Coenzyme Q10 Attenuates Human Platelet Aggregation Induced by SARS-CoV-2 Spike Protein via Reducing Oxidative Stress In Vitro
Abstract
:1. Introduction
2. Results
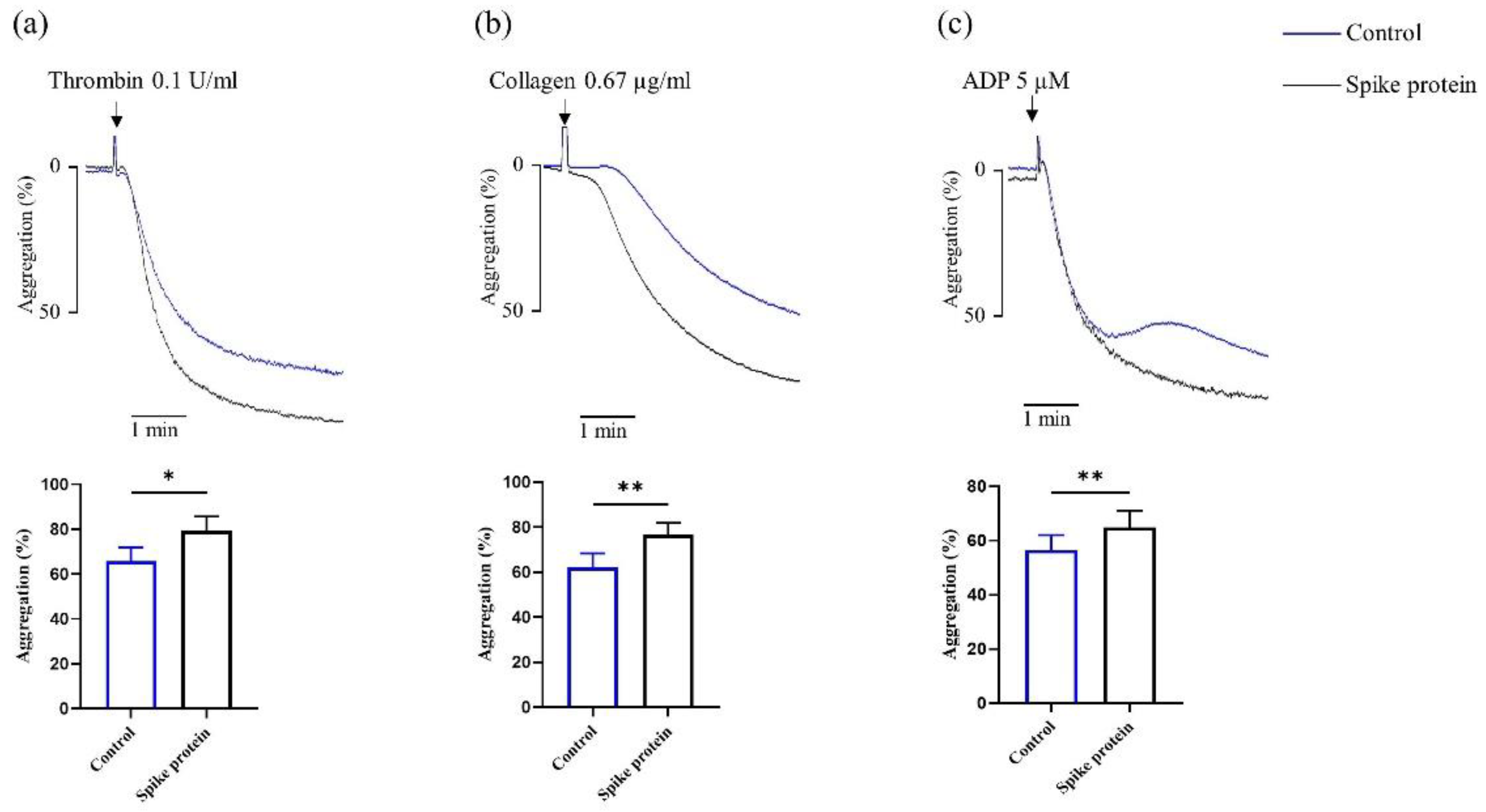
2.1. Spike Protein Potentiates Platelet Aggregation
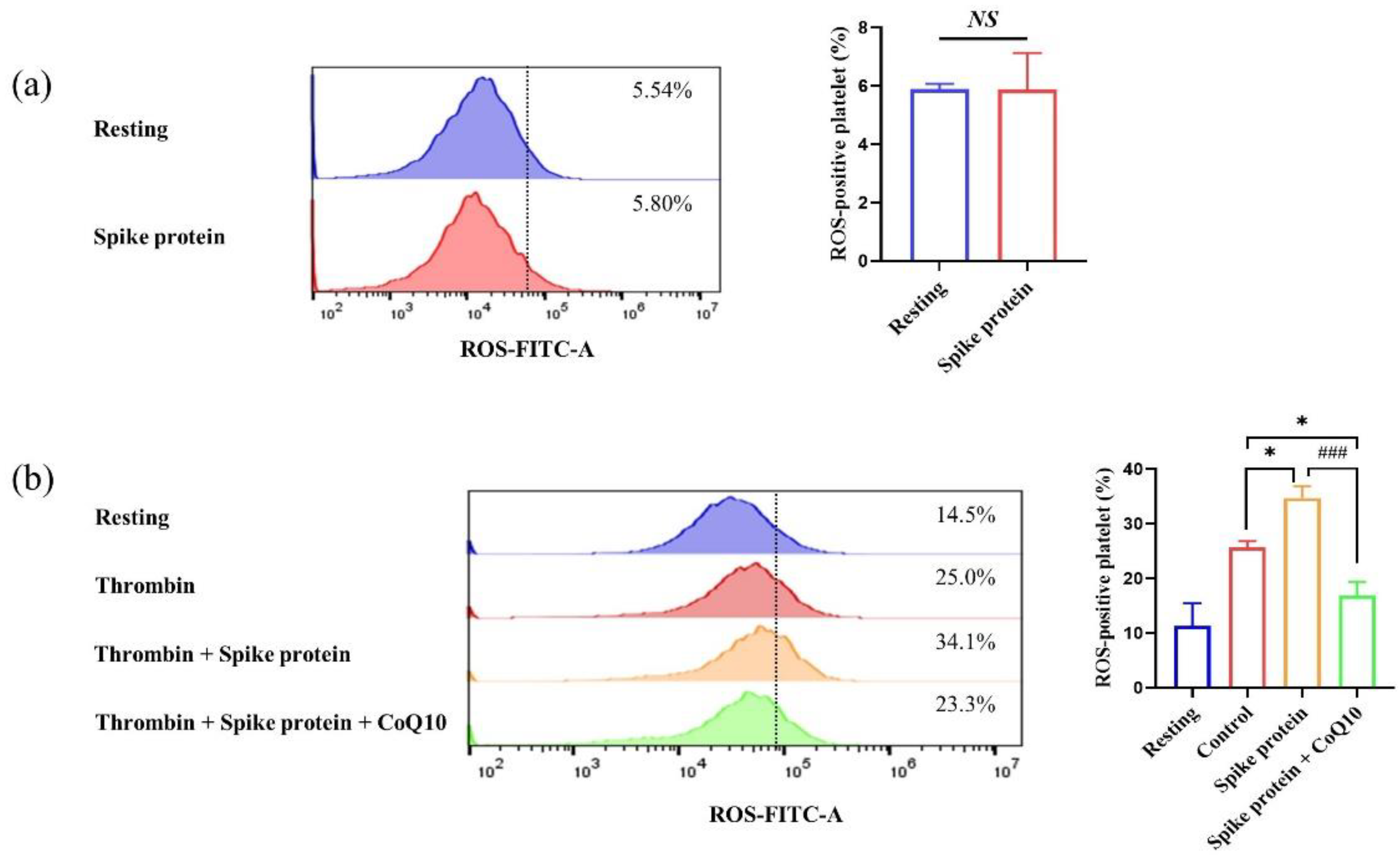
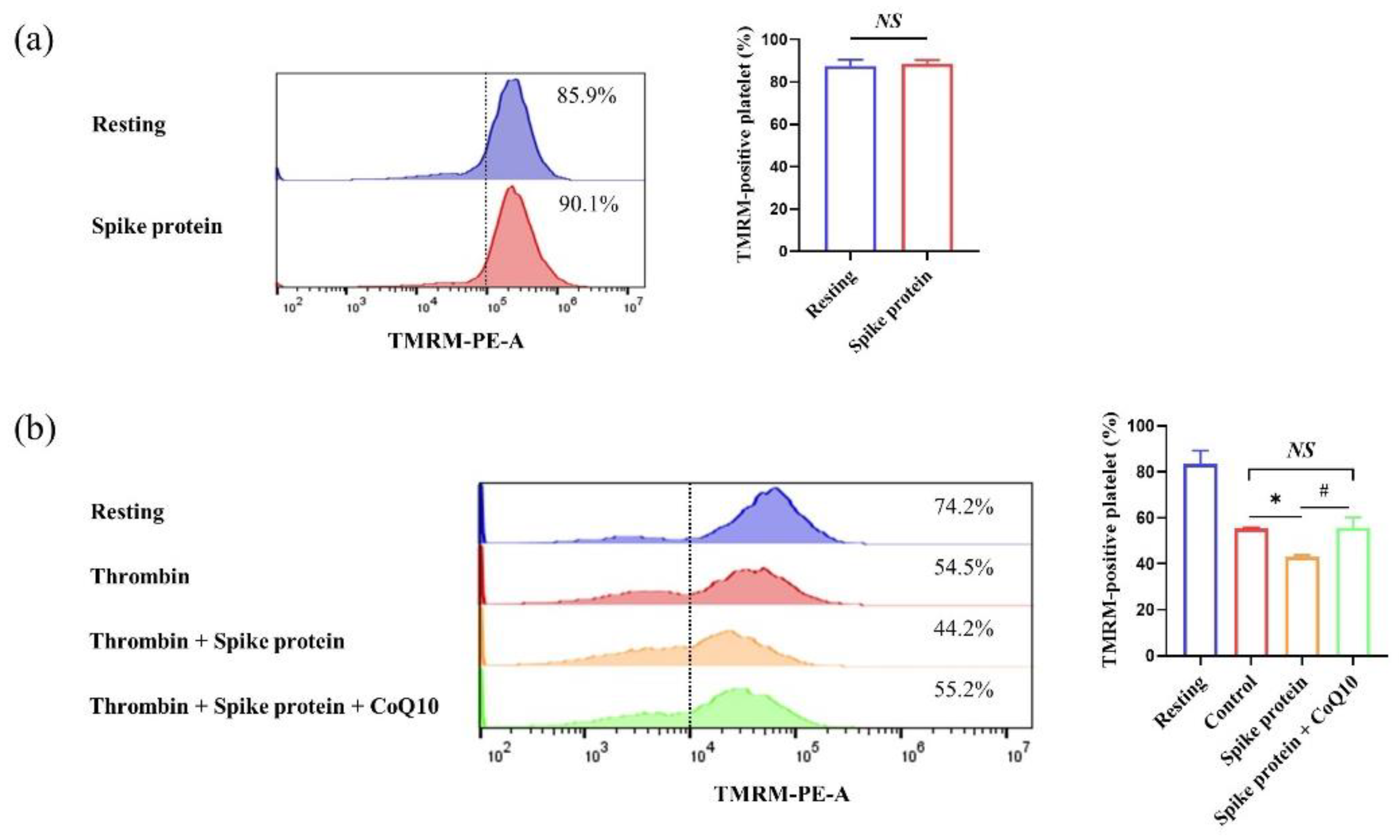
2.2. Spike Protein Does Not Affect Platelet Activation
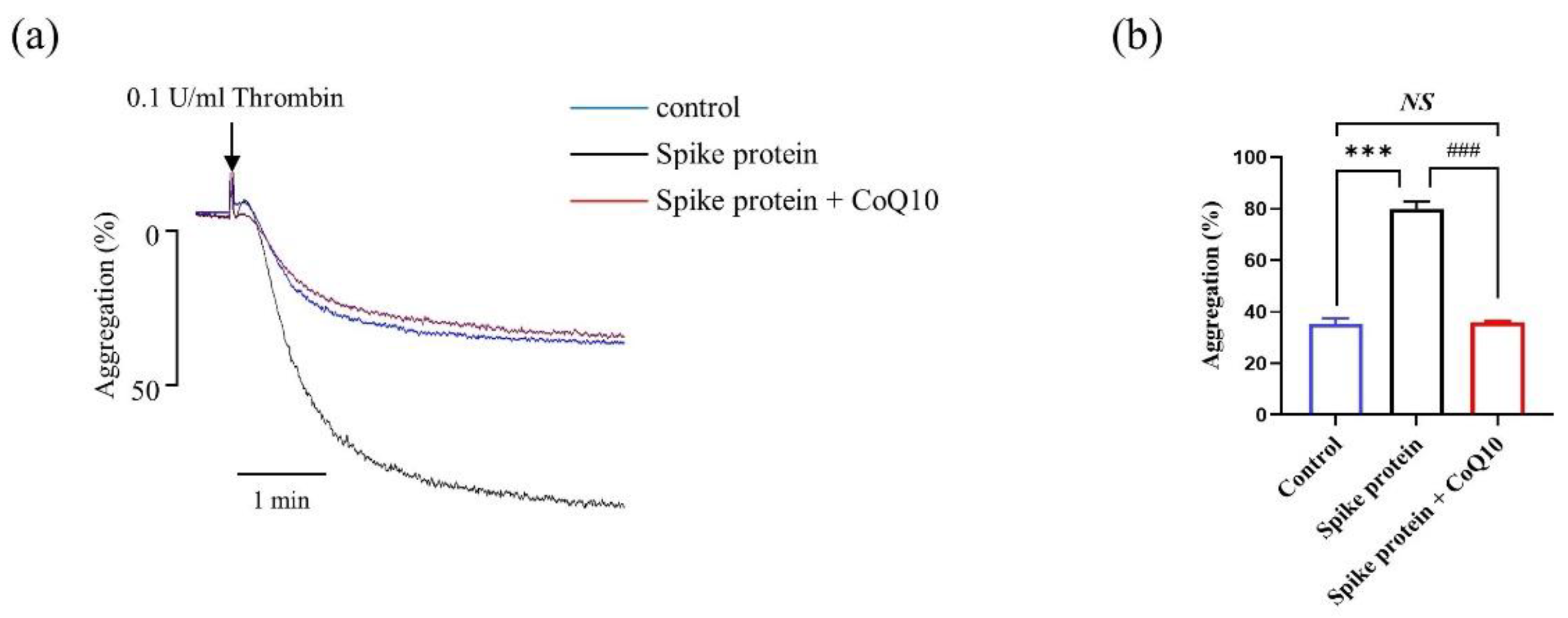
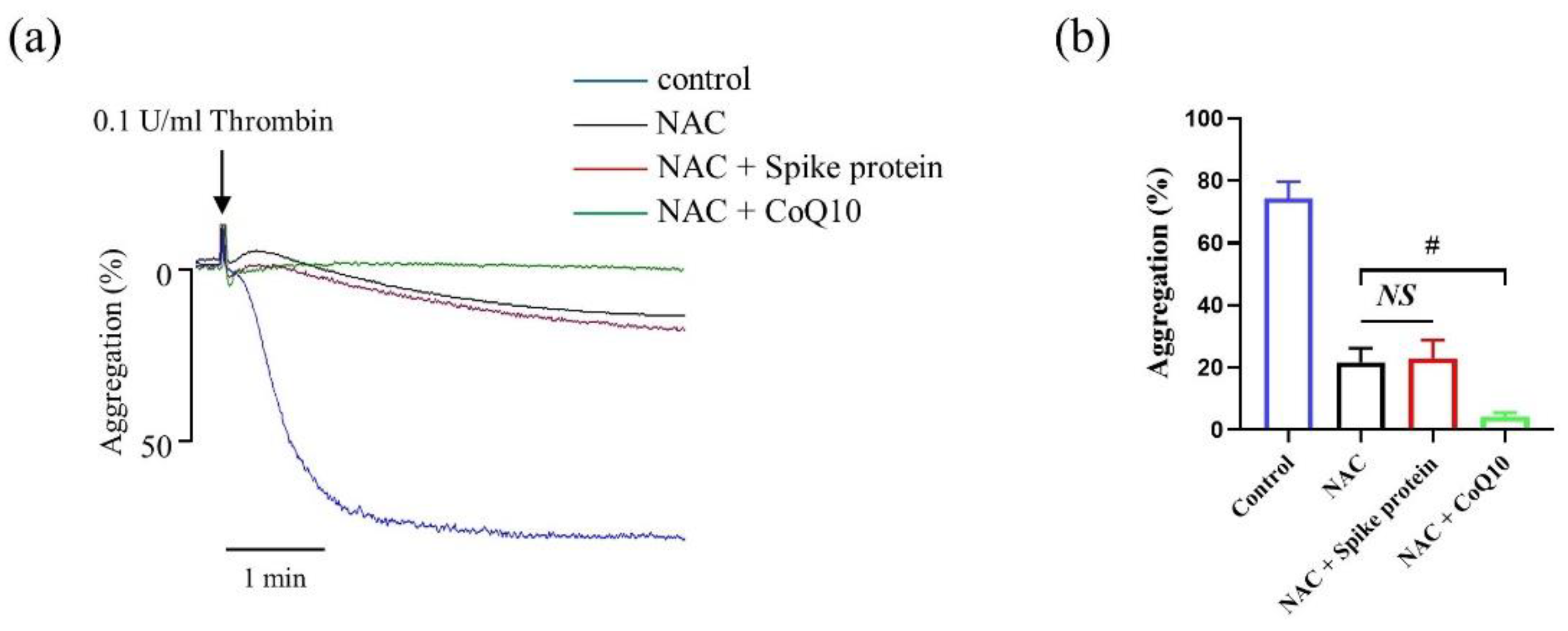
2.3. CoQ10 Attenuates Spike Protein-Potentiated Platelet Aggregation
2.4. CoQ10 Inhibits Spike Protein-Potentiated Platelet Oxidative Stress
2.5. CoQ10 Inhibits Platelet Oxidative Lipid Damage Induced by Spike Protein
2.6. CoQ10 Improves Platelet Antioxidative Activity Reduced by Spike Protein
2.7. The Effect of Spike Protein on Potentiating Platelet Aggregation Is via ROS-Mediated Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Human Platelets
4.3. Platelet Aggregation and Activation Assessment
4.4. Measurement of Platelet Intracellular ROS
4.5. Measurement of Platelet Mitochondrial Membrane Potential
4.6. Platelet MDA, 8-iso-PGF2α, SOD Activity, and TAC Levels Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Spike Protein Does Not Affect Platelet Apoptosis

References
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pao, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Li, Y.; Wang, Z.; Ma, F.; Luo, R.; Xu, X.; Zhou, G.; Wang, J.; Niu, J.; et al. Platelets mediate inflammatory monocyte activation by SARS-CoV-2 spike protein. J. Clin. Investig. 2022, 132, e150101. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M. Platelet Oxidative Stress and its Relationship with Cardiovascular Diseases in Type 2 Diabetes Mellitus Patients. Curr. Med. Chem. 2019, 26, 4145–4165. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Pietraforte, D.; Vona, R.; Marchesi, A.; de Jacobis, I.T.; Villani, A.; Del Principe, D.; Straface, E. Redox control of platelet functions in physiology and pathophysiology. Antioxid. Redox Signal. 2014, 21, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Guerra-Valle, M.; Casas-Forero, N.; Sobral, M.M.C.; Viegas, O.; Alarcon-Enos, J.; Ferreira, I.M.; Pinho, O. Western Dietary Pattern Antioxidant Intakes and Oxidative Stress: Importance During the SARS-CoV-2/COVID-19 Pandemic. Adv. Nutr. 2021, 12, 670–681. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Sune-Negre, J.M.; Garcia-Montoya, E. Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef]
- Ya, F.; Xu, X.R.; Shi, Y.; Gallant, R.C.; Song, F.; Zuo, X.; Zhao, Y.; Tian, Z.; Zhang, C.; Xu, X.; et al. Coenzyme Q10 Upregulates Platelet cAMP/PKA Pathway and Attenuates Integrin alphaIIbbeta3 Signaling and Thrombus Growth. Mol. Nutr. Food Res. 2019, 63, e1900662. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.F.; Jacob, R.F.; Jeffers, B.; Ghadanfar, M.M.; Preston, G.M.; Buch, J.; Mason, R.P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: A longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004, 44, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Xuan, Y.; Gao, X.; Holleczek, B.; Brenner, H.; Schottker, B. Prediction of myocardial infarction, stroke and cardiovascular mortality with urinary biomarkers of oxidative stress: Results from a large cohort study. Int. J. Cardiol. 2018, 273, 223–229. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Trachtenburg, J.; Ryan, U.S.; Abendschein, D.R. Potentiation of endogenous nitric oxide with superoxide dismutase inhibits platelet-mediated thrombosis in injured and stenotic arteries. J. Am. Coll. Cardiol. 1995, 25, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P. The growing complexity of platelet aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puhm, F.; Allaeys, I.; Lacasse, E.; Dubuc, I.; Galipeau, Y.; Zaid, Y.; Khalki, L.; Belleannee, C.; Durocher, Y.; Brisson, A.R.; et al. Platelet activation by SARS-CoV-2 implicates the release of active tissue factor by infected cells. Blood Adv. 2022, 6, 3593–3605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef]
- Chen, S.; Su, Y.; Wang, J. ROS-mediated platelet generation: A microenvironment-dependent manner for megakaryocyte proliferation, differentiation, and maturation. Cell Death Dis. 2013, 4, e722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.J.; Salido, G.M.; Gomez-Arteta, E.; Rosado, J.A.; Pariente, J.A. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J. Thromb. Haemost. 2007, 5, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin alphaIIbbeta3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenwaelder, S.M.; Yuan, Y.; Josefsson, E.C.; White, M.J.; Yao, Y.; Mason, K.D.; O’Reilly, L.A.; Henley, K.J.; Ono, A.; Hsiao, S.; et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 2009, 114, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Sumbalova, Z.; Kucharska, J.; Palacka, P.; Rausova, Z.; Langsjoen, P.H.; Langsjoen, A.M.; Gvozdjakova, A. Platelet mitochondrial function and endogenous coenzyme Q10 levels are reduced in patients after COVID-19. Bratisl. Lek. Listy 2022, 123, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Arauna, D.; Fuentes, F.; Araya-Maturana, R.; Palomo, I.; Alarcon, M.; Sebastian, D.; Zorzano, A.; Fuentes, E. Mitoquinone (MitoQ) Inhibits Platelet Activation Steps by Reducing ROS Levels. Int. J. Mol. Sci. 2020, 21, 6192. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pignatelli, P. Platelet NOX, a novel target for anti-thrombotic treatment. Thromb. Haemost. 2014, 111, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Carnevale, R.; Di Santo, S.; Bartimoccia, S.; Sanguigni, V.; Lenti, L.; Finocchi, A.; Mendolicchio, L.; Soresina, A.R.; Plebani, A.; et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, J.E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s11–s16. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Huang, X.Y.; Liu, N.; Liu, M.M.; Sun, C.R.; Qi, B.Y.; Sun, K.; Wei, X.; Ma, Y.; Zhu, L.G. Discovering the Potential Value of Coenzyme Q10 in Oxidative Stress: Enlightenment From a Synthesis of Clinical Evidence Based on Various Population. Front. Pharmacol. 2022, 13, 936233. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Wu, J.; Sun, H. CoenzymeQ10-Induced Activation of AMPK-YAP-OPA1 Pathway Alleviates Atherosclerosis by Improving Mitochondrial Function, Inhibiting Oxidative Stress and Promoting Energy Metabolism. Front. Pharmacol. 2020, 11, 1034. [Google Scholar] [CrossRef] [PubMed]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in oxidative markers in COVID-19 patients. Arch. Med. Res. 2021, 52, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Xu, X.C.; Liu, T.; Yuan, S. Mitochondrion-Permeable Antioxidants to Treat ROS-Burst-Mediated Acute Diseases. Oxid. Med. Cell Longev. 2016, 2016, 6859523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Las Heras, N.; Martin Gimenez, V.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Chase, M.; Cocchi, M.N.; Liu, X.; Andersen, L.W.; Holmberg, M.J.; Donnino, M.W. Coenzyme Q10 in acute influenza. Influenza Other Respir. Viruses 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Israel, A.; Schaffer, A.A.; Cicurel, A.; Cheng, K.; Sinha, S.; Schiff, E.; Feldhamer, I.; Tal, A.; Lavie, G.; Ruppin, E. Identification of drugs associated with reduced severity of COVID-19—A case-control study in a large population. Elife 2021, 10, 68165. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Luo, L.; Hu, Y. Thrombocytopenia and thrombosis in hospitalized patients with COVID-19. J. Hematol. Oncol. 2020, 13, 161. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A. Risk assessment for coenzyme Q10 (Ubiquinone). Regul. Toxicol. Pharmacol. 2006, 45, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ya, F.; Xu, X.R.; Tian, Z.; Gallant, R.C.; Song, F.; Shi, Y.; Wu, Y.; Wan, J.; Zhao, Y.; Adili, R.; et al. Coenzyme Q10 attenuates platelet integrin alphaIIbbeta3 signaling and platelet hyper-reactivity in ApoE-deficient mice. Food Funct. 2020, 11, 139–152. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Chen, Y.; Tian, Z.; Zhu, M.; Zhang, B.; Du, S.; Li, Y.; Liu, Z.; Hou, S.; Yang, Y. Coenzyme Q10 Attenuates Human Platelet Aggregation Induced by SARS-CoV-2 Spike Protein via Reducing Oxidative Stress In Vitro. Int. J. Mol. Sci. 2022, 23, 12345. https://doi.org/10.3390/ijms232012345
Wang R, Chen Y, Tian Z, Zhu M, Zhang B, Du S, Li Y, Liu Z, Hou S, Yang Y. Coenzyme Q10 Attenuates Human Platelet Aggregation Induced by SARS-CoV-2 Spike Protein via Reducing Oxidative Stress In Vitro. International Journal of Molecular Sciences. 2022; 23(20):12345. https://doi.org/10.3390/ijms232012345
Chicago/Turabian StyleWang, Ruijie, Yiting Chen, Zezhong Tian, Meiyan Zhu, Bingying Zhang, Sijin Du, Yanzhang Li, Zhihao Liu, Shanshan Hou, and Yan Yang. 2022. "Coenzyme Q10 Attenuates Human Platelet Aggregation Induced by SARS-CoV-2 Spike Protein via Reducing Oxidative Stress In Vitro" International Journal of Molecular Sciences 23, no. 20: 12345. https://doi.org/10.3390/ijms232012345




