4.2. Chemical Composition of Meat
4.3. pH and Meat Color
4.4. Cooking Loss and Shear Force
4.5. Proteomics
4.5.1. Extraction and Precipitation of Proteins
4.5.2. Protein Separation by Two-Dimensional Electrophoresis (2D-PAGE)
4.5.3. Tryptic Digestion of Protein Spots and Identification of Proteins by ESI-MS/MS
4.5.4. Bioinformatics
4.5.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, M.; Ripoll, G.; Delavaud, C.; Casasús, I. Performance, carcass and meat quality of young bulls, steers and heifers slaughtered at a common body weight. Livest. Sci. 2020, 240, 104156. [Google Scholar] [CrossRef]
- Mueller, L.F.; Balieiro, J.C.D.C.; Ferrinho, A.; Martins, T.; Corte, R.R.P.D.S.; De Amorim, T.R.; Furlan, J.D.J.M.; Baldi, F.; Pereira, A.S.C. Gender status effect on carcass and meat quality traits of feedlot Angus × Nellore cattle. Anim. Sci. J. 2019, 90, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Andreo, N.; Bridi, A.M.; Soares, A.L.; Prohmann, P.E.F.; Peres, L.M.; Tarsitano, M.A.; Giangareli, B.D.L.; Takabayashi, A.A. Fatty acid profile of beef from immunocastrated (BOPRIVA ®) Nellore bulls. Meat Sci. 2016, 117, 12–17. [Google Scholar] [CrossRef]
- Miguel, G.Z.; Faria, M.H.; Roca, R.; Santos, C.T.; Suman, S.; Faitarone, A.B.; Delbem, N.L.; Girao, L.; Homem, J.M.; Barbosa, E.K.; et al. Immunocastration improves carcass traits and beef color attributes in Nellore and Nellore×Aberdeen Angus crossbred animals finished in feedlot. Meat Sci. 2014, 96, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.C. Novilho superprecoce: Técnicas de nutrição e manejo. Simpósio Goiano sobre Manejo e Nutrição de bovinos de corte e leite. Goiânia Colégio Bras. De Nutr. Anim. 2003, 5, 153–166. [Google Scholar]
- Needham, T.; Lambrechts, H.; Hoffman, L. Castration of male livestock and the potential of immunocastration to improve animal we. S. Afr. J. Anim. Sci. 2017, 47, 731. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.C.; Mullen, A.M.; Franco, D.; Warner, R.D.; Lorenzo, J.M.; Purslow, P.P.; Gerrard, D.; Hopkins, D.L.; Troy, D.; et al. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi-platform proteomics studies. Meat Sci. 2021, 172, 108311. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Zhu, Y. Proteomics Advances in Beef Production. In Food Proteomics; Academic Press: Cambridge, MA, USA, 2022; pp. 151–182. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, C.; Richardson, I.; Hocquette, J.-F.; Picard, B. The associations between proteomic biomarkers and beef tenderness depend on the end-point cooking temperature, the country origin of the panelists and breed. Meat Sci. 2019, 157, e107871. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Micol, D.; Cassar-Malek, I.; Hocquette, J.F.; Terlouw, C.E. Inverse relationships between biomarkers and beef tenderness according to contractile and metabolic properties of the muscle. J. Agric. Food Chem. 2014, 62, 9808–9818. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Picard, B. Proteomics to Explain and Predict Meat Quality; Woodhead Publishing: UK, 2022; pp. 393–431. [Google Scholar] [CrossRef]
- Munekata, P.E.; Pateiro, M.; López-Pedrouso, M.; Gagaoua, M.; Lorenzo, J.M. Foodomics in meat quality. Curr. Opin. Food Sci. 2021, 38, 79–85. [Google Scholar] [CrossRef]
- Purslow, P.P.; Gagaoua, M.; Warner, R.D. Insights on meat quality from combining traditional studies and proteomics. Meat Sci. 2020, 174, 108423. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M. Meta-proteomics for the discovery of protein biomarkers of beef tenderness: An overview of integrated studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M. Proteomic Investigations of Beef Tenderness. In Proteomics in Food Science: From Farm to Fork; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Joseph, P.; Nair, M.N.; Suman, S.P. Application of proteomics to characterize and improve color and oxidative stability of muscle foods. Food Res. Int. 2015, 76, 938–945. [Google Scholar] [CrossRef]
- Gagaoua, M.; Hughes, J.; Terlouw, E.C.; Warner, R.D.; Purslow, P.P.; Lorenzo, J.M.; Picard, B. Proteomic biomarkers of beef colour. Trends Food Sci. Technol. 2020, 101, 234–252. [Google Scholar] [CrossRef]
- Yang, B.; Liu, X. Application of proteomics to understand the molecular mechanisms determining meat quality of beef muscles during postmortem aging. PLoS ONE 2021, 16, e0246955. [Google Scholar] [CrossRef]
- Di Luca, A.; Mullen, A.M.; Elia, G.; Davey, G.; Hamill, R. Centrifugal drip is an accessible source for protein indicators of pork ageing and water-holding capacity. Meat Sci. 2011, 88, 261–270. [Google Scholar] [CrossRef]
- Gagaoua, M.; Warner, R.D.; Purslow, P.; Ramanathan, R.; Mullen, A.M.; López-Pedrouso, M.; Franco, D.; Lorenzo, J.M.; Tomasevic, I.; Picard, B.; et al. Dark-cutting beef: A brief review and an integromics meta-analysis at the proteome level to decipher the underlying pathways. Meat Sci. 2021, 181, 108611. [Google Scholar] [CrossRef]
- De Andrade, T.S.; Albertini, T.Z.; Barioni, L.G.; De Medeiros, S.R.; Millen, D.D.; Dos Santos, A.C.R.; Goulart, R.S.; Lanna, D.P.D. Perception of consultants, feedlot owners, and packers regarding management and marketing decisions on feedlots: A national survey in Brazil (Part II). Can. J. Anim. Sci. 2020, 100, 759–770. [Google Scholar] [CrossRef]
- Ferraz, J.B.S.; de Felício, P.E. Production systems—An example from Brazil. Meat Sci. 2010, 84, 238–243. [Google Scholar] [CrossRef]
- Bazile, J.; Picard, B.; Chambon, C.; Valais, A.; Bonnet, M. Pathways and biomarkers of marbling and carcass fat deposition in bovine revealed by a combination of gel-based and gel-free proteomic analyses. Meat Sci. 2019, 156, 146–155. [Google Scholar] [CrossRef]
- Silva, L.; Rodrigues, R.; Assis, D.E.; Benedeti, P.D.; Duarte, M.S.; Chizzotti, M. Explaining meat quality of bulls and steers by differential proteome and phosphoproteome analysis of skeletal muscle. J. Proteom. 2019, 199, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Poleti, M.D.; Regitano, L.C.A.; Souza, G.H.M.F.; Cesar, A.S.M.; Simas, R.C.; Silva-Vignato, B.; Oliveira, G.B.; Andrade, S.C.S.; Cameron, L.C.; Coutinho, L.L.; et al. Longissimus dorsi muscle label-free quantitative proteomic reveals biological mechanisms associated with intramuscular fat deposition. J. Proteom. 2018, 179, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Dashty, M. A quick look at biochemistry: Carbohydrate metabolism. Clin. Biochem. 2013, 46, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef]
- Baldassini, W.A.; Braga, C.P.; Chardulo, L.A.L.; Silva, J.A.I.V.; Malheiros, J.M.; de Albuquerque, L.G.; Fernandes, T.T.; Padilha, P.D.M. Bioanalytical methods for the metalloproteomics study of bovine longissimus thoracis muscle tissue with different grades of meat tenderness in the Nellore breed (Bos indicus). Food Chem. 2015, 57, 363–370. [Google Scholar] [CrossRef]
- Antonelo, D.S.; Gómez, J.F.; Silva, S.L.; Beline, M.; Zhang, X.; Wang, Y.; Pavan, B.; Koulicoff, L.A.; Rosa, A.F.; Goulart, R.S.; et al. Proteome basis for the biological variations in color and tenderness of longissimus thoracis muscle from beef cattle differing in growth rate and feeding regime. Food Res. Int. 2022, 153, 110947. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Muscle Fiber Properties in Cattle and Their Relationships with Meat Qualities: An Overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Al-Jammas, M.; De Koning, L.; Valais, A.; Bonnet, M. Beef tenderness and intramuscular fat proteomic biomarkers: Muscle type effect. PeerJ 2018, 6, e4891. [Google Scholar] [CrossRef]
- Wu, W.; Gao, X.-G.; Dai, Y.; Fu, Y.; Li, X.-M.; Dai, R.-T. Post-mortem changes in sarcoplasmic proteome and its relationship to meat color traits in M. semitendinosus of Chinese Luxi yellow cattle. Food Res. Int. 2015, 72, 98–105. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zan LSen Wang, H.B.; Xin, Y.P.; Adoligbe, C.M.; Ujan, J.A. Effect of sex on meat quality characteristics of Qinchuan cattle. Afr. J. Biotechnol. 2010, 9, 28. [Google Scholar] [CrossRef]
- Shen, Y.N.; Kim, S.H.; Yoon, D.H.; Lee, H.G.; Kang, H.S.; Seo, K.S. Proteome Analysis of Bovine Longissimus dorsi Muscle Associated with the Marbling Score. Asian-Australasian J. Anim. Sci. 2012, 25, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Couvreur, S.; Le Bec, G.; Aminot, G.; Picard, B. Associations among Protein Biomarkers and pH and Color Traits in Longissimus thoracis and Rectus abdominis Muscles in Protected Designation of Origin Maine-Anjou Cull Cows. J Agric Food Chem 2017, 65, 3569–3580. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Zhao, D.; He, G.; Nian, Y.; Da, D.; Yan, J.; Li, C. Acetylation and Phosphorylation of Proteins Affect Energy Metabolism and Pork Quality. J. Agric. Food Chem. 2020, 68, 7259–7268. [Google Scholar] [CrossRef] [PubMed]
- della Malva, A.; Gagaoua, M.; Santillo, A.; De Palo, P.; Sevi, A.; Albenzio, M. First insights about the underlying mechanisms of Martina Franca donkey meat tenderization during aging: A proteomic approach. Meat Sci. 2022, 193, 108925. [Google Scholar] [CrossRef]
- Nelson, D.; Cox, M. Lehninger—Principios de Bioquimica, 5th ed.; Freeman, W.H., Ed.; MACMILLAN: New York Basingstoke, NY, USA, 2009. [Google Scholar]
- Ryu, Y.; Kim, B. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005, 71, 351–357. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Higher drip loss is associated with protein oxidation. Meat Sci. 2012, 90, 917–924. [Google Scholar] [CrossRef]
- Cho, J.H.; Jeong, J.Y.; Lee, R.H.; Na Park, M.; Kim, S.-H.; Park, S.-M.; Shin, J.-C.; Jeon, Y.-J.; Shim, J.-H.; Choi, N.-J.; et al. Regional Differences of Proteins Expressing in Adipose Depots Isolated from Cows, Steers and Bulls as Identified by a Proteomic Approach. Asian-Australasian J. Anim. Sci. 2016, 29, 1197–1206. [Google Scholar] [CrossRef]
- Stricher, F.; Macri, C.; Ruff, M.; Muller, S. HSPA8/HSC70 chaperone protein. Autophagy 2013, 9, 1937–1954. [Google Scholar] [CrossRef]
- Chen, E.; Xue, D.; Zhang, W.; Lin, F.; Pan, Z. Extracellular heat shock protein 70 promotes osteogenesis of human mesenchymal stem cells through activation of the ERK signaling pathway. FEBS Lett. 2015, 589, 4088–4096. [Google Scholar] [CrossRef]
- Gagaoua, M.; Monteils, V.; Couvreur, S.; Picard, B. Identification of Biomarkers Associated with the Rearing Practices, Carcass Characteristics, and Beef Quality: An Integrative Approach. J. Agric. Food Chem. 2017, 65, 8264–8278. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Zolla, L. Meat science: From proteomics to integrated omics towards system biology. J. Proteom. 2013, 78, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, L.; Lebret, B.; Ecolan, P.; Louveau, I.; Damon, M.; Prunier, A.; Billon, Y.; Sellier, P.; Gilbert, H. Muscle characteristics and meat quality traits are affected by divergent selection on residual feed intake in pigs1. J. Anim. Sci. 2011, 89, 996–1010. [Google Scholar] [CrossRef]
- Paim, T.D.P.; Viana, P.; Van Tilburg, M.F.; Moura, A.D.A.; De Souza, J.R.; McManus, C.; Abdalla, A.L.; Louvandini, H. Feeding effects of cottonseed and its co-products on the meat proteome from ram lambs. Sci. Agricola 2019, 76, 463–472. [Google Scholar] [CrossRef]
- Lana, A.; Zolla, L. Proteolysis in meat tenderization from the point of view of each single protein: A proteomic perspective. J. Proteom. 2016, 147, 85–97. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.M.C.; Boudjellal, A.; Picard, B. Coherent correlation networks among protein biomarkers of beef tenderness: What they reveal. J. Proteom. 2015, 128, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Aldana, S.; Beggs, M.; Birkey, J.; Conquest, A.; Conway, R.; Hemminger, T.; Herrick, J.; Hurley, C.; Ionita, C.; et al. Determination of Fat, Moisture, and Protein in Meat and Meat Products by Using the FOSS FoodScan Near-Infrared Spectrophotometer with FOSS Artificial Neural Network Calibration Model and Associated Database: Collaborative Study. J. AOAC Int. 2007, 90, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.L.; Koohmaraie, M.; Cundiff, L.V.; Dikeman, M.E. Effects of cooking and shearing methodology on variation in Warner-Bratzler shear force values in beef2. J. Anim. Sci. 1994, 72, 2325–2330. [Google Scholar] [CrossRef]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat; American Meat Science Association Educational Foundation: Savoy, IL, USA, 2015. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Paredi, G.; Mori, F.; de Marino, M.G.; Raboni, S.; Marchi, L.; Galati, S.; Buschini, A.; Fiego, D.P.L.; Mozzarelli, A. Is the protein profile of pig Longissimus dorsi affected by gender and diet? J. Proteom. 2019, 206, 103437. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Götz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2012, 41, D808–D815. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Troy, D.; Mullen, A.M. The Extent and Rate of the Appearance of the Major 110 and 30 kDa Proteolytic Fragments during Post-Mortem Aging of Beef Depend on the Glycolysing Rate of the Muscle and Aging Time: An LC–MS/MS Approach to Decipher Their Proteome and Associated Pathways. J. Agric. Food Chem. 2021, 69, 602–614. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Al Jammas, M.; Bonnet, M. Beef tenderness and intramuscular fat proteomic biomarkers: Effect of gender and rearing practices. J. Proteom. 2019, 200, 1–10. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; Ellies-Oury, M.-P.; De Koning, L.; Picard, B. Reverse phase protein arrays for the identification/validation of biomarkers of beef texture and their use for early classification of carcasses. Food Chem. 2018, 250, 245–252. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; De Koning, L.; Picard, B. Reverse Phase Protein array for the quantification and validation of protein biomarkers of beef qualities: The case of meat color from Charolais breed. Meat Sci. 2018, 145, 308–319. [Google Scholar] [CrossRef]




| Variables 1 | Heifers | Steers | SEM | p-Value |
|---|---|---|---|---|
| IBW (kg) | 289.40 | 284.00 | 9.57 | 0.78 |
| FBW (kg) | 373.75 | 391.13 | 10.41 | 0.57 |
| HCW (kg) | 202.18 | 219.25 | 5.97 | 0.09 |
| CY (%) | 54.81 | 55.82 | 0.00 | 0.53 |
| BFT (mm) | 14.90 | 10.15 | 1.10 | 0.04 |
| REA (cm2) | 75.99 | 90.48 | 1.66 | 0.06 |
| Variables 1 | Heifers | Steers | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ageing (Days) | Ageing (Days) | SEM | Gender | Ageing Time | |||||
| 3 | 10 | 17 | 3 | 10 | 17 | ||||
| L* | 33.76 | 34.78 | 36.54 | 32.94 | 34.50 | 34.84 | 0.49 | 0.24 | 0.06 |
| a* | 16.26 | 16.55 | 16.73 | 15.94 | 16.73 | 15.99 | 0.14 | 0.45 | 0.53 |
| b* | 6.08 | 6.18 | 6.53 | 5.93 | 6.21 | 6.05 | 0.08 | 0.41 | 0.63 |
| pH | 5.60 | 5.71 | 5.73 | 5.60 | 5.68 | 5.75 | 0.02 | 0.65 | <0.01 |
| WBSF (N) | 55.70 | 40.40 | 31.28 | 54.42 | 40.79 | 37.26 | 3.62 | 0.42 | <0.01 |
| EL (%) | 24.27 | 20.43 | 17.10 | 21.46 | 17.47 | 17.14 | 0.01 | 0.01 | <0.01 |
| DL (%) | 6.69 | 4.49 | 4.28 | 7.13 | 6.54 | 5.22 | 0.00 | 0.02 | 0.003 |
| CL (%) | 30.96 | 24.92 | 21.38 | 28.59 | 24.01 | 22.36 | 0.01 | 0.25 | <0.01 |
| QTL Linked to QTLdb 1 | Gene Symboles | UniProtID (Bovine) | Chr. |
|---|---|---|---|
| Marbling score (n = 4) | HSPA8; MYBPC1; PGM1; PYGM | A6QP89; P19120; P79334; Q08DP0 | Chr.15; Chr.5; Chr.3; Chr.29 |
| Fat thickness at the 12th rib (n = 4) | ATP5F1B; MB; MYBPC1; PGM1 | P00829; P02192; A6QP89; Q08DP0 | Chr.5; Chr.5; Chr.5; Chr.3 |
| Intramuscular fat (n = 1) | ATP5F1B | P00829 | Chr.5; |
| Juiciness (n = 2) | ENO1; PYGM | P79334; Q9XSJ4 | Chr.16; Chr.29 |
| Shear force (n = 5) | ALB; ALDOA; HSPA8; PEBP1; PYGM | P02769; A6QLL8; P19120; P13696; P79334 | Chr.6; Chr.25; Chr.15; Chr.17; Chr.29 |
| Tenderness score (n = 2) | ALDOA; PYGM | A6QLL8; P79334 | Chr.25; Chr.29 |
| Adhesion (n = 1) | TPM2 | Q5KR48 | Chr.8 |
| Muscle pH (n = 1) | PKM | A5D984 | Chr.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severino, M.; Gagaoua, M.; Baldassini, W.; Ribeiro, R.; Torrecilhas, J.; Pereira, G.; Curi, R.; Chardulo, L.A.; Padilha, P.; Neto, O.M. Proteomics Unveils Post-Mortem Changes in Beef Muscle Proteins and Provides Insight into Variations in Meat Quality Traits of Crossbred Young Steers and Heifers Raised in Feedlot. Int. J. Mol. Sci. 2022, 23, 12259. https://doi.org/10.3390/ijms232012259
Severino M, Gagaoua M, Baldassini W, Ribeiro R, Torrecilhas J, Pereira G, Curi R, Chardulo LA, Padilha P, Neto OM. Proteomics Unveils Post-Mortem Changes in Beef Muscle Proteins and Provides Insight into Variations in Meat Quality Traits of Crossbred Young Steers and Heifers Raised in Feedlot. International Journal of Molecular Sciences. 2022; 23(20):12259. https://doi.org/10.3390/ijms232012259
Chicago/Turabian StyleSeverino, Mariane, Mohammed Gagaoua, Welder Baldassini, Richard Ribeiro, Juliana Torrecilhas, Guilherme Pereira, Rogério Curi, Luis Artur Chardulo, Pedro Padilha, and Otávio Machado Neto. 2022. "Proteomics Unveils Post-Mortem Changes in Beef Muscle Proteins and Provides Insight into Variations in Meat Quality Traits of Crossbred Young Steers and Heifers Raised in Feedlot" International Journal of Molecular Sciences 23, no. 20: 12259. https://doi.org/10.3390/ijms232012259
APA StyleSeverino, M., Gagaoua, M., Baldassini, W., Ribeiro, R., Torrecilhas, J., Pereira, G., Curi, R., Chardulo, L. A., Padilha, P., & Neto, O. M. (2022). Proteomics Unveils Post-Mortem Changes in Beef Muscle Proteins and Provides Insight into Variations in Meat Quality Traits of Crossbred Young Steers and Heifers Raised in Feedlot. International Journal of Molecular Sciences, 23(20), 12259. https://doi.org/10.3390/ijms232012259









