Glucocorticoid-Regulated Kinase CAMKIγ in the Central Amygdala Controls Anxiety-like Behavior in Mice
Abstract
1. Introduction
2. Results
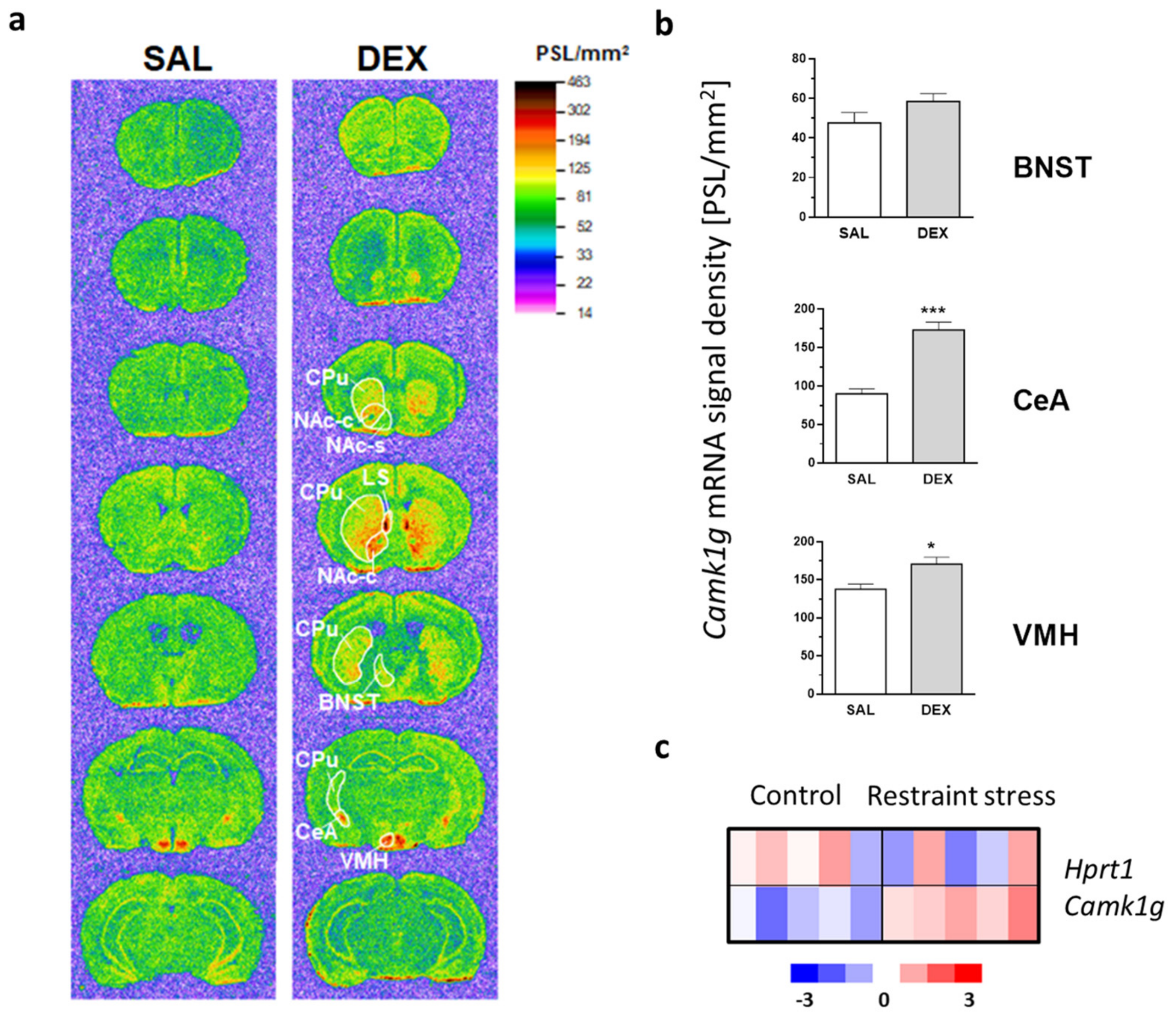
2.1. Camk1g Distribution, Regulation and Induction after Stress
2.2. Behavioral Consequences of Camk1g Knockdown in CeA
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Dexamethasone Administration and In Situ Hybridization
4.3. Restraint Stress and Microarray Analysis
4.4. Cloning and Lentiviral Vectors Generation
4.5. Primary Cultures
4.6. Stereotactic Surgery
4.7. Immunostaining of Transduced Brain Slices
4.8. Fear Conditioning
4.9. Light/Dark Box
4.10. Y Maze
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal Relationship Between Stressful Life Events and the Onset of Major Depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Davis, C.G.; Kendler, K.S. Childhood Adversity and Adult Psychiatric Disorder in the US National Comorbidity Survey. Psychol. Med. 1997, 27, 1101–1119. [Google Scholar] [CrossRef] [PubMed]
- Shalev, A.Y. Posttraumatic Stress Disorder and Stress-Related Disorders. Psychiatr. Clin. 2009, 32, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.J.G.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.M.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W.J.H. Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity: Results from a Large Cohort Study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Mackin, P. HPA Axis Function in Mood Disorders. Psychiatry 2006, 5, 166–170. [Google Scholar] [CrossRef]
- Daviu, N.; Bruchas, M.R.; Moghaddam, B.; Sandi, C.; Beyeler, A. Neurobiological Links between Stress and Anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Zhang, W.-H.; Zheng, Z.-H.; Zou, J.-X.; Liu, X.-X.; Huang, S.-H.; You, W.-J.; He, Y.; Zhang, J.-Y.; Wang, X.-D.; et al. Identification of a Prefrontal Cortex-to-Amygdala Pathway for Chronic Stress-Induced Anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef]
- Pêgo, J.M.; Sousa, J.C.; Almeida, O.F.X.; Sousa, N. Stress and the Neuroendocrinology of Anxiety Disorders. Curr. Top. Behav. Neurosci. 2010, 2, 97–117. [Google Scholar] [CrossRef]
- Rao, R.P.; Anilkumar, S.; McEwen, B.S.; Chattarji, S. Glucocorticoids Protect Against the Delayed Behavioral and Cellular Effects of Acute Stress on the Amygdala. Biol. Psychiatry 2012, 72, 466–475. [Google Scholar] [CrossRef]
- Kolber, B.J.; Roberts, M.S.; Howell, M.P.; Wozniak, D.F.; Sands, M.S.; Muglia, L.J. Central Amygdala Glucocorticoid Receptor Action Promotes Fear-Associated CRH Activation and Conditioning. Proc. Natl. Acad. Sci. USA 2008, 105, 12004–12009. [Google Scholar] [CrossRef]
- Wang, Q.; Verweij, E.W.E.; Krugers, H.J.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Distribution of the Glucocorticoid Receptor in the Human Amygdala; Changes in Mood Disorder Patients. Brain Struct. Funct. 2014, 219, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Piechota, M.; Korostynski, M.; Golda, S.; Ficek, J.; Jantas, D.; Barbara, Z.; Przewlocki, R. Transcriptional Signatures of Steroid Hormones in the Striatal Neurons and Astrocytes. BMC Neurosci. 2017, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Sunkin, S.M.; Ng, L.; Lau, C.; Dolbeare, T.; Gilbert, T.L.; Thompson, C.L.; Hawrylycz, M.; Dang, C. Allen Brain Atlas: An Integrated Spatio-Temporal Portal for Exploring the Central Nervous System. Nucleic Acids Res. 2013, 41, D996–D1008. [Google Scholar] [CrossRef] [PubMed]
- Skupio, U.; Tertil, M.; Bilecki, W.; Barut, J.; Korostynski, M.; Golda, S.; Kudla, L.; Wiktorowska, L.; Sowa, J.E.; Siwiec, M.; et al. Astrocytes Determine Conditioned Response to Morphine via Glucocorticoid Receptor-Dependent Regulation of Lactate Release. Neuropsychopharmacology 2020, 45, 404–415. [Google Scholar] [CrossRef]
- Cohen, S.M.; Ma, H.; Kuchibhotla, K.V.; Watson, B.O.; Buzsáki, G.; Froemke, R.C.; Tsien, R.W. Excitation-Transcription Coupling in Parvalbumin-Positive Interneurons Employs a Novel CaM Kinase-Dependent Pathway Distinct from Excitatory Neurons. Neuron 2016, 90, 292–307. [Google Scholar] [CrossRef]
- Takemoto-Kimura, S.; Terai, H.; Takamoto, M.; Ohmae, S.; Kikumura, S.; Segi, E.; Arakawa, Y.; Furuyashiki, T.; Narumiya, S.; Bito, H. Molecular Cloning and Characterization of CLICK-III/CaMKIgamma, a Novel Membrane-Anchored Neuronal Ca2+/Calmodulin-Dependent Protein Kinase (CaMK). J. Biol. Chem. 2003, 278, 18597–18605. [Google Scholar] [CrossRef]
- Dong, H.W.; Petrovich, G.D.; Swanson, L.W. Topography of Projections from Amygdala to Bed Nuclei of the Stria Terminalis. Brain Res. Brain Res. Rev. 2001, 38, 192–246. [Google Scholar] [CrossRef]
- Möller, C.; Wiklund, L.; Sommer, W.; Thorsell, A.; Heilig, M. Decreased Experimental Anxiety and Voluntary Ethanol Consumption in Rats Following Central but Not Basolateral Amygdala Lesions. Brain Res. 1997, 760, 94–101. [Google Scholar] [CrossRef]
- Szklarczyk, K.; Korostynski, M.; Golda, S.; Piechota, M.; Ficek, J.; Przewlocki, R. Endogenous Opioids Regulate Glucocorticoid-Dependent Stress-Coping Strategies in Mice. Neuroscience 2016, 330, 121–137. [Google Scholar] [CrossRef]
- Keifer, O.P.; Hurt, R.C.; Ressler, K.J.; Marvar, P.J. The Physiology of Fear: Reconceptualizing the Role of the Central Amygdala in Fear Learning. Physiology 2015, 30, 389–401. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Zhang, J.-Y.; Holmes, A.; Pan, B.-X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol. Psychiatry 2021, 89, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.M. Corticosteroids in Relation to Fear, Anxiety and Psychopathology. Neurosci. Biobehav. Rev. 2001, 25, 117–142. [Google Scholar] [CrossRef]
- Korostynski, M.; Piechota, M.; Dzbek, J.; Mlynarski, W.; Szklarczyk, K.; Ziolkowska, B.; Przewlocki, R. Novel Drug-Regulated Transcriptional Networks in Brain Reveal Pharmacological Properties of Psychotropic Drugs. BMC Genomics 2013, 14, 606. [Google Scholar] [CrossRef]
- Korostynski, M.; Piechota, M.; Kaminska, D.; Solecki, W.; Przewlocki, R. Morphine Effects on Striatal Transcriptome in Mice. Genome Biol. 2007, 8, R128. [Google Scholar] [CrossRef]
- Lisman, J.E.; Goldring, M.A. Feasibility of Long-Term Storage of Graded Information by the Ca2+/Calmodulin-Dependent Protein Kinase Molecules of the Postsynaptic Density. Proc. Natl. Acad. Sci. USA 1988, 85, 5320–5324. [Google Scholar] [CrossRef]
- Bito, H.; Deisseroth, K.; Tsien, R.W. CREB Phosphorylation and Dephosphorylation: A Ca2+-and Stimulus Duration–Dependent Switch for Hippocampal Gene Expression. Cell 1996, 87, 1203–1214. [Google Scholar] [CrossRef]
- Kang, H.; Sun, L.D.; Atkins, C.M.; Soderling, T.R.; Wilson, M.A.; Tonegawa, S. An Important Role of Neural Activity-Dependent CaMKIV Signaling in the Consolidation of Long-Term Memory. Cell 2001, 106, 771–783. [Google Scholar] [CrossRef]
- Rodriguez-López, J.; Arrojo, M.; Paz, E.; Páramo, M.; Costas, J. Identification of Relevant Hub Genes for Early Intervention at Gene Coexpression Modules with Altered Predicted Expression in Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 98, 109815. [Google Scholar] [CrossRef]
- Davis, M. The Role of the Amygdala in Fear and Anxiety. Annu. Rev. Neurosci. 1992, 15, 353–375. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Iwata, J.; Cicchetti, P.; Reis, D.J. Different Projections of the Central Amygdaloid Nucleus Mediate Autonomic and Behavioral Correlates of Conditioned Fear. J. Neurosci. Off. J. Soc. Neurosci. 1988, 8, 2517–2529. [Google Scholar] [CrossRef]
- Canteras, N.S.; Simerly, R.B.; Swanson, L.W. Organization of Projections from the Medial Nucleus of the Amygdala: A PHAL Study in the Rat. J. Comp. Neurol. 1995, 360, 213–245. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-W.; Swanson, L.W. Projections from Bed Nuclei of the Stria Terminalis, Posterior Division: Implications for Cerebral Hemisphere Regulation of Defensive and Reproductive Behaviors. J. Comp. Neurol. 2004, 471, 396–433. [Google Scholar] [CrossRef] [PubMed]
- Risold, P.Y.; Thompson, R.H.; Swanson, L.W. The Structural Organization of Connections between Hypothalamus and Cerebral Cortex. Brain Res. Brain Res. Rev. 1997, 24, 197–254. [Google Scholar] [CrossRef]
- Canteras, N.S. The Medial Hypothalamic Defensive System: Hodological Organization and Functional Implications. Pharmacol. Biochem. Behav. 2002, 71, 481–491. [Google Scholar] [CrossRef]
- Dielenberg, R.A.; Hunt, G.E.; McGregor, I.S. “When a Rat Smells a Cat”: The Distribution of Fos Immunoreactivity in Rat Brain Following Exposure to a Predatory Odor. Neuroscience 2001, 104, 1085–1097. [Google Scholar] [CrossRef]
- Fietta, P.; Fietta, P.; Delsante, G. Central Nervous System Effects of Natural and Synthetic Glucocorticoids. Psychiatry Clin. Neurosci. 2009, 63, 613–622. [Google Scholar] [CrossRef]
- Fox, A.S.; Oler, J.A.; Tromp, D.P.M.; Fudge, J.L.; Kalin, N.H. Extending the Amygdala in Theories of Threat Processing. Trends Neurosci. 2015, 38, 319–329. [Google Scholar] [CrossRef]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral Methods to Study Anxiety in Rodents. Dialogues Clin. Neurosci. 2017, 19, 181–191. [Google Scholar] [CrossRef]
- Zalcman, G.; Federman, N.; Romano, A. CaMKII Isoforms in Learning and Memory: Localization and Function. Front. Mol. Neurosci. 2018, 11, 445. [Google Scholar] [CrossRef]
- Srivastava, T.; Fortin, D.A.; Nygaard, S.; Kaech, S.; Sonenberg, N.; Edelman, A.M.; Soderling, T.R. Regulation of Neuronal MRNA Translation by CaM-Kinase I Phosphorylation of EIF4GII. J. Neurosci. 2012, 32, 5620–5630. [Google Scholar] [CrossRef] [PubMed]
- Young, W.S.; Bonner, T.I.; Brann, M.R. Mesencephalic Dopamine Neurons Regulate the Expression of Neuropeptide MRNAs in the Rat Forebrain. Proc. Natl. Acad. Sci. USA 1986, 83, 9827–9831. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McLaughlin, R.J.; Pan, B.; Fitzgerald, M.L.; Roberts, C.J.; Lee, T.T.-Y.; Karatsoreos, I.N.; Mackie, K.; Viau, V.; Pickel, V.M.; et al. Recruitment of Prefrontal Cortical Endocannabinoid Signaling by Glucocorticoids Contributes to Termination of the Stress Response. J. Neurosci. 2011, 31, 10506–10515. [Google Scholar] [CrossRef] [PubMed]
- Slezak, M.; Korostynski, M.; Gieryk, A.; Golda, S.; Dzbek, J.; Piechota, M.; Wlazlo, E.; Bilecki, W.; Przewlocki, R. Astrocytes Are a Neural Target of Morphine Action via Glucocorticoid Receptor-Dependent Signaling. Glia 2013, 61, 623–635. [Google Scholar] [CrossRef]
- Jantas-Skotniczna, D.; Kajta, M.; Lasoń, W. Memantine Attenuates Staurosporine-Induced Activation of Caspase-3 and LDH Release in Mouse Primary Neuronal Cultures. Brain Res. 2006, 1069, 145–153. [Google Scholar] [CrossRef]
- Tertil, M.; Skupio, U.; Barut, J.; Dubovyk, V.; Wawrzczak-Bargiela, A.; Soltys, Z.; Golda, S.; Kudla, L.; Wiktorowska, L.; Szklarczyk, K.; et al. Glucocorticoid Receptor Signaling in Astrocytes Is Required for Aversive Memory Formation. Transl. Psychiatry 2018, 8, 255. [Google Scholar] [CrossRef]
- Wietrzych, M.; Meziane, H.; Sutter, A.; Ghyselinck, N.; Chapman, P.F.; Chambon, P.; Krezel, W. Working Memory Deficits in Retinoid X Receptor Gamma-Deficient Mice. Learn. Mem. Learn. Mem. 2005, 12, 318–326. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piechota, M.; Skupio, U.; Borczyk, M.; Ziółkowska, B.; Gołda, S.; Szumiec, Ł.; Szklarczyk-Smolana, K.; Bilecki, W.; Rodriguez Parkitna, J.M.; Korostyński, M. Glucocorticoid-Regulated Kinase CAMKIγ in the Central Amygdala Controls Anxiety-like Behavior in Mice. Int. J. Mol. Sci. 2022, 23, 12328. https://doi.org/10.3390/ijms232012328
Piechota M, Skupio U, Borczyk M, Ziółkowska B, Gołda S, Szumiec Ł, Szklarczyk-Smolana K, Bilecki W, Rodriguez Parkitna JM, Korostyński M. Glucocorticoid-Regulated Kinase CAMKIγ in the Central Amygdala Controls Anxiety-like Behavior in Mice. International Journal of Molecular Sciences. 2022; 23(20):12328. https://doi.org/10.3390/ijms232012328
Chicago/Turabian StylePiechota, Marcin, Urszula Skupio, Małgorzata Borczyk, Barbara Ziółkowska, Sławomir Gołda, Łukasz Szumiec, Klaudia Szklarczyk-Smolana, Wiktor Bilecki, Jan Manuel Rodriguez Parkitna, and Michał Korostyński. 2022. "Glucocorticoid-Regulated Kinase CAMKIγ in the Central Amygdala Controls Anxiety-like Behavior in Mice" International Journal of Molecular Sciences 23, no. 20: 12328. https://doi.org/10.3390/ijms232012328
APA StylePiechota, M., Skupio, U., Borczyk, M., Ziółkowska, B., Gołda, S., Szumiec, Ł., Szklarczyk-Smolana, K., Bilecki, W., Rodriguez Parkitna, J. M., & Korostyński, M. (2022). Glucocorticoid-Regulated Kinase CAMKIγ in the Central Amygdala Controls Anxiety-like Behavior in Mice. International Journal of Molecular Sciences, 23(20), 12328. https://doi.org/10.3390/ijms232012328





