AMPK Amplifies IL2–STAT5 Signaling to Maintain Stability of Regulatory T Cells in Aged Mice
Abstract
:1. Introduction
2. Results
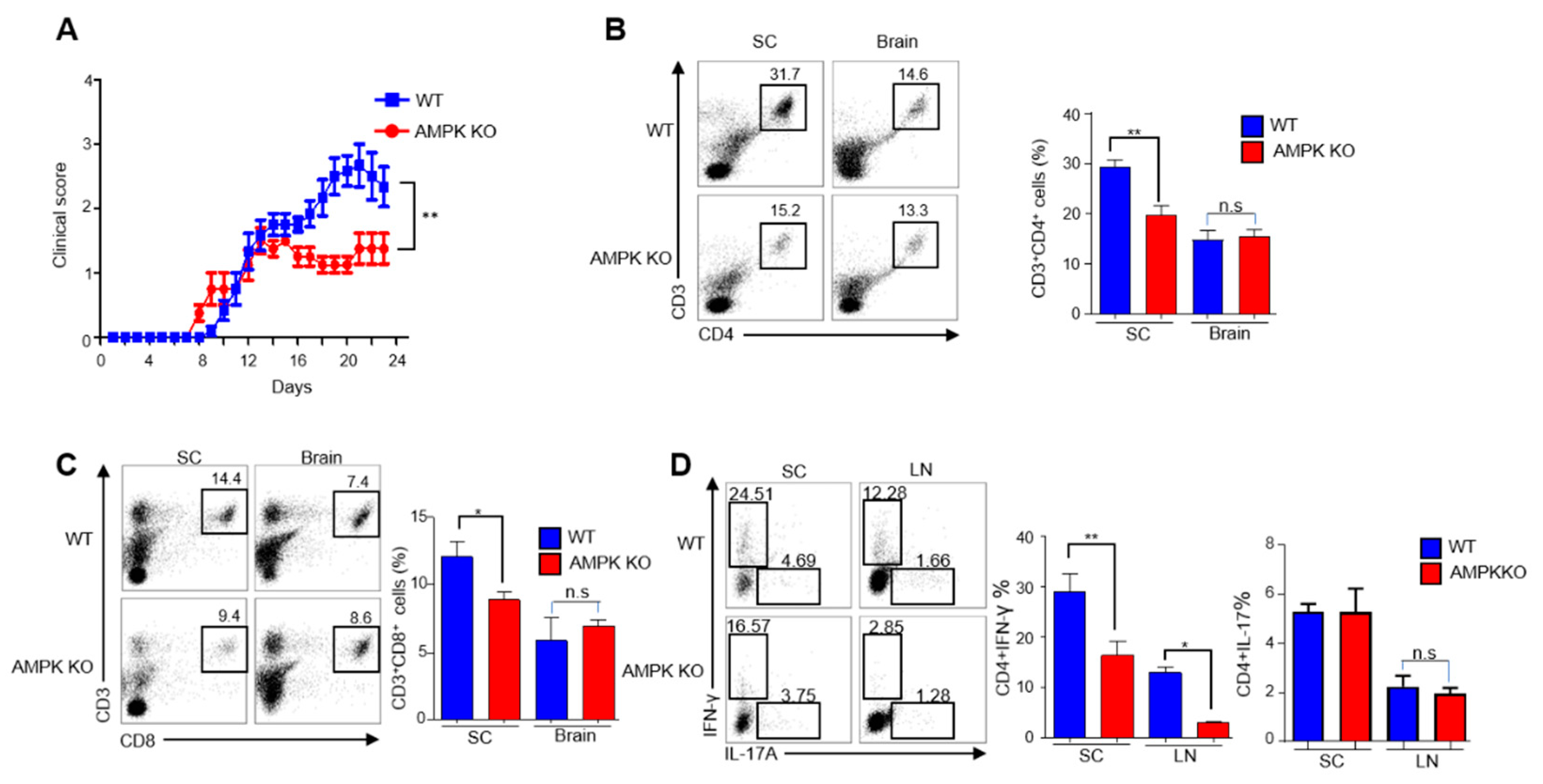
2.1. Young AMPKα1fl/flFoxp3yfp-cre (AMPK-KO) and Foxp3ypf-cre (WT) Mice Are Phenotypically Similar
2.2. Young AMPK-KO Mice Showed a Less Inflammatory Phenotype Than WT Mice
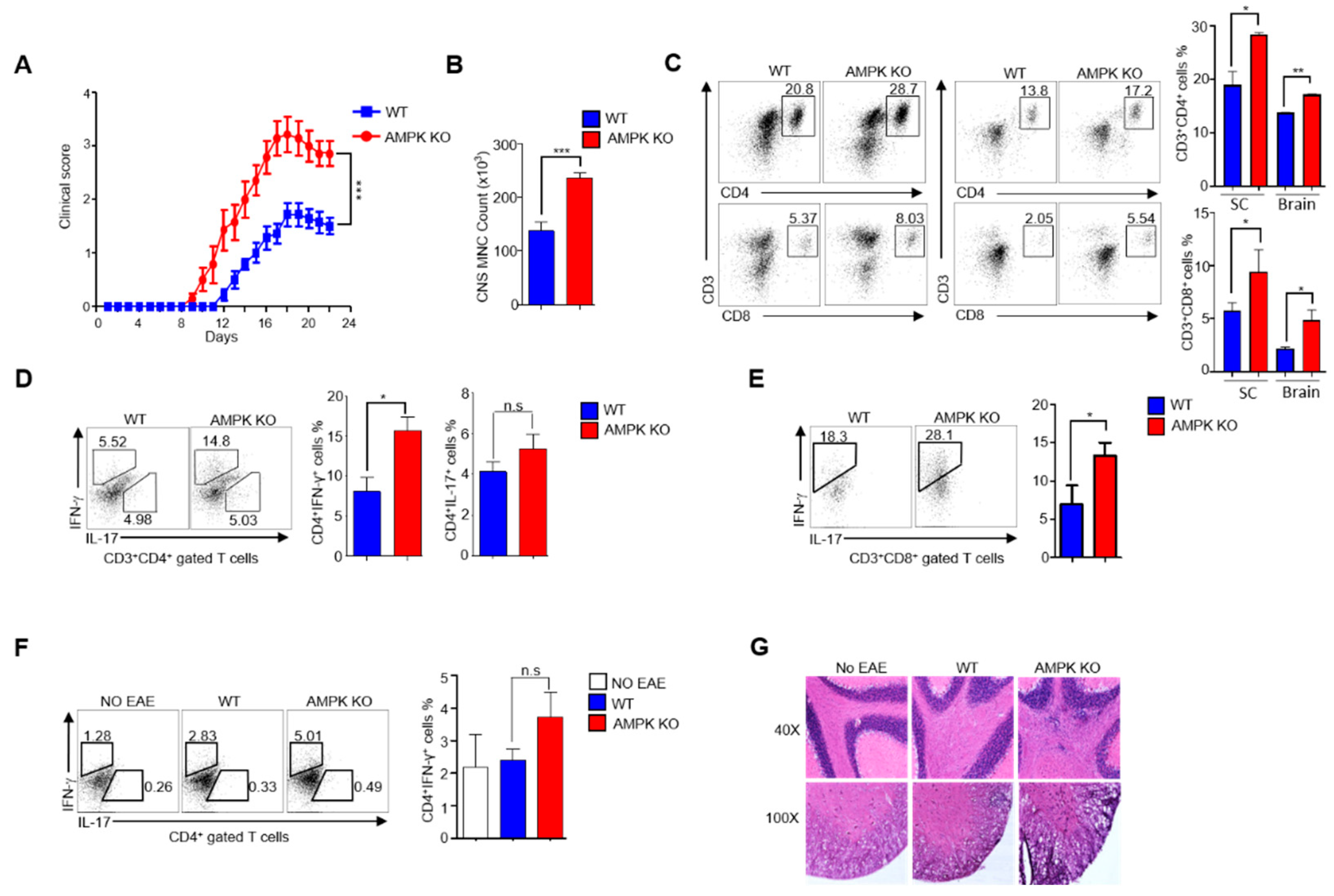
2.3. AMPK Loss Abolishes the Ability of Tregs to Resolve EAE in Aged Mice
2.4. Treg-Specific AMPK Deletion Develops Spontaneous Systemic Lymphoproliferative Disease with Age
2.5. Substantial Decrease of CD25 Expression in AMPK-KO Tregs with Age
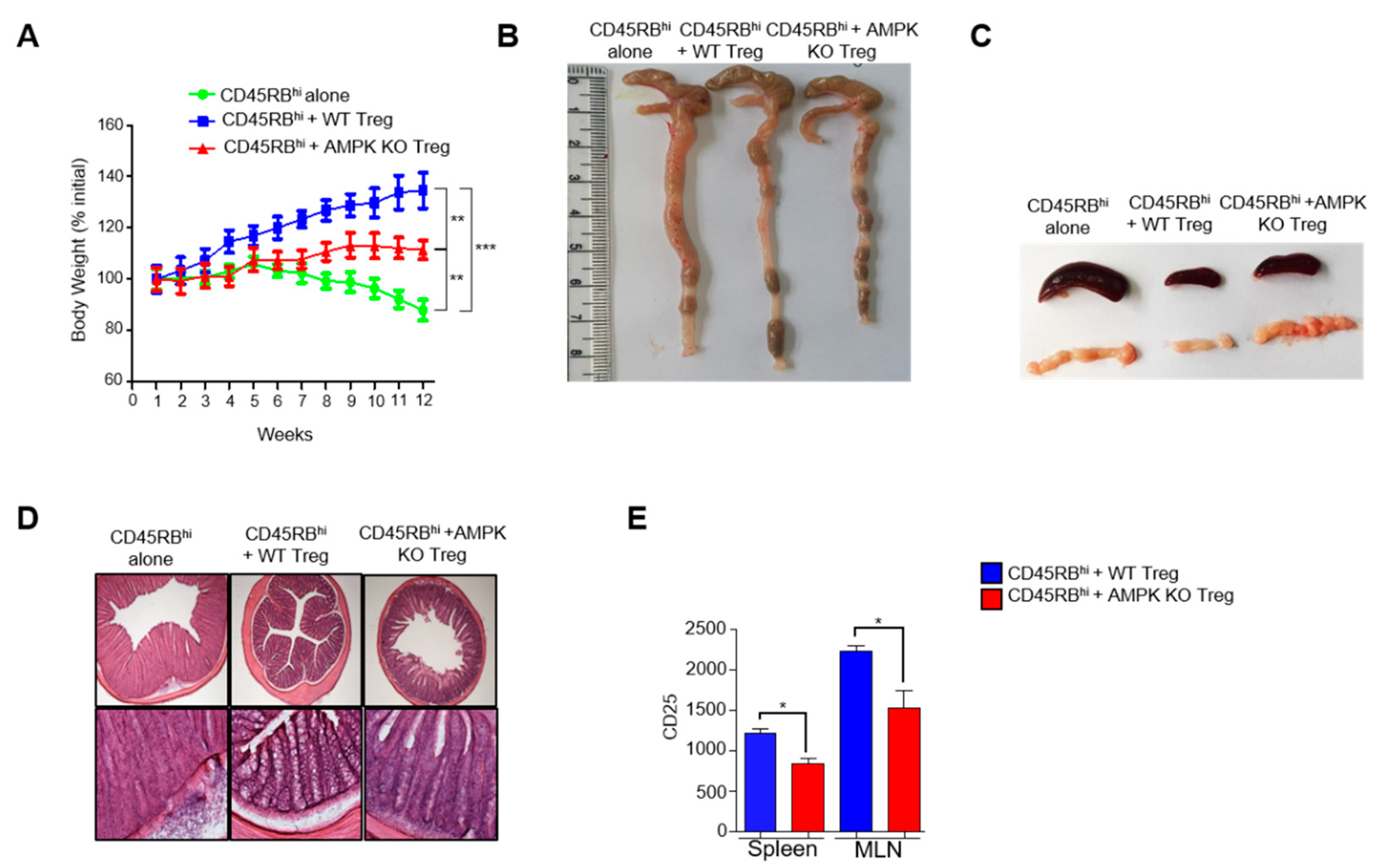
2.6. AMPK Supports Suppressive Activity of Tregs
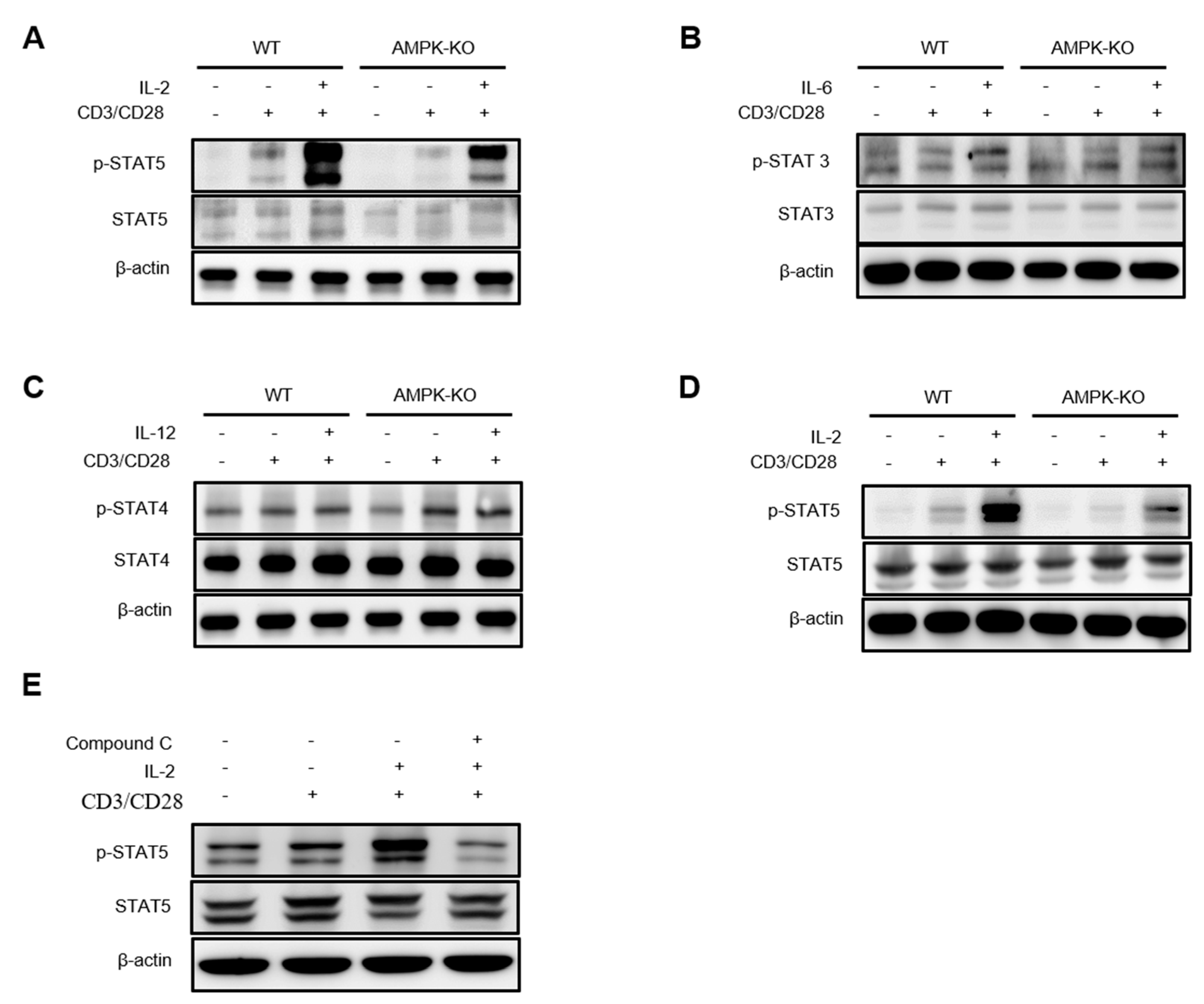
2.7. AMPK Amplifies IL-2–STAT5 Signaling in Tregs
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. EAE Models
4.3. Cell Purification and Culture
4.4. Flow Cytometry and Cell Sorting
4.5. Total RNA Isolation and Quantitative Real-Time PCR
4.6. T Cell Transfer Colitis
4.7. Histopathology
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. RNA Sequencing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, N.; Fan, W.; Henriquez, B.; Yu, R.T.; Atkins, A.R.; Liddle, C.; Zheng, Y.; Downes, M.; Evans, R.M. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc. Natl. Acad. Sci. USA 2017, 114, 12542–12547. [Google Scholar] [CrossRef] [Green Version]
- Barbi, J.; Pardoll, D.M.; Pan, F. Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 2014, 259, 115–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Jagger, A.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T cells and the immune aging process: A mini-review. Gerontology 2014, 60, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardie, D. Adenosine monophosphate–activated protein kinase: A central regulator of metabolism with roles in diabetes, cancer, and viral infection. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2011. [Google Scholar]
- Hardie, D.G. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.H.; Poffenberger, M.C.; Wong, A.H.-T.; Jones, R.G. The role of AMPK in T cell metabolism and function. Curr. Opin. Immunol. 2017, 46, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Stancu, A.L. AMPK activation can delay aging. Discoveries 2015, 3, e53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK mediated pathways in autophagy and aging. Biochimie 2021, 195, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- McNerlan, S.E.; Armstrong, M.; Ross, O.A.; Rea, I.M. Cytokine expression and production changes in very old age. In Handbook on Immunosenescence; Springer: Berlin/Heidelberg, Germany, 2009; pp. 771–781. [Google Scholar]
- Cevenini, E.; Monti, D.; Franceschi, C. Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 14–20. [Google Scholar] [CrossRef]
- Pokhrel, R.H.; Acharya, S.; Ahn, J.-H.; Gu, Y.; Pandit, M.; Kim, J.-O.; Park, Y.-Y.; Kang, B.; Ko, H.-J.; Chang, J.-H. AMPK promotes antitumor immunity by downregulating PD-1 in regulatory T cells via the HMGCR/p38 signaling pathway. Mol. Cancer 2021, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zmijewski, J.W.; Lorne, E.; Liu, G.; Park, Y.-J.; Tsuruta, Y.; Abraham, E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L497–L504. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, Y.; Nakae, S.; Matsuki, T.; Nambu, A.; Ishigame, H.; Kakuta, S.; Sudo, K.; Iwakura, Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 566–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Permanyer, M.; Bošnjak, B.; Glage, S.; Friedrichsen, M.; Floess, S.; Huehn, J.; Patzer, G.E.; Odak, I.; Eckert, N.; Zargari, R.; et al. Efficient IL-2R signaling differentially affects the stability, function, and composition of the regulatory T-cell pool. Cell. Mol. Immunol. 2021, 18, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.; Boldt, A.; Freitag, M.-T.; Gruhn, B.; Köhl, U.; Sack, U. Evaluating STAT5 phosphorylation as a mean to assess T cell proliferation. Front. Immunol. 2019, 10, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antov, A.; Yang, L.; Vig, M.; Baltimore, D.; Van Parijs, L. Essential role for STAT5 signaling in CD25+ CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J. Immunol. 2003, 171, 3435–3441. [Google Scholar] [CrossRef]
- Lin, J.-X.; Li, P.; Liu, D.; Jin, H.T.; He, J.; Rasheed, M.A.U.; Rochman, Y.; Wang, L.; Cui, K.; Liu, C.; et al. Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity 2012, 36, 586–599. [Google Scholar] [CrossRef] [Green Version]
- Rocamora-Reverte, L.; Melzer, F.L.; Würzner, R.; Weinberger, B. The complex role of regulatory T cells in immunity and aging. Front. Immunol. 2021, 11, 616949. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Activation of immunosuppressive network in the aging process. Ageing Res. Rev. 2020, 57, 100998. [Google Scholar] [CrossRef] [PubMed]
- Sarode, A.Y.; Jha, M.K.; Zutshi, S.; Ghosh, S.K.; Mahor, H.; Sarma, U.; Saha, B. Residue-Specific Message Encoding in CD40-Ligand. Iscience 2020, 23, 101441. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, Y.; Vogel, P.; Chi, H. Control of IL-17 receptor signaling and tissue inflammation by the p38α–MKP-1 signaling axis in a mouse model of multiple sclerosis. Sci. Signal. 2015, 8, ra24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Ferrucci, L.; Semba, R.D.; Guralnik, J.M.; Ershler, W.B.; Bandinelli, S.; Patel, K.V.; Sun, K.; Woodman, R.C.; Andrews, N.C.; Cotter, R.J.; et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood J. Am. Soc. Hematol. 2010, 115, 3810–3816. [Google Scholar] [CrossRef] [Green Version]
- Peairs, A.; Radjavi, A.; Davis, S.; Li, L.; Ahmed, A.; Giri, S.; Reilly, C.M. Activation of AMPK inhibits inflammation in MRL/lpr mouse mesangial cells. Clin. Exp. Immunol. 2009, 156, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, E.; Zhao, K.; Wu, C.; Yang, B. The suppressive effect of CD25+ Treg cells on Th1 differentiation requires cell—cell contact partially via TGF-β production. Cell Biol. Int. 2011, 35, 705–712. [Google Scholar] [CrossRef]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhrel, R.H.; Kang, B.; Timilshina, M.; Chang, J.-H. AMPK Amplifies IL2–STAT5 Signaling to Maintain Stability of Regulatory T Cells in Aged Mice. Int. J. Mol. Sci. 2022, 23, 12384. https://doi.org/10.3390/ijms232012384
Pokhrel RH, Kang B, Timilshina M, Chang J-H. AMPK Amplifies IL2–STAT5 Signaling to Maintain Stability of Regulatory T Cells in Aged Mice. International Journal of Molecular Sciences. 2022; 23(20):12384. https://doi.org/10.3390/ijms232012384
Chicago/Turabian StylePokhrel, Ram Hari, Ben Kang, Maheshwor Timilshina, and Jae-Hoon Chang. 2022. "AMPK Amplifies IL2–STAT5 Signaling to Maintain Stability of Regulatory T Cells in Aged Mice" International Journal of Molecular Sciences 23, no. 20: 12384. https://doi.org/10.3390/ijms232012384






