The Third Extracellular Loop of Mammalian Odorant Receptors Is Involved in Ligand Binding
Abstract
:1. Introduction
2. Results
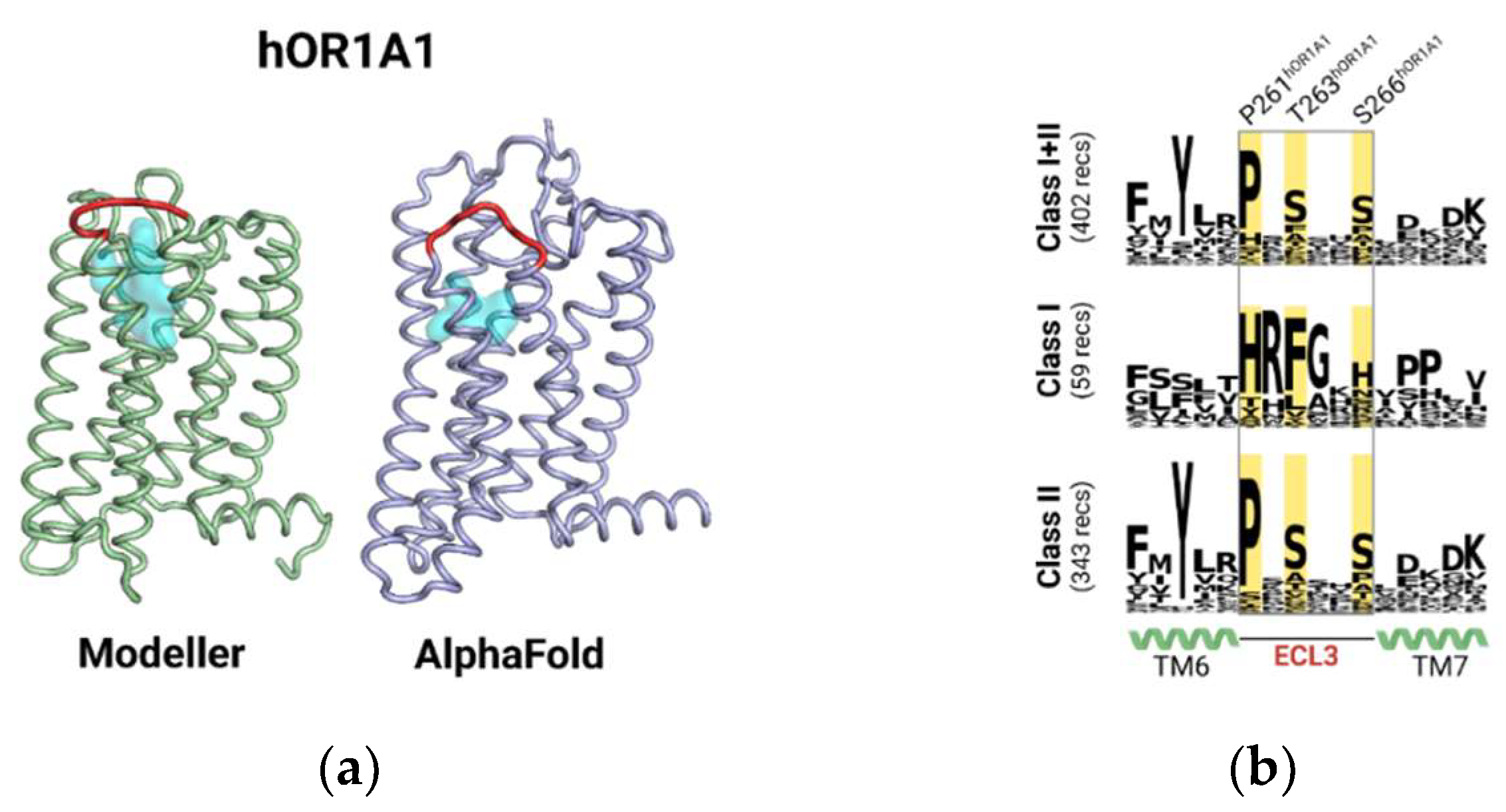
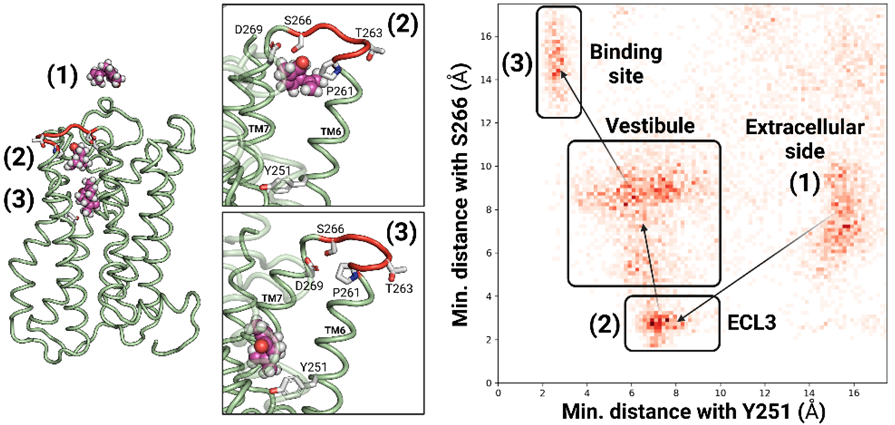
2.1. The Highly Variable ECL3 Sequence Acts as a Vestibular Binding Pocket for Ligands
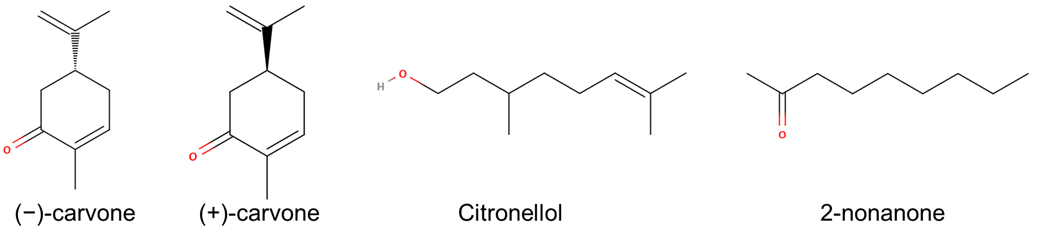
2.2. In Vitro Functional Assays Highlight the Differential Interactions with Diverse Ligands
3. Discussion
4. Materials and Methods
4.1. In Silico Experiments
4.1.1. Molecular Modeling
4.1.2. Molecular Dynamics Preparation
4.1.3. Molecular Dynamics Protocol
4.2. In Vitro Experiments
4.2.1. Cell Culture
4.2.2. DNA Constructs, Site-Directed Mutagenesis, and Gene Transfection
4.2.3. cAMP Luminescence Assay
4.2.4. Functional Expression
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debat, H.; Eloit, C.; Blon, F.; Sarazin, B.; Henry, C.; Huet, J.C.; Trotier, D.; Pernollet, J.C. Identification of human olfactory cleft mucus proteins using proteomic analysis. J. Proteome Res. 2007, 6, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, A.; Touhara, K. Enzymatic conversion of odorants in nasal mucus affects olfactory glomerular activation patterns and odor perception. J. Neurosci. 2010, 30, 16391–16398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelosi, P.; Knoll, W. Odorant-binding proteins of mammals. Biol. Rev. Camb. Philos. Soc. 2022, 97, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Malnic, B.; Godfrey, P.A.; Buck, L.B. The human olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2584–2589. [Google Scholar] [CrossRef] [Green Version]
- Muratore, A.; Clinet, J.C.; Dunach, E. Synthesis of new exo- and endo-3,8-dihydro-beta-santalols and other norbornyl-derived alcohols. Chem. Biodivers. 2010, 7, 623–638. [Google Scholar] [CrossRef]
- Kraft, P.; Frater, G. Enantioselectivity of the musk odor sensation. Chirality 2001, 13, 388–394. [Google Scholar] [CrossRef]
- Trimmer, C.; Keller, A.; Murphy, N.R.; Snyder, L.L.; Willer, J.R.; Nagai, M.H.; Katsanis, N.; Vosshall, L.B.; Matsunami, H.; Mainland, J.D. Genetic variation across the human olfactory receptor repertoire alters odor perception. Proc. Natl. Acad. Sci. USA 2019, 116, 9475–9480. [Google Scholar] [CrossRef] [Green Version]
- Gerkin, R.C.; Castro, J.B. The number of olfactory stimuli that humans can discriminate is still unknown. Elife 2015, 4, e08127. [Google Scholar] [CrossRef]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef]
- Lawson, Z.; Wheatley, M. The third extracellular loop of G-protein-coupled receptors: More than just a linker between two important transmembrane helices. Biochem. Soc. Trans. 2004, 32, 1048–1050. [Google Scholar] [CrossRef]
- Wheatley, M.; Wootten, D.; Conner, M.T.; Simms, J.; Kendrick, R.; Logan, R.T.; Poyner, D.R.; Barwell, J. Lifting the lid on GPCRs: The role of extracellular loops. Br. J. Pharmacol. 2012, 165, 1688–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, M.C.; van Westen, G.J.; Li, Q.; AP, I.J. Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol. Sci. 2011, 32, 35–42. [Google Scholar] [CrossRef]
- Wheatley, M.; Simms, J.; Hawtin, S.R.; Wesley, V.J.; Wootten, D.; Conner, M.; Lawson, Z.; Conner, A.C.; Baker, A.; Cashmore, Y.; et al. Extracellular loops and ligand binding to a subfamily of Family A G-protein-coupled receptors. Biochem. Soc. Trans. 2007, 35, 717–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dror, R.O.; Arlow, D.H.; Maragakis, P.; Mildorf, T.J.; Pan, A.C.; Xu, H.; Borhani, D.W.; Shaw, D.E. Activation mechanism of the beta2-adrenergic receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 18684–18689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushdid, C.; de March, C.A.; Topin, J.; Do, M.; Matsunami, H.; Golebiowski, J. Mammalian class I odorant receptors exhibit a conserved vestibular-binding pocket. Cell Mol. Life Sci. 2019, 76, 995–1004. [Google Scholar] [CrossRef]
- Charlier, L.; Topin, J.; Ronin, C.; Kim, S.K.; Goddard, W.A., 3rd; Efremov, R.; Golebiowski, J. How broadly tuned olfactory receptors equally recognize their agonists. Human OR1G1 as a test case. Cell Mol. Life Sci. 2012, 69, 4205–4213. [Google Scholar] [CrossRef]
- de March, C.A.; Kim, S.K.; Antonczak, S.; Goddard, W.A., 3rd; Golebiowski, J. G protein-coupled odorant receptors: From sequence to structure. Protein Sci. 2015, 24, 1543–1548. [Google Scholar] [CrossRef] [Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Jakubik, J.; Randakova, A.; Zimcik, P.; El-Fakahany, E.E.; Dolezal, V. Binding of N-methylscopolamine to the extracellular domain of muscarinic acetylcholine receptors. Sci. Rep. 2017, 7, 40381. [Google Scholar] [CrossRef]
- Geithe, C.; Noe, F.; Kreissl, J.; Krautwurst, D. The Broadly Tuned Odorant Receptor OR1A1 is Highly Selective for 3-Methyl-2,4-nonanedione, a Key Food Odorant in Aged Wines, Tea, and Other Foods. Chem. Senses 2017, 42, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Geithe, C.; Krautwurst, D. Chirality Matters—Enantioselective Orthologous Odorant Receptors for Related Terpenoid Structures. ACS Sym. Ser. 2015, 1212, 161–181. [Google Scholar]
- Geithe, C.; Protze, J.; Kreuchwig, F.; Krause, G.; Krautwurst, D. Structural determinants of a conserved enantiomer-selective carvone binding pocket in the human odorant receptor OR1A1. Cell Mol. Life Sci. 2017, 74, 4209–4229. [Google Scholar] [CrossRef] [PubMed]
- de March, C.A.; Yu, Y.; Ni, M.J.; Adipietro, K.A.; Matsunami, H.; Ma, M.; Golebiowski, J. Conserved Residues Control Activation of Mammalian G Protein-Coupled Odorant Receptors. J. Am. Chem. Soc. 2015, 137, 8611–8616. [Google Scholar] [CrossRef] [Green Version]
- Schmiedeberg, K.; Shirokova, E.; Weber, H.P.; Schilling, B.; Meyerhof, W.; Krautwurst, D. Structural determinants of odorant recognition by the human olfactory receptors OR1A1 and OR1A2. J. Struct. Biol. 2007, 159, 400–412. [Google Scholar] [CrossRef] [PubMed]
- de March, C.A.; Topin, J.; Bruguera, E.; Novikov, G.; Ikegami, K.; Matsunami, H.; Golebiowski, J. Odorant Receptor 7D4 Activation Dynamics. Angew. Chem. Int. Ed. Engl. 2018, 57, 4554–4558. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Meyerowitz, J.G.; Panova, O.; Borrelli, K.; Skiniotis, G. Plasticity in ligand recognition at somatostatin receptors. Nat. Struct. Mol. Biol. 2022, 29, 210–217. [Google Scholar] [CrossRef]
- Asada, H.; Horita, S.; Hirata, K.; Shiroishi, M.; Shiimura, Y.; Iwanari, H.; Hamakubo, T.; Shimamura, T.; Nomura, N.; Kusano-Arai, O.; et al. Crystal structure of the human angiotensin II type 2 receptor bound to an angiotensin II analog. Nat. Struct. Mol. Biol. 2018, 25, 570–576. [Google Scholar] [CrossRef]
- Vu, O.; Bender, B.J.; Pankewitz, L.; Huster, D.; Beck-Sickinger, A.G.; Meiler, J. The Structural Basis of Peptide Binding at Class A G Protein-Coupled Receptors. Molecules 2021, 27, 210. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2014, 1137, 1–15. [Google Scholar] [CrossRef]
- Cook, B.L.; Steuerwald, D.; Kaiser, L.; Graveland-Bikker, J.; Vanberghem, M.; Berke, A.P.; Herlihy, K.; Pick, H.; Vogel, H.; Zhang, S. Large-scale production and study of a synthetic G protein-coupled receptor: Human olfactory receptor 17-4. Proc. Natl. Acad. Sci. USA 2009, 106, 11925–11930. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006, 15, 2507–2524. [Google Scholar] [CrossRef] [Green Version]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively.y expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.; Sondergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef] [PubMed]
- Schott-Verdugo, S.; Gohlke, H. PACKMOL-Memgen: A Simple-To-Use, Generalized Workflow for Membrane-Protein-Lipid-Bilayer System Building. J. Chem. Inf. Model 2019, 59, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Cerutti, D.S.; Mermelstein, D.; Lin, C.; LeGrand, S.; Giese, T.J.; Roitberg, A.; Case, D.A.; Walker, R.C.; York, D.M. GPU-Accelerated Molecular Dynamics and Free Energy Methods in Amber18: Performance Enhancements and New Features. J. Chem. Inf Model 2018, 58, 2043–2050. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Wang, J.M.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. 3rd. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Shepard, B.D.; Natarajan, N.; Protzko, R.J.; Acres, O.W.; Pluznick, J.L. A cleavable N-terminal signal peptide promotes widespread olfactory receptor surface expression in HEK293T cells. PLoS ONE 2013, 8, e68758. [Google Scholar] [CrossRef] [Green Version]
- Hogrefe, H.H.; Cline, J.; Youngblood, G.L.; Allen, R.M. Creating randomized amino acid libraries with the QuikChange Multi Site-Directed Mutagenesis Kit. Biotechniques 2002, 33, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Matsunami, H. Evaluating cell-surface expression and measuring activation of mammalian odorant receptors in heterologous cells. Nat. Protoc. 2008, 3, 1402–1413. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, T.; Pacalon, J.; Kim, W.-C.; Cong, X.; Topin, J.; Golebiowski, J.; Moon, C. The Third Extracellular Loop of Mammalian Odorant Receptors Is Involved in Ligand Binding. Int. J. Mol. Sci. 2022, 23, 12501. https://doi.org/10.3390/ijms232012501
Shim T, Pacalon J, Kim W-C, Cong X, Topin J, Golebiowski J, Moon C. The Third Extracellular Loop of Mammalian Odorant Receptors Is Involved in Ligand Binding. International Journal of Molecular Sciences. 2022; 23(20):12501. https://doi.org/10.3390/ijms232012501
Chicago/Turabian StyleShim, Tammy, Jody Pacalon, Won-Cheol Kim, Xiaojing Cong, Jérémie Topin, Jérôme Golebiowski, and Cheil Moon. 2022. "The Third Extracellular Loop of Mammalian Odorant Receptors Is Involved in Ligand Binding" International Journal of Molecular Sciences 23, no. 20: 12501. https://doi.org/10.3390/ijms232012501
APA StyleShim, T., Pacalon, J., Kim, W.-C., Cong, X., Topin, J., Golebiowski, J., & Moon, C. (2022). The Third Extracellular Loop of Mammalian Odorant Receptors Is Involved in Ligand Binding. International Journal of Molecular Sciences, 23(20), 12501. https://doi.org/10.3390/ijms232012501






