In Vitro Assessment of Poly-N-Vinylpyrrolidone/Acrylic Acid Nanoparticles Biocompatibility in a Microvascular Endothelium Model
Abstract
:1. Introduction
2. Results and Discussion
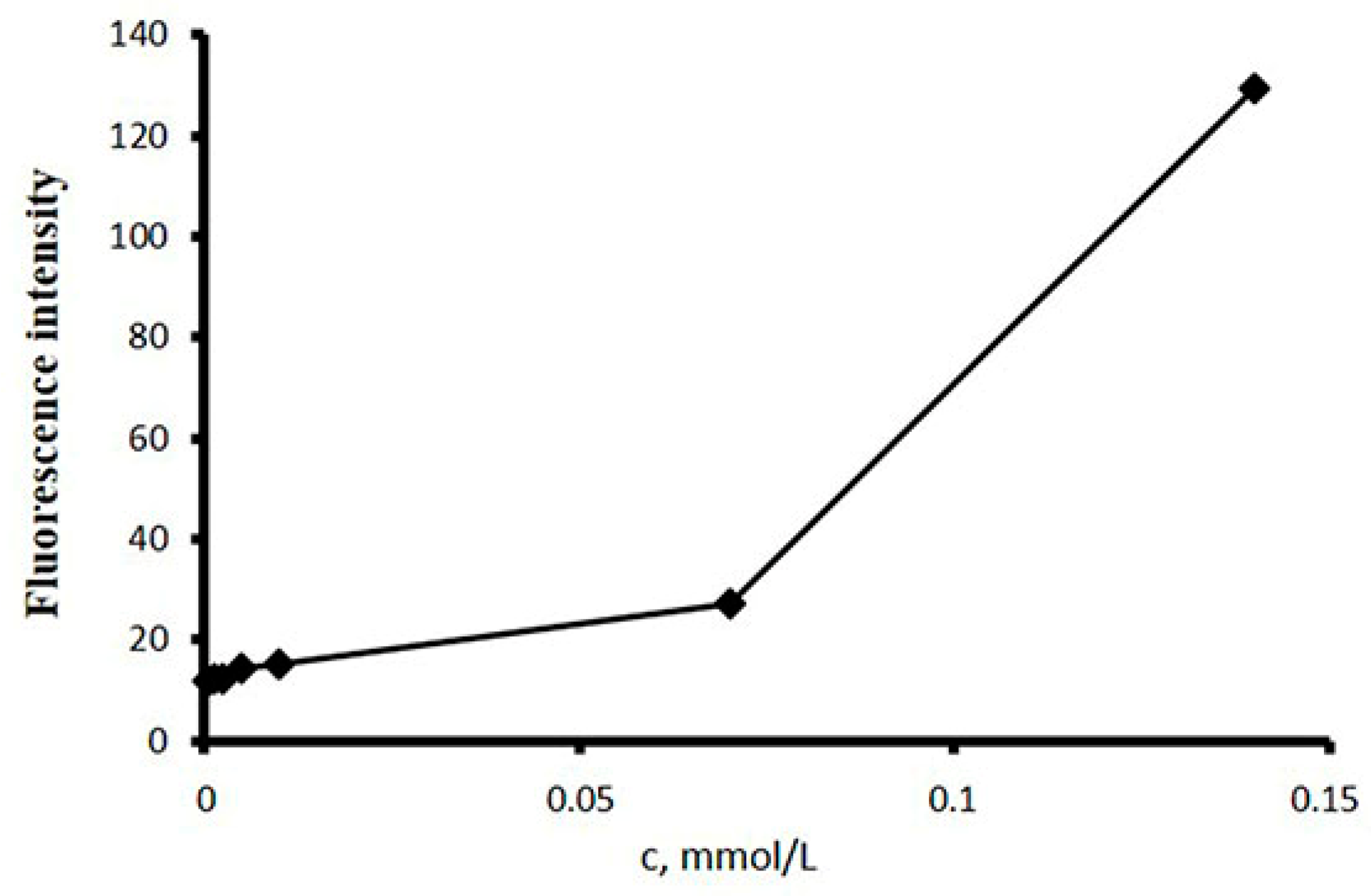
2.1. Preparation and Characterization of p(VP-AA) NPs Carrying Fluorophores
2.2. Effect of p(VP-AA) NPs and DNPs on Healthy and Challenged with LPS HMEC-1 Cell Viability
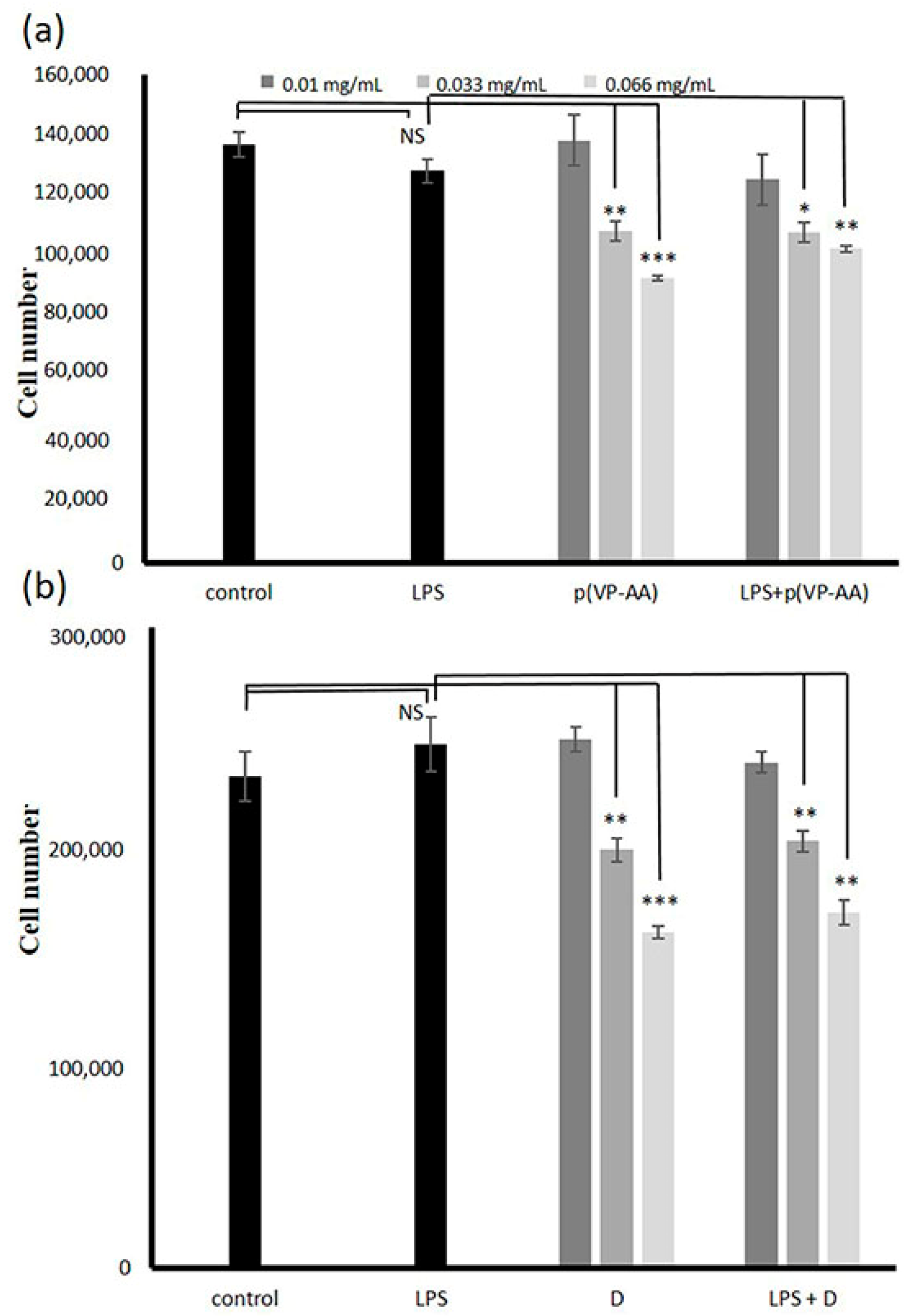
2.3. Effect of p(VP-AA) NPs on Normal and Activated with LPS HMEC-1 Cell Growth
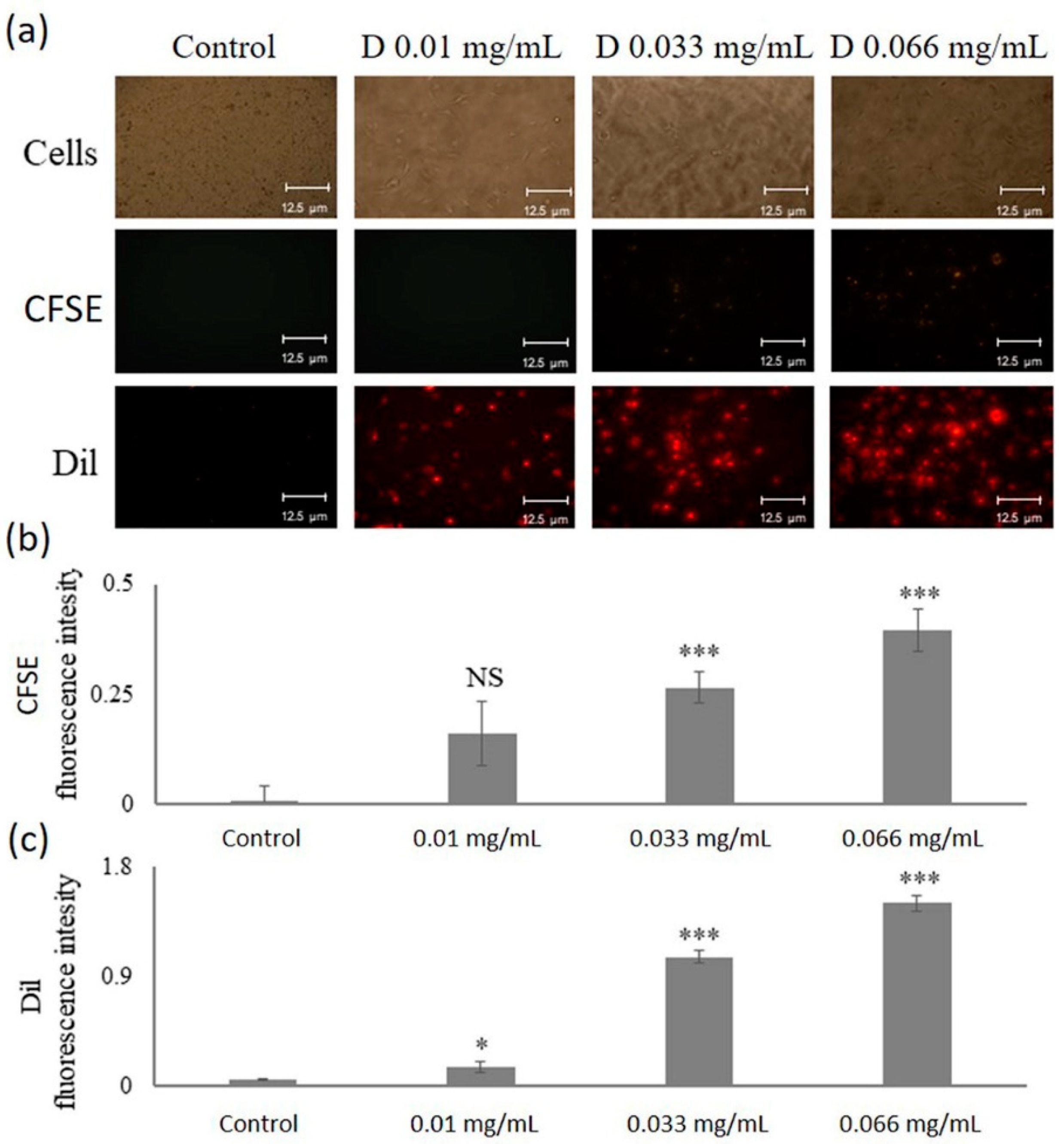
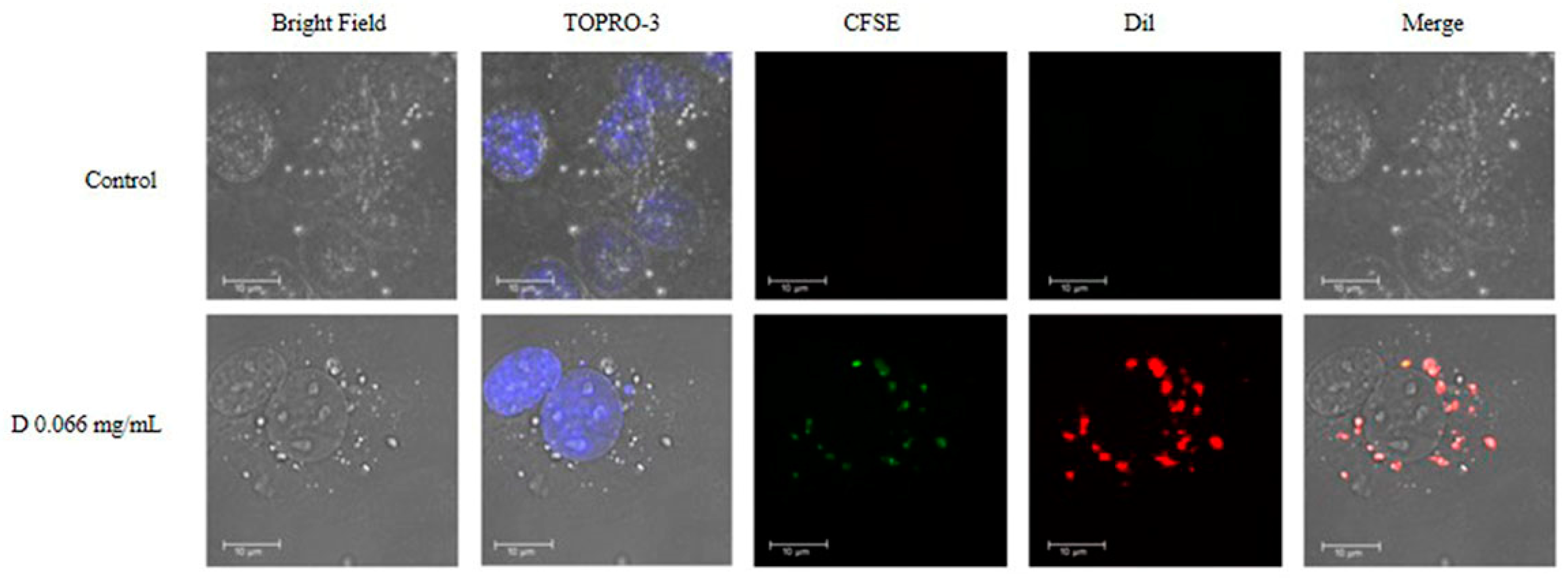
2.4. Uptake of DNPs by Microvascular Endothelial Cells
2.5. Uptake of p(VP-AA) NPs by Living HMEC-1 Cells
2.6. Intracellular Localization of DNPs
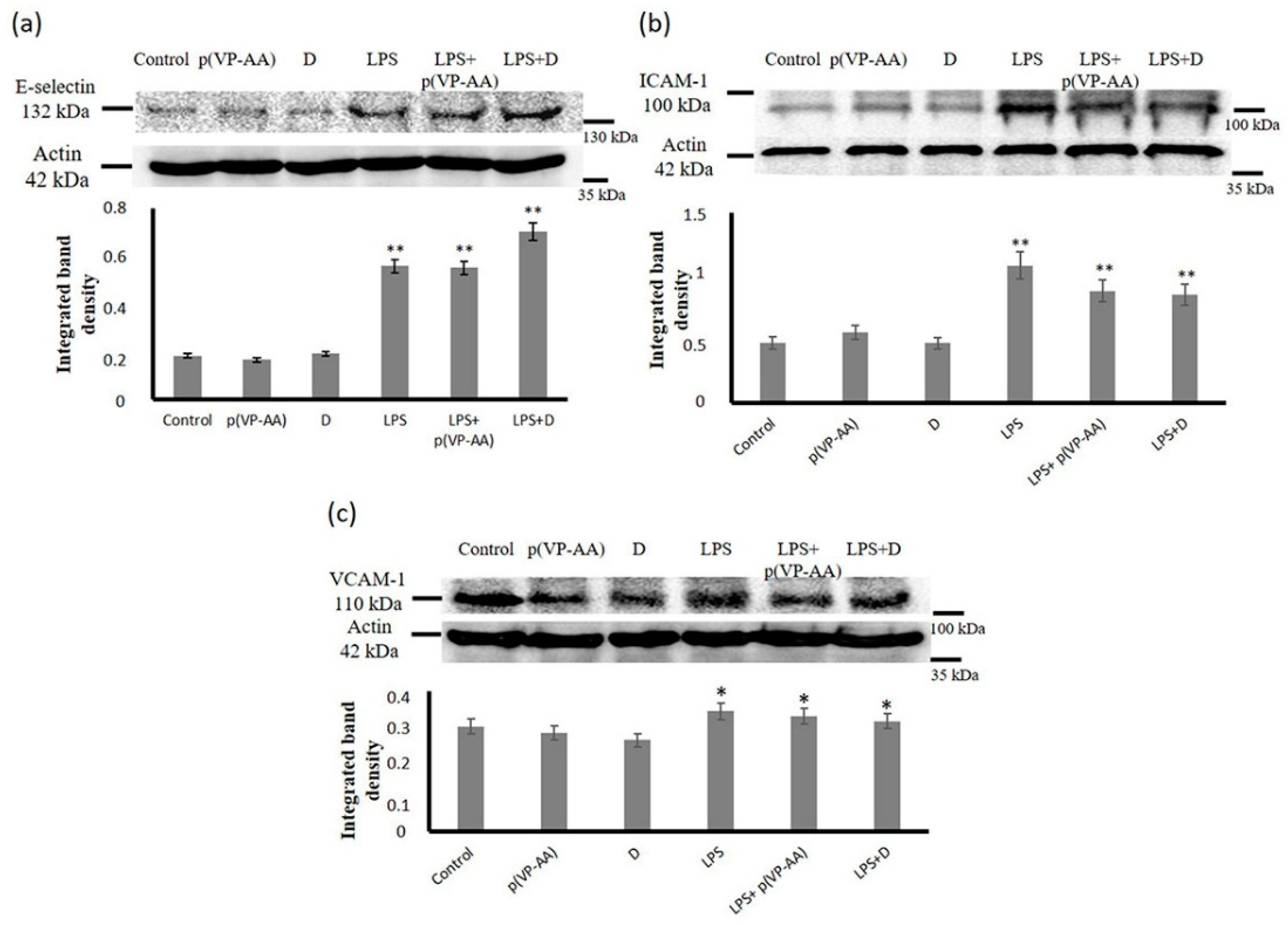
2.7. Effect of p(PV-AA) NPs and DNPs on HMEC-1 Cells Adhesion Molecule Expression
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Amphiphilic Poly-N-Vinylpyrrolidone-Based Polymeric Materials
3.3. Modification of p(VP-AA) by CFSE
3.4. Preparation and Characterization of p(VP-AA) NPs Carrying Fluorophores
3.5. Cell Culture
3.6. Fluorescence Microscopy
3.7. Measurement of Nanoparticle Uptake Utilizing Fluorescence
3.8. Confocal Microscopy
3.9. MTT Assay
3.10. Cell Growth Assay
3.11. Western Blot
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Matteis, V.; Rizzello, L. Noble metals and soft bio-inspired nanoparticles in retinal diseases treatment: A perspective. Cells 2020, 9, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich-Noack, P.; Nikitovic, D.; Neagu, M.; Docea, A.O.; Engin, A.B.; Gelperina, S.; Shtilman, M.; Mitsias, P.; Tzanakakis, G.; Gozes, I.; et al. The blood-brain barrier and beyond: Nano-based neuropharmacology and the role of extracellular matrix. Nanomedicine 2019, 17, 359–379. [Google Scholar] [CrossRef]
- Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Cetin, M.; Ugur, A.B.; Galateanu, B.; Mezhuev, Y.; Okkay, U.; Taspinar, N.; Taspinar, M.; Uyanik, A.; et al. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: In vitro and in vivo studies. Nanomedicine 2018, 13, 1595–1606. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Kulikov, P.P.; Goryachaya, A.V.; Tzatzarakis, M.N.; Docea, A.O.; Velonia, K.; Shtilman, M.I.; Tsatsakis, A.M. Amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for non-steroidal, anti-inflammatory drugs: In vitro cytotoxicity and in vivo acute toxicity study. Nanomedicine 2017, 13, 1021–1030. [Google Scholar] [CrossRef]
- Basyreva, L.Y.; Voinova, E.V.; Gusev, A.A.; Mikhalchik, E.V.; Kuskov, A.N.; Goryachaya, A.V.; Gusev, S.A.; Shtilman, M.I.; Velonia, K.; Tsatsakis, A.M. Fluorouracil neutrophil extracellular traps formation inhibited by polymer nanoparticle shielding. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110382. [Google Scholar] [CrossRef]
- Kuskov, A.; Selina, O.; Kulikov, P.; Imatdinov, I.; Balysheva, V.; Kryukov, A.; Shtilman, M.; Markvicheva, E. Amphiphilic poly (N-vinylpyrrolidone) nanoparticles loaded with DNA plasmids encoding Gn and Gc glycoproteins of the Rift valley fever virus: Preparation and in vivo evaluation. ACS Appl. Bio. Mater. 2021, 4, 6084–6092. [Google Scholar] [CrossRef]
- Yamskov, I.A.; Kuskov, A.N.; Babievsky, K.K.; Berezin, B.B.; Krayukhina, M.A.; Samoylova, N.A.; Tikhonov, V.E.; Shtilman, M.I. Novel liposomal forms of antifungal antibiotics modified by amphiphilic polymers. Appl. Biochem. Microbiol. 2008, 44, 624–628. [Google Scholar] [CrossRef]
- Yagolovich, A.; Kuskov, A.; Kulikov, P.; Kurbanova, L.; Bagrov, D.; Artykov, A.; Gasparian, M.; Sizova, S.; Oleinikov, V.; Gileva, A.; et al. Amphiphilic poly(N-vinylpyrrolidone) nanoparticles conjugated with DR5-specific antitumor cytokine DR5-B for targeted delivery to cancer cells. Pharmaceutics 2021, 13, 1413. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Voskresenskaya, A.A.; Goryachaya, A.V.; Artyukhov, A.A.; Shtilman, M.I.; Tsatsakis, A.M. Preparation and characterization of amphiphilic poly-N-vinylpyrrolidone nanoparticles containing indomethacin. J. Mater. Sci. Mater. Med. 2010, 21, 1521–1530. [Google Scholar] [CrossRef]
- Berdiaki, A.; Perisynaki, E.; Stratidakis, A.; Kulikov, P.P.; Kuskov, A.N.; Stivaktakis, P.; Henrich-Noack, P.; Luss, A.L.; Shtilman, M.M.; Tzanakakis, G.N.; et al. Assessment of amphiphilic poly-N-vinylpyrrolidone nanoparticles’ biocompatibility with endothelial cells in vitro and delivery of an anti-inflammatory drug. Mol. Pharm. 2020, 17, 4212–4225. [Google Scholar] [CrossRef] [PubMed]
- Kuskov, A.N.; Kulikov, P.P.; Shtilman, M.I.; Rakitskii, V.N.; Tsatsakis, A.M. Amphiphilic poly-N-vynilpyrrolidone nanoparticles: Cytotoxicity and acute toxicity study. Food Chem. Toxicol. 2016, 96, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Villemson, A.L.; Kuskov, A.N.; Shtilman, M.I.; Galebskaya, L.V.; Ryumina, E.V.; Larionova, N.I. Interaction of polymer aggregates based on stearoyl-poly-N-vinylpyrrolidone with blood components. Biochemistry 2004, 69, 621–628. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Stratidakis, A.K.; Goryachaya, A.V.; Tzatzarakis, M.N.; Stivaktakis, P.D.; Docea, A.O.; Berdiaki, A.; Nikitovic, D.; Velonia, K.; Shtilman, M.I.; et al. In vitro blood compatibility and in vitro cytotoxicity of amphiphilic poly-N-vinylpyrrolidone nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kuskov, A.; Nikitovic, D.; Berdiaki, A.; Shtilman, M.; Tsatsakis, A. Amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for nonsteroidal, anti-inflammatory drugs: Pharmacokinetic, anti-inflammatory, and ulcerogenic activity study. Pharmaceutics 2022, 14, 925. [Google Scholar] [CrossRef]
- Giodini, L.; Re, F.L.; Campagnol, D.; Marangon, E.; Posocco, B.; Dreussi, E.; Toffoli, G. Nanocarriers in cancer clinical practice: A pharmacokinetic issue. Nanomedicine 2017, 13, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Kirf, D.; Higginbotham, C.L.; Rowan, N.J.; Devery, S.M. Cyto- and genotoxicological assessment and functional characterization of N-vinyl-2-pyrrolidone-acrylic acid-based copolymeric hydrogels with potential for future use in wound healing applications. Biomed. Mater. 2010, 5, 35002. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, Y.; Wang, L.; Ge, X.; Fu, M.; Wang, P.; Wang, Q. High-strength nanocomposite hydrogels with swelling-resistant and anti-dehydration properties. Polymers 2018, 10, 1025. [Google Scholar] [CrossRef] [Green Version]
- Thurmer, M.B.; Diehl, C.E.; Brum, F.J.; dos Santos, L.A. Development of dual-setting calcium phosphate cement using absorbable polymer. Artif. Organs 2013, 37, 992–997. [Google Scholar] [CrossRef]
- Estifeeva, T.M.; Barmin, R.A.; Rudakovskaya, P.G.; Nechaeva, A.M.; Luss, A.L.; Mezhuev, Y.O.; Chernyshev, V.S.; Krivoborodov, E.G.; Klimenko, O.A.; Sindeeva, O.A.; et al. Hybrid (bovine serum albumin)/poly(N-vinyl-2-pyrrolidone-co-acrylic acid)-shelled microbubbles as advanced ultrasound contrast agents. ACS Appl. Bio. Mater. 2022, 5, 3338–3348. [Google Scholar] [CrossRef]
- Kizilbey, K.; Mansuroglu, B.; Derman, S.; Mustafaeva Akdeste, Z. An in vivo study: Adjuvant activity of poly-n-vinyl-2-pyrrolidone-co-acrylic acid on immune responses against Melanoma synthetic peptide. Bioengineered 2018, 9, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, M.; Hadlak, S.; Gotze, C.; Sokolov, M.; Kulikov, P.; Kuskov, A.; Shtilman, M.; Sabel, B.A.; Henrich-Noack, P. Live in-vivo neuroimaging reveals the transport of lipophilic cargo through the blood-retina barrier with modified amphiphilic poly-N-vinylpyrrolidone nanoparticles. J. Biomed. Nanotechnol. 2021, 17, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zou, P.; Tyner, K.; Lee, S. Physiologically based pharmacokinetic (PBPK) modeling of pharmaceutical nanoparticles. AAPS J. 2017, 19, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: The cell and immune system. Part. Fibre Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Furuse, M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010, 2, a002907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the vascular barrier: Endothelial signaling processes controlling extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and mechanoregulation of endothelial cell functions. Compr. Physiol. 2019, 9, 873–904. [Google Scholar]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Nagel, T.; Topper, J.N. Biomechanical activation: An emerging paradigm in endothelial adhesion biology. J. Clin. Investig. 1997, 100, S61-5. [Google Scholar] [CrossRef]
- Hattori, Y.; Hattori, K.; Machida, T.; Matsuda, N. Vascular endotheliitis associated with infections: Its pathogenetic role and therapeutic implication. Biochem. Pharmacol. 2022, 197, 114909. [Google Scholar] [CrossRef]
- Piga, R.; Naito, Y.; Kokura, S.; Handa, O.; Yoshikawa, T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis 2007, 193, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.; Pelaez, L.F.; Rojas, M.; Narvaez-Sanchez, R.; Velasquez, J.A.; Escudero, C.; San Martin, S.; Cadavid, A.P. Differences in endothelial activation and dysfunction induced by antiphospholipid antibodies among groups of patients with thrombotic, refractory, and non-refractory antiphospholipid syndrome. Front. Physiol. 2021, 12, 764702. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric nanoparticles for drug delivery. Methods Mol. Biol. 2010, 624, 163–175. [Google Scholar] [PubMed]
- Dhawan, A.; Sharma, V. Toxicity assessment of nanomaterials: Methods and challenges. Anal. Bioanal. Chem. 2010, 398, 589–605. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of nanoparticles in medicine. Curr. Drug. Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Tapeinos, C.; Tziveleka, L.A.; Boukos, N.; Kordas, G. pH- and thermo-responsive microcontainers as potential drug delivery systems: Morphological characteristic, release and cytotoxicity studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 271–277. [Google Scholar] [CrossRef]
- Tapeinos, C.; Efthimiadou, E.K.; Boukos, N.; Kordas, G. Sustained release profile of quatro stimuli nanocontainers as a multi sensitive vehicle exploiting cancer characteristics. Colloids Surf. B Biointerfaces 2016, 148, 95–103. [Google Scholar] [CrossRef]
- Kumar, R.; Kulkarni, A.; Nabulsi, J.; Nagesha, D.K.; Cormack, R.; Makrigiorgos, M.G.; Sridhar, S. Facile synthesis of PEGylated PLGA nanoparticles encapsulating doxorubicin and its in vitro evaluation as potent drug delivery vehicle. Drug Deliv. Transl. Res. 2013, 3, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, C.; Miao, X.Q.; Chow, S.F.; Wu, W.J.; Yan, R.; Liao, Y.H.; Chow, A.H.; Zheng, Y. Particle size effect of curcumin nanosuspensions on cytotoxicity, cellular internalization, in vivo pharmacokinetics and biodistribution. Nanomedicine 2017, 13, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hou, Z.; Zhang, H.; Kong, H.; Li, X.; Wang, H.; Xie, W. An efficient Trojan delivery of tetrandrine by poly(N-vinylpyrrolidone)-block-poly(epsilon-caprolactone) (PVP-b-PCL) nanoparticles shows enhanced apoptotic induction of lung cancer cells and inhibition of its migration and invasion. Int. J. Nanomed. 2014, 9, 231–242. [Google Scholar]
- Kuskov, A.N.; Kulikov, P.P.; Goryachaya, A.V.; Shtilman, M.I.; Tzatzarakis, M.N.; Tsatsakis, A.M.; Velonia, K. Self-assembled amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for hydrophobic drugs: Stability aspects. J. Appl. Polym. Sci. 2018, 135, 45637. [Google Scholar] [CrossRef]
- Schulz, M.; Schmoldt, A.; Andresen-Streichert, H.; Iwersen-Bergmann, S. Revisited: Therapeutic and toxic blood concentrations of more than 1100 drugs and other xenobiotics. Crit. Care 2020, 24, 195. [Google Scholar] [CrossRef]
- Opal, S.M.; van der Poll, T. Endothelial barrier dysfunction in septic shock. J. Intern. Med. 2015, 277, 277–293. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Levi, M.; van der Poll, T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc. Med. 2005, 15, 254–259. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Schafer, K. Role of endothelial cells in acute and chronic thrombosis. Hamostaseologie 2019, 39, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Tamma, R.; Annese, T. The role of vascular niche and endothelial cells in organogenesis and regeneration. Exp. Cell Res. 2021, 398, 112398. [Google Scholar] [CrossRef]
- Laroia, S.T.; Ganti, A.K.; Laroia, A.T.; Tendulkar, K.K. Endothelium and the lipid metabolism: The current understanding. Int. J. Cardiol. 2003, 88, 1–9. [Google Scholar] [CrossRef]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, M.; Iwersen-Bergmann, S.; Andresen, H.; Schmoldt, A. Therapeutic and toxic blood concentrations of nearly 1000 drugs and other xenobiotics. Crit. Care 2012, 16, R136. [Google Scholar] [CrossRef] [PubMed]
- Aberg, C.; Kim, J.A.; Salvati, A.; Dawson, K.A. Reply to ‘The interface of nanoparticles with proliferating mammalian cells’. Nat. Nanotechnol. 2017, 12, 600–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliyandi, A.; Satchell, S.; Unger, R.E.; Bartosch, B.; Parent, R.; Zuhorn, I.S.; Salvati, A. Effect of endothelial cell heterogeneity on nanoparticle uptake. Int. J. Pharm. 2020, 587, 119699. [Google Scholar] [CrossRef]
- Aliyandi, A.; Reker-Smit, C.; Bron, R.; Zuhorn, I.S.; Salvati, A. Correlating corona composition and cell uptake to identify proteins affecting nanoparticle entry into endothelial cells. ACS Biomater. Sci. Eng. 2021, 7, 5573–5584. [Google Scholar] [CrossRef]
- Mohamed, T.; Matou-Nasri, S.; Farooq, A.; Whitehead, D.; Azzawi, M. Polyvinylpyrrolidone-coated gold nanoparticles inhibit endothelial cell viability, proliferation, and ERK1/2 phosphorylation and reduce the magnitude of endothelial-independent dilator responses in isolated aortic vessels. Int. J. Nanomed. 2017, 12, 8813–8830. [Google Scholar] [CrossRef] [Green Version]
- Pulido-Reyes, G.; Briffa, S.M.; Hurtado-Gallego, J.; Yudina, T.; Leganés, F.; Puntes, V.; Valsami-Jones, E.; Rosal, R.; Fernández-Piñas, F. Internalization and toxicological mechanisms of uncoated and PVP-coated cerium oxide nanoparticles in the freshwater alga Chlamydomonas reinhardtii. Environ. Sci. Nano 2019, 6, 1959–1972. [Google Scholar] [CrossRef]
- Radeva, M.Y.; Waschke, J. Mind the gap: Mechanisms regulating the endothelial barrier. Acta Physiol. 2018, 222, e12860. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Nadar, S.; Blann, A.D.; Lip, G.Y. Endothelial dysfunction: Methods of assessment and application to hypertension. Curr. Pharm. Des. 2004, 10, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.D. Mechanism of diapedesis: Importance of the transcellular route. Adv. Immunol. 2016, 129, 25–53. [Google Scholar] [PubMed] [Green Version]
- Montane, X.; Bajek, A.; Roszkowski, K.; Montornes, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for cancer therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef]
- Stanescu, P.O.; Radu, I.C.; Leu Alexa, R.; Hudita, A.; Tanasa, E.; Ghitman, J.; Stoian, O.; Tsatsakis, A.; Ginghina, O.; Zaharia, C.; et al. Novel chitosan and bacterial cellulose biocomposites tailored with polymeric nanoparticles for modern wound dressing development. Drug Deliv. 2021, 28, 1932–1950. [Google Scholar] [CrossRef] [PubMed]
- Cooney, L.; Loke, Y.K.; Golder, S.; Kirkham, J.; Jorgensen, A.; Sinha, I.; Hawcutt, D. Overview of systematic reviews of therapeutic ranges: Methodologies and recommendations for practice. BMC Med. Res. Methodol. 2017, 17, 84. [Google Scholar] [CrossRef]
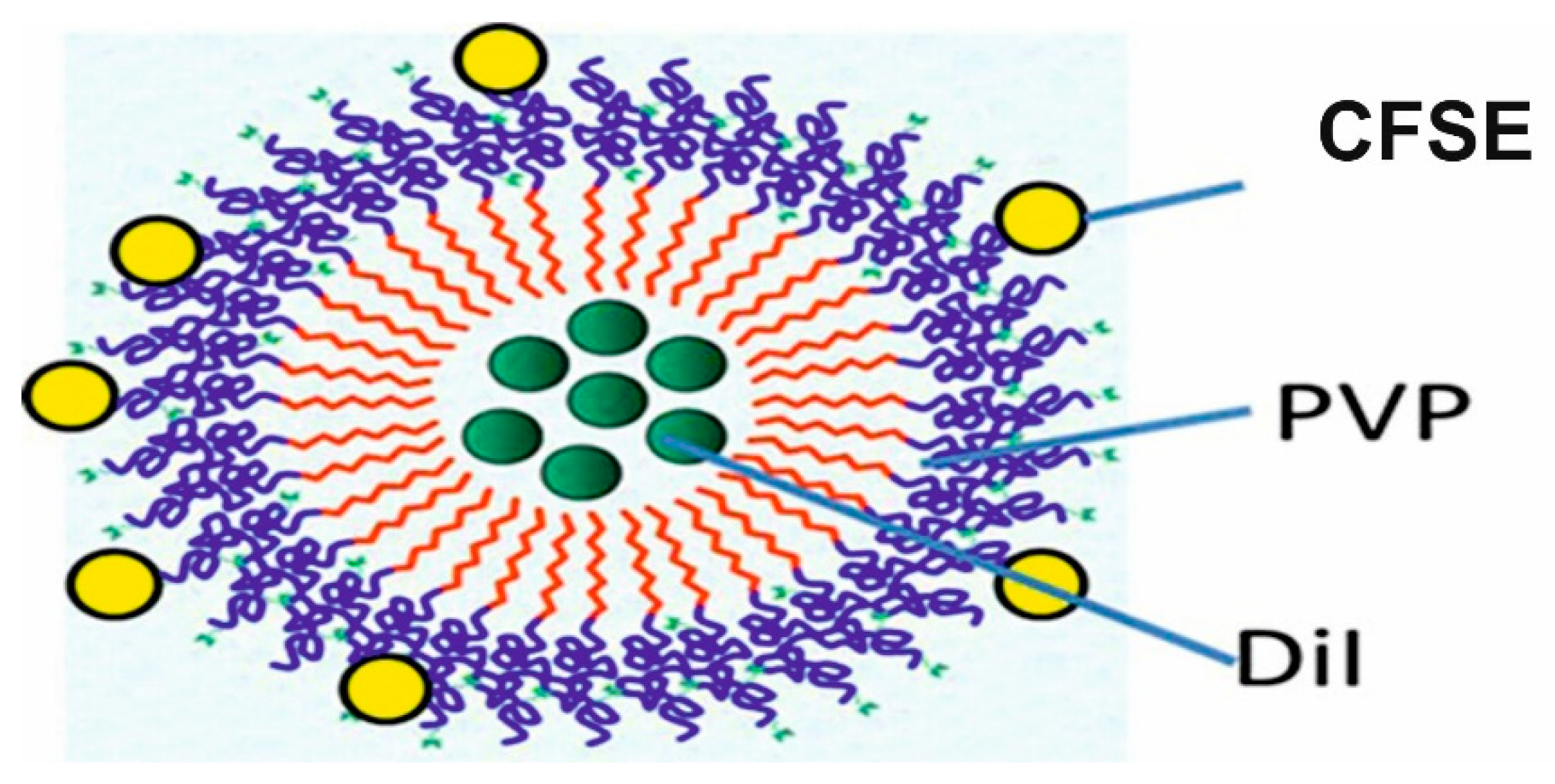

| NPs | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Hollow p(VP-AA) | 155 ± 8 | 0.174 ± 0.025 | −17.9 ± 0.91 |
| DiI loaded p(VP-AA) linked with CFSE nanoparticles (DNPs) | 172 ± 10 | 0.156 ± 0.031 | −15.2 ± 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdiaki, A.; Kuskov, A.N.; Kulikov, P.P.; Thrapsanioti, L.-N.; Giatagana, E.-M.; Stivaktakis, P.; Shtilman, M.I.; Tsatsakis, A.; Nikitovic, D. In Vitro Assessment of Poly-N-Vinylpyrrolidone/Acrylic Acid Nanoparticles Biocompatibility in a Microvascular Endothelium Model. Int. J. Mol. Sci. 2022, 23, 12446. https://doi.org/10.3390/ijms232012446
Berdiaki A, Kuskov AN, Kulikov PP, Thrapsanioti L-N, Giatagana E-M, Stivaktakis P, Shtilman MI, Tsatsakis A, Nikitovic D. In Vitro Assessment of Poly-N-Vinylpyrrolidone/Acrylic Acid Nanoparticles Biocompatibility in a Microvascular Endothelium Model. International Journal of Molecular Sciences. 2022; 23(20):12446. https://doi.org/10.3390/ijms232012446
Chicago/Turabian StyleBerdiaki, Aikaterini, Andrey N. Kuskov, Pavel P. Kulikov, Lydia-Nefeli Thrapsanioti, Eirini-Maria Giatagana, Polychronis Stivaktakis, Mikhail I. Shtilman, Aristidis Tsatsakis, and Dragana Nikitovic. 2022. "In Vitro Assessment of Poly-N-Vinylpyrrolidone/Acrylic Acid Nanoparticles Biocompatibility in a Microvascular Endothelium Model" International Journal of Molecular Sciences 23, no. 20: 12446. https://doi.org/10.3390/ijms232012446








