Individual and Combined Effect of Bisphenol A and Bisphenol AF on Prostate Cell Proliferation through NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Results
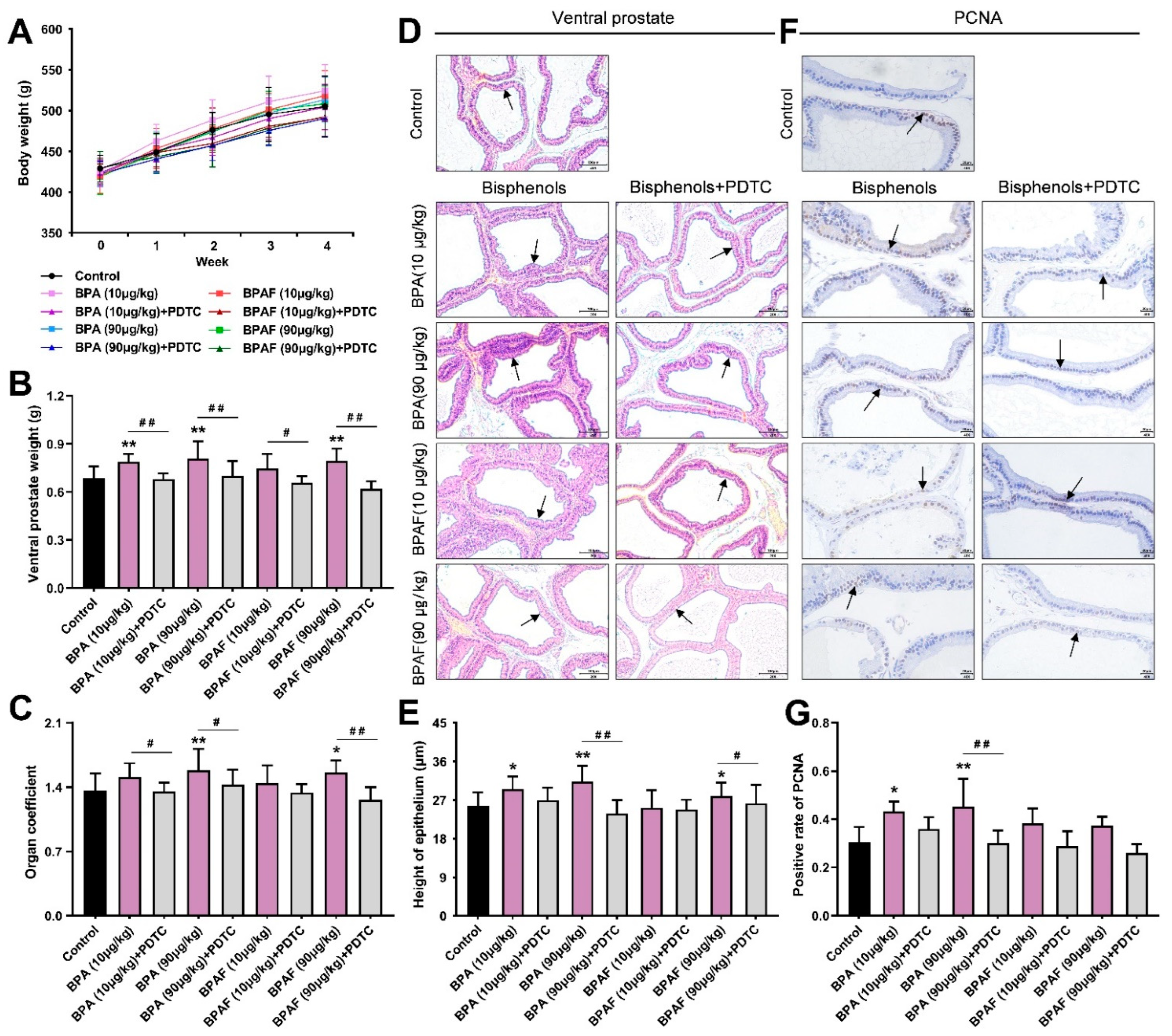
2.1. Anatomical Analysis of Prostates in the Rats Treated with BPA, BPAF and PDTC
2.2. BPA- and BPAF-Facilitated Pathological Hyperplasia of the Prostate in Rats
2.3. Localization and Qualification of the Critical Modulators of NF-κB Signaling Pathway
2.4. BPA and BPAF Stimulated Expressions of NF-κB p65, COX-2, TNF-α and EGFR
2.5. Combined Exposure to BPA and BPAF Upregulated Prostate Cell Viability
2.6. Inhibition of NF-κB Inhibited Proliferation and Stimulative Apoptosis of Prostate Cells
2.7. In Vitro Validation of the Modulation of NF-κB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
4.2. Pathological Evaluation
4.3. IHC Analysis
4.4. Tissue-Based ELISA
4.5. Cell Cultivation
4.6. Cell Proliferation Assay
4.7. Cell Apoptosis Detection
4.8. Cell-Based ELISA
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, Z.; Liu, Y.; Yan, K.; Wu, S.; Han, Z.; Guo, R.; Chen, M.; Yang, Q.; Zhang, S.; Chen, J. Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: Occurrence, distribution, source apportionment, and ecological and human health risk. Chemosphere 2017, 184, 318–328. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, Q.; Zhang, M.; Zhang, N.; Zhang, W.; Zhan, M.; Xu, W.; Lu, L.; Fan, J.; Wang, Q. Occurrence of bisphenol A and its alternatives in paired urine and indoor dust from Chinese university students: Implications for human exposure. Chemosphere 2020, 247, 125987. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Niu, Y.; Wang, B.; Liu, J.; Zhao, Y.; Zhang, J.; Wang, Y.; Shao, B. Estimation of lactating mothers’ daily intakes of bisphenol A using breast milk. Environ. Pollut. 2021, 286, 117545. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Kolossa-Gehring, M.; Schroter-Kermani, C.; Angerer, J.; Bruning, T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: A retrospective exposure evaluation. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, G.; Lee, P.M.Y.; Zhao, S.; Ho, W.M.; Lam, A.T.; Lee, M.K.; Poon, P.K.M.; Ng, S.S.M.; Li, W.; He, Y.; et al. Joint effect between bisphenol A and alcohol consumption on benign prostatic hyperplasia: A case-control study in Hong Kong Chinese males. Prostate 2021, 81, 1214–1224. [Google Scholar] [CrossRef]
- Taylor, J.A.; Jones, M.B.; Besch-Williford, C.L.; Berendzen, A.F.; Ricke, W.A.; vom Saal, F.S. Interactive effects of perinatal BPA or DES and adult testosterone and estradiol exposure on adult urethral obstruction and bladder, kidney, and prostate pathology in male mice. Int. J. Mol. Sci. 2020, 21, 3902. [Google Scholar] [CrossRef]
- Olukole, S.G.; Ajani, S.O.; Ola-Davies, E.O.; Lanipekun, D.O.; Aina, O.O.; Oyeyemi, M.O.; Oke, B.O. Melatonin ameliorates bisphenol A-induced perturbations of the prostate gland of adult Wistar rats. Biomed. Pharm. 2018, 105, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.S.; Galvao, A.L.; Rodriguez, D.A.; Biancardi, M.F.; Marques, M.R.; Vilamaior, P.S.; Santos, F.C.; Taboga, S.R. Prepubertal exposure to bisphenol-A induces ERα upregulation and hyperplasia in adult gerbil female prostate. Int. J. Exp. Pathol. 2015, 96, 188–195. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Zhou, P.; Su, X.; Yang, R.; Shao, C.; Wu, J. Bisphenol A exposure triggers the malignant transformation of prostatic hyperplasia in beagle dogs via cfa-miR-204/KRAS axis. Ecotoxicol. Environ. Saf. 2022, 235, 113430. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Liu, F. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 2021, 262, 127880. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, L.; Meng, X.; Qin, H.; Xiang, Z.; Gong, W.; Luo, W.; Li, D.; Han, X. Chronic exposure to microcystin-LR increases the risk of prostate cancer and induces malignant transformation of human prostate epithelial cells. Chemosphere 2021, 263, 128295. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan, J. Bisphenol-A induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef]
- Yin, Z.; Hua, L.; Chen, L.; Hu, D.; Li, J.; An, Z.; Tian, T.; Ning, H.; Ge, Y. Bisphenol-A exposure induced neurotoxicity and associated with synapse and cytoskeleton in Neuro-2a cells. Toxicol. In Vitro 2020, 67, 104911. [Google Scholar] [CrossRef] [PubMed]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-a review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Li, J.; Chen, M.; Peng, D.; Liang, Y.; Song, M.; Zhang, J.; Jiang, G. Exposure to bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2016, 31, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiao, Z.; Zheng, S.; Li, M.; Zhang, J.; Feng, Y.; Yin, J.; Shao, B. Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere 2015, 128, 252–257. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, J.; Jiao, Z.; Shi, J.; Li, M.; Shao, B. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol. Lett. 2012, 211, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Li, Y.; Zhang, Y.; Liang, S.; Li, S.; Liu, D. Comprehensive analysis based in silico study of alternative bisphenols—Environmental explanation of prostate cancer progression. Toxicology 2022, 465, 153051. [Google Scholar] [CrossRef]
- Prins, G.S.; Hu, W.Y.; Shi, G.B.; Hu, D.P.; Majumdar, S.; Li, G.; Huang, K.; Nelles, J.L.; Ho, S.M.; Walker, C.L.; et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 2014, 155, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Hu, W.Y.; Xie, L.; Shi, G.B.; Hu, D.P.; Birch, L.; Bosland, M.C. Evaluation of bisphenol A (BPA) exposures on prostate stem cell homeostasis and prostate cancer risk in the NCTR-Sprague-Dawley rat: An NIEHS/FDA CLARITY-BPA consortium study. Environ. Health Perspect. 2018, 126, 117001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Burns, K.A.; Arao, Y.; Luh, C.J.; Korach, K.S. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ. Health Perspect. 2012, 120, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Huang, D.; Su, X.; Yan, H.; Ma, A.; Li, L.; Wu, J.; Sun, Z. The prostaglandin synthases, COX-2 and L-PGDS, mediate prostate hyperplasia induced by low-dose bisphenol A. Sci. Rep. 2020, 10, 13108. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, D.; Su, X.; Yan, H.; Wu, J.; Sun, Z. Oral exposure to low-dose bisphenol A induces hyperplasia of dorsolateral prostate and upregulates EGFR expression in adult Sprague-Dawley rats. Toxicol. Ind. Health 2019, 35, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, M.P.; Maihofner, C.; Bhambra, U.; Lightfoot, T.; Gooderham, N.J. The Colorectal Cancer Study Group. Upregulation of cyclooxygenase-2 is accompanied by increased expression of nuclear factor-κB and IκB kinase-α in human colorectal cancer epithelial cells. Br. J. Cancer 2003, 88, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Pal, A.; Kumar, R.; Dwivedi, P.D.; Das, M.; Ansari, K.M. EGFR-mediated Akt and MAPKs signal pathways play a crucial role in patulin-induced cell proliferation in primary murine keratinocytes via modulation of Cyclin D1 and COX-2 expression. Mol. Carcinog. 2014, 53, 988–998. [Google Scholar] [CrossRef]
- Austin, D.C.; Strand, D.W.; Love, H.L.; Franco, O.E.; Jang, A.; Grabowska, M.M.; Miller, N.L.; Hameed, O.; Clark, P.E.; Fowke, J.H.; et al. NF-κB and androgen receptor variant expression correlate with human BPH progression. Prostate 2016, 76, 491–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pande, V.; Sharma, R.K.; Inoue, J.-I.; Otsuka, M.; Ramos, M.J. A molecular modeling study of inhibitors of nuclear factor kappa-B (p50)—DNA binding. J. Comput. Aided Mol. Des. 2003, 17, 825–836. [Google Scholar] [CrossRef]
- Ho, S.M.; Rao, R.; To, S.; Schoch, E.; Tarapore, P. Bisphenol A and its analogues disrupt centrosome cycle and microtubule dynamics in prostate cancer. Endocr. Relat. Cancer 2017, 24, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rodriguez, D.; Franssen, D.; Bakker, J.; Lomniczi, A.; Parent, A.S. Cellular and molecular features of EDC exposure: Consequences for the GnRH network. Nat. Rev. Endocrinol. 2021, 17, 83–96. [Google Scholar] [CrossRef]
- Manfo, F.P.; Jubendradass, R.; Nantia, E.A.; Moundipa, P.F.; Mathur, P.P. Adverse effects of bisphenol A on male reproductive function. Rev. Environ. Contam. Toxicol. 2014, 228, 57–82. [Google Scholar] [PubMed]
- Prins, G.S.; Ye, S.H.; Birch, L.; Zhang, X.; Cheong, A.; Lin, H.; Calderon-Gierszal, E.; Groen, J.; Hu, W.Y.; Ho, S.M.; et al. Prostate cancer risk and DNA methylation signatures in aging rats following developmental BPA exposure: A dose-response analysis. Environ. Health Perspect. 2017, 125, 077007. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.L.; Ricke, E.A.; Liu, T.T.; Gerona, R.; MacGillivray, L.; Wang, Z.; Timms, B.G.; Bjorling, D.E.; Vom Saal, F.S.; Ricke, W.A. Bisphenol-A analogs induce lower urinary tract dysfunction in male mice. Biochem. Pharmacol. 2022, 197, 114889. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, T.; Yang, P.; Li, M.; Yang, Y.; Wang, Y.; Du, J.; Pan, K.; Zhang, K. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRgamma signals. Toxicol. In Vitro 2015, 30, 521–528. [Google Scholar] [PubMed]
- Skledar, D.G.; Carino, A.; Trontelj, J.; Troberg, J.; Distrutti, E.; Marchiano, S.; Tomasic, T.; Zega, A.; Finel, M.; Fiorucci, S.; et al. Endocrine activities and adipogenic effects of bisphenol AF and its main metabolite. Chemosphere 2019, 215, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Mentor, A.; Wann, M.; Brunstrom, B.; Jonsson, M.; Mattsson, A. Bisphenol AF and bisphenol F induce similar feminizing effects in chicken embryo testis as bisphenol A. Toxicol. Sci. 2020, 178, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xia, M.; Zhang, L.; Cheng, W.; Yan, J.; Sun, Y.; Wang, Y.; Jiang, H. Individual and combined effects of BPA, BPS and BPAF on the cardiomyocyte differentiation of embryonic stem cells. Ecotoxicol. Environ. Saf. 2021, 220, 112366. [Google Scholar] [CrossRef]
- de Miguel, M.P.; Royuela, M.; Bethencourt, F.R.; Santamaria, L.; Fraile, B.; Paniagua, R. Immunoexpression of tumour necrosis factor-α and its receptors 1 and 2 correlates with proliferation/apoptosis equilibrium in normal, hyperplasic and carcinomatous human prostate. Cytokine 2000, 12, 535–538. [Google Scholar] [CrossRef]
- Rohr, H.P.; Bartsch, G. Human benign prostatic hyperplasia: A stromal disease? New perspectives by quantitative morphology. Urology 1980, 16, 625–633. [Google Scholar] [CrossRef]
- Lim, A.; Shin, K.; Zhao, C.; Kawano, S.; Beachy, P.A. Spatially restricted Hedgehog signalling regulates HGF-induced branching of the adult prostate. Nat. Cell Biol. 2014, 16, 1135–1145. [Google Scholar] [CrossRef]
- Tarapore, P.; Ying, J.; Ouyang, B.; Burke, B.; Bracken, B.; Ho, S.M. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS ONE 2014, 9, e90332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Gierszal, E.L.; Prins, G.S. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol A exposure. PLoS ONE 2015, 10, e0133238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, W.; Zhou, M.; Xiang, Z.; Han, X.; Li, D. Combined effects of nonylphenol and bisphenol a on the human prostate epithelial cell line RWPE-1. Int. J. Environ. Res. Public Health 2015, 12, 4141–4155. [Google Scholar] [CrossRef] [PubMed]
- Pollock, T.; Weaver, R.E.; Ghasemi, R.; de Catanzaro, D. A mixture of five endocrine-disrupting chemicals modulates concentrations of bisphenol A and estradiol in mice. Chemosphere 2018, 193, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Akhtar, T.; Hameed, N.; Sheikh, N. In vivo assessment of bisphenol A induced histopathological alterations and inflammatory gene expression in lungs of male Wistar rats. Hum. Exp. Toxicol. 2021, 40, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, Z.; Ji, W. Bisphenol A induces apoptosis, oxidative stress and inflammatory response in colon and liver of mice in a mitochondria-dependent manner. Biomed. Pharmacother. 2019, 117, 109182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, L.; Chen, H.; Zhang, Y.; Zhang, Z.; Lin, Z.; Shi, M.; Zhang, W.; Li, X.; Tang, Z.; et al. Bisphenol F promotes the secretion of pro-inflammatory cytokines in macrophages by enhanced glycolysis through PI3K-AKT signaling pathway. Toxicol. Lett. 2021, 350, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, Y.Y.; Park, J.; Lee, Y. Korean Red Ginseng suppresses bisphenol A-induced expression of cyclooxygenase-2 and cellular migration of A549 human lung cancer cell through inhibition of reactive oxygen species. J. Ginseng. Res. 2021, 45, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Y.; Li, H.; Zhang, X. Low dose of bisphenol A activates NF-κB/IL-6 signals to increase malignancy of neuroblastoma cells. Cell Mol. Neurobiol. 2017, 37, 1095–1103. [Google Scholar] [CrossRef]
- Zang, L.; Tian, F.; Yao, Y.; Chen, Y.; Shen, Y.; Han, M.; Meng, Z.; Fan, S.; Zhang, X.; Cai, T.; et al. Qianliexin capsule exerts anti-inflammatory activity in chronic non-bacterial prostatitis and benign prostatic hyperplasia via NF-κB and inflammasome. J. Cell Mol. Med. 2021, 25, 5753–5768. [Google Scholar] [CrossRef]
- Xie, C.; Sun, X.; Chen, J.; Ng, C.F.; Lau, K.M.; Cai, Z.; Jiang, X.; Chan, H.C. Down-regulated CFTR during aging contributes to benign prostatic hyperplasia. J. Cell Physiol. 2015, 230, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, X. Positive and negative signaling components involved in TNFalpha-induced NF-kappaB activation. Cytokine 2008, 41, 1–8. [Google Scholar] [CrossRef]
- Chopra, D.P.; Menard, R.E.; Januszewski, J.; Mattingly, R.R. TNF-α-mediated apoptosis in normal human prostate epithelial cells and tumor cell lines. Cancer Lett. 2004, 203, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ramu, A.; Kathiresan, S.; Ramadoss, H.; Nallu, A.; Kaliyan, R.; Azamuthu, T. Gramine attenuates EGFR-mediated inflammation and cell proliferation in oral carcinogenesis via regulation of NF-kappaB and STAT3 signaling. Biomed. Pharm. 2018, 98, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Deng, Z.; Zhou, Z.; Jiang, B.; Jiang, C.Y.; Zhao, R.Z.; Sun, F.; Cui, D.; Sun, M.H.; Sun, Q.; et al. Heat injured stromal cells-derived exosomal EGFR enhances prostatic wound healing after thulium laser resection through EMT and NF-κB signaling. Prostate 2019, 79, 1238–1255. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Huang, D.; Zhou, P.; Su, X.; Yang, R.; Shao, C.; Ma, A.; Wu, J. Individual and Combined Effect of Bisphenol A and Bisphenol AF on Prostate Cell Proliferation through NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12283. https://doi.org/10.3390/ijms232012283
Wang K, Huang D, Zhou P, Su X, Yang R, Shao C, Ma A, Wu J. Individual and Combined Effect of Bisphenol A and Bisphenol AF on Prostate Cell Proliferation through NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(20):12283. https://doi.org/10.3390/ijms232012283
Chicago/Turabian StyleWang, Kaiyue, Dongyan Huang, Ping Zhou, Xin Su, Rongfu Yang, Congcong Shao, Aicui Ma, and Jianhui Wu. 2022. "Individual and Combined Effect of Bisphenol A and Bisphenol AF on Prostate Cell Proliferation through NF-κB Signaling Pathway" International Journal of Molecular Sciences 23, no. 20: 12283. https://doi.org/10.3390/ijms232012283




