The Manufacture of Xeno- and Feeder-Free Clinical-Grade Human Embryonic Stem Cell Lines: First Step for Cell Therapy
Abstract
1. Introduction
2. Results
2.1. Development of a System for the Manufacture and QC of Xeno-Free, Feeder-Free Clinical-Grade hESC Lines According to cGMP
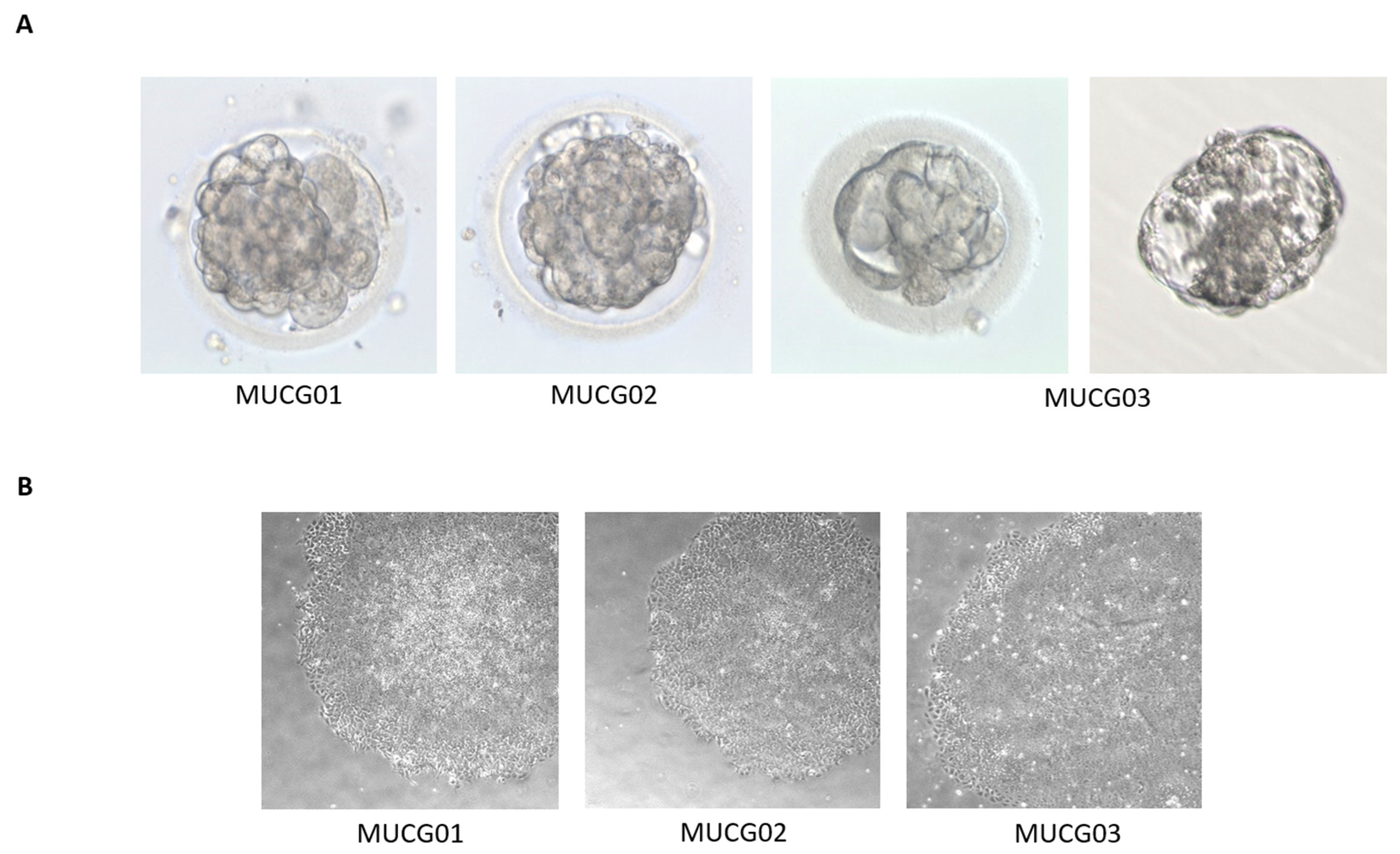
2.2. Xeno-Free and Feeder-Free Derivation and Culture of Clinical-Grade hESC Lines According to cGMP
2.3. Characterization of the Clinical-Grade hESC Lines
2.4. Sterility
2.5. Genetic Characterization
3. Discussion
4. Materials and Methods
4.1. Donor Testing
4.2. Preparation and Transport of Embryos
4.3. Derivation
4.4. Culture Conditions
4.5. Freezing
4.6. Mycoplasma Testing
4.7. Environmental and Personnel Monitoring
4.8. Endotoxin Testing
4.9. HLA Analysis
4.10. After-Thawing Recovery
4.11. Sterility
4.12. Cancer Predisposition Sequencing
4.13. Flow Cytometry
4.14. Cell Differentiation
4.15. Immunocytochemistry
4.16. Karyotyping
4.17. STR Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomson, J.A. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Plaza Reyes, A.; Petrus-Reurer, S.; Antonsson, L.; Stenfelt, S.; Bartuma, H.; Panula, S.; Mader, T.; Douagi, I.; André, H.; Hovatta, O.; et al. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Stem. Cell Rep. 2016, 6, 9–17. [Google Scholar] [CrossRef]
- Kirkeby, A.; Nolbrant, S.; Tiklova, K.; Heuer, A.; Kee, N.; Cardoso, T.; Ottosson, D.R.; Lelos, M.J.; Rifes, P.; Dunnett, S.B.; et al. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of HESC-Based Therapy for Parkinson’s Disease. Cell Stem. Cell 2017, 20, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, L.K.; Harjumäki, R.; Peltoniemi, P.; Bogacheva, M.S.; Salmi, T.; Porola, P.; Niklander, J.; Smutný, T.; Urtti, A.; Yliperttula, M.L.; et al. Laminin-511 and Laminin-521-Based Matrices for Efficient Hepatic Specification of Human Pluripotent Stem Cells. Biomaterials 2016, 103, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Verginer, L.; Riccaboni, M. Stem Cell Legislation and Its Impact on the Geographic Preferences of Stem Cell Researchers. Eurasian Bus Rev. 2021, 11, 163–189. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A Bioengineered Retinal Pigment Epithelial Monolayer for Advanced, Dry Age-Related Macular Degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell–Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef]
- Kobold, S.; Guhr, A.; Mah, N.; Bultjer, N.; Seltmann, S.; Seiler Wulczyn, A.E.M.; Stacey, G.; Jie, H.; Liu, W.; Löser, P.; et al. A Manually Curated Database on Clinical Studies Involving Cell Products Derived from Human Pluripotent Stem Cells. Stem. Cell Rep. 2020, 15, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Ogilvie, C. Pluripotent Stem Cells in Clinical Setting—New Developments and Overview of Current Status. Stem. Cells 2022, 40, sxac040. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Devito, L.; Miere, C.; Codognotto, S. Human Embryonic and Induced Pluripotent Stem Cells in Clinical Trials. Br. Med. Bull. 2015, 116, 19–27. [Google Scholar] [CrossRef]
- Desgres, M.; Menasché, P. Clinical Translation of Pluripotent Stem Cell Therapies: Challenges and Considerations. Cell Stem. Cell 2019, 25, 594–606. [Google Scholar] [CrossRef] [PubMed]
- EudraLex—Volume 4. Available online: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 22 August 2022).
- Carpenter, M.K. Chapter 6—Regulatory Considerations for Pluripotent Stem Cell Therapies. In Progress in Brain Research; Dunnett, S.B., Björklund, A., Eds.; Functional Neural Transplantation IV.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 230, pp. 151–163. [Google Scholar]
- Tannenbaum, S.E.; Reubinoff, B.E. Advances in HPSC Expansion towards Therapeutic Entities: A Review. Cell Prolif. 2022, 55, e13247. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, P.A.; Downie, J.M.; Tye, B.J.; Bruce, K.; Dand, P.; Dhanjal, S.; Serhal, P.; Harper, J.; Turner, M.; Bateman, M. Development and Production of Good Manufacturing Practice Grade Human Embryonic Stem Cell Lines as Source Material for Clinical Application. Stem Cell Res. 2016, 17, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.M.; Stacey, G.N. Setting Quality Standards for Stem Cell Banking, Research and Translation: The International Stem Cell Banking Initiative. In Stem Cell Banking; Ilic, D., Ed.; Stem Cell Biology and Regenerative Medicine; Springer: New York, NY, USA, 2014; pp. 3–9. ISBN 978-1-4939-0584-3. [Google Scholar]
- Abranches, E.; Spyrou, S.; Ludwig, T. GMP Banking of Human Pluripotent Stem Cells: A US and UK Perspective. Stem. Cell Res. 2020, 45, 101805. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A.; Bongso, A. Embryonic Stem Cell Lines from Human Blastocysts: Somatic Differentiation in Vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Pekkanen-Mattila, M.; Kerkelä, E.; Tanskanen, J.M.A.; Pietilä, M.; Pelto-Huikko, M.; Hyttinen, J.; Skottman, H.; Suuronen, R.; Aalto-Setälä, K. Substantial Variation in the Cardiac Differentiation of Human Embryonic Stem Cell Lines Derived and Propagated under the Same Conditions—A Comparison of Multiple Cell Lines. Ann. Med. 2009, 41, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Skottman, H.; Mikkola, M.; Lundin, K.; Olsson, C.; Strömberg, A.; Tuuri, T.; Otonkoski, T.; Hovatta, O.; Lahesmaa, R. Gene Expression Signatures of Seven Individual Human Embryonic Stem Cell Lines. Stem. Cells 2005, 23, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, S.E.; Turetsky, T.T.; Singer, O.; Aizenman, E.; Kirshberg, S.; Ilouz, N.; Gil, Y.; Berman-Zaken, Y.; Perlman, T.S.; Geva, N.; et al. Derivation of Xeno-Free and GMP-Grade Human Embryonic Stem Cells—Platforms for Future Clinical Applications. PLoS ONE 2012, 7, e35325. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Bates, N.; Soteriou, D.; Grady, L.; Edmond, C.; Ross, A.; Kerby, A.; Lewis, P.A.; Adeniyi, T.; Wright, R.; et al. High Quality Clinical Grade Human Embryonic Stem Cell Lines Derived from Fresh Discarded Embryos. Stem Cell Res. Ther. 2017, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.M.; Peura, T.T.; Kravets, L.; Bosman, A.G.; Buzzard, J.J.; Horne, R.; Hentze, H.; Dunn, N.R.; Zweigerdt, R.; Chua, F.; et al. The Generation of Six Clinical-Grade Human Embryonic Stem Cell Lines. Cell Stem. Cell 2007, 1, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Albalushi, H.; Kurek, M.; Karlsson, L.; Landreh, L.; Kjartansdóttir, K.R.; Söder, O.; Hovatta, O.; Stukenborg, J.-B. Laminin 521 Stabilizes the Pluripotency Expression Pattern of Human Embryonic Stem Cells Initially Derived on Feeder Cells. Available online: https://www.hindawi.com/journals/sci/2018/7127042/ (accessed on 10 January 2019).
- Närvä, E.; Pursiheimo, J.-P.; Laiho, A.; Rahkonen, N.; Emani, M.R.; Viitala, M.; Laurila, K.; Sahla, R.; Lund, R.; Lähdesmäki, H.; et al. Continuous Hypoxic Culturing of Human Embryonic Stem Cells Enhances SSEA-3 and MYC Levels. PLoS ONE 2013, 8, e78847. [Google Scholar] [CrossRef]
- Forristal, C.E.; Wright, K.L.; Hanley, N.A.; Oreffo, R.O.C.; Houghton, F.D. Hypoxia Inducible Factors Regulate Pluripotency and Proliferation in Human Embryonic Stem Cells Cultured at Reduced Oxygen Tensions. Reproduction 2010, 139, 85–97. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Off. J. Eur. Union 2006, 38, 40.
- Soukupová, J.; Zemánková, P.; Kleiblová, P.; Janatová, M.; Kleibl, Z. CZECANCA: CZEch CAncer paNel for Clinical Application—Design and Optimization of the Targeted Sequencing Panel for the Identification of Cancer Susceptibility in High-risk Individuals from the Czech Republic. Klin. Onkol. 2016, 29, S46–S54. [Google Scholar] [CrossRef]
- Main, H.; Hedenskog, M.; Acharya, G.; Hovatta, O.; Lanner, F. Karolinska Institutet Human Embryonic Stem Cell Bank. Stem. Cell Res. 2020, 45, 101810. [Google Scholar] [CrossRef] [PubMed]
- Kawase, E.; Takada, K.; Nakatani, R.; Yamazaki, S.; Suemori, H. Generation of Clinical-Grade Human Embryonic Stem Cell Line KthES11 According to Japanese Regulations. Stem. Cell Res. 2021, 54, 102383. [Google Scholar] [CrossRef]
- Rodin, S.; Antonsson, L.; Niaudet, C.; Simonson, O.E.; Salmela, E.; Hansson, E.M.; Domogatskaya, A.; Xiao, Z.; Damdimopoulou, P.; Sheikhi, M.; et al. Clonal Culturing of Human Embryonic Stem Cells on Laminin-521/E-Cadherin Matrix in Defined and Xeno-Free Environment. Nat. Commun. 2014, 5, 3195. [Google Scholar] [CrossRef]
- ESHRE Guideline Group on Good Practice in IVF Labs; De los Santos, M.J.; Apter, S.; Coticchio, G.; Debrock, S.; Lundin, K.; Plancha, C.E.; Prados, F.; Rienzi, L.; Verheyen, G.; et al. Revised Guidelines for Good Practice in IVF Laboratories (2015)†. Hum. Reprod. 2016, 31, 685–686. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.E.; Park, J.H.; Lee, J.B.; Kim, J.M.; Yoon, B.S.; Song, J.M.; Roh, S.I.; Kim, C.G.; Yoon, H.S. Efficient Derivation of New Human Embryonic Stem Cell Lines. Mol. Cells 2005, 19, 46–53. [Google Scholar] [PubMed]
- Strom, S.; Inzunza, J.; Grinnemo, K.-H.; Holmberg, K.; Matilainen, E.; Stromberg, A.-M.; Blennow, E.; Hovatta, O. Mechanical Isolation of the Inner Cell Mass Is Effective in Derivation of New Human Embryonic Stem Cell Lines. Hum. Reprod. 2007, 22, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Ghosh, S.; Genovese, G.; Handsaker, R.E.; Kashin, S.; Meyer, D.; Karczewski, K.J.; O’Dushlaine, C.; Pato, C.; Pato, M.; et al. Whole-Genome Analysis of Human Embryonic Stem Cells Enables Rational Line Selection Based on Genetic Variation. Cell Stem. Cell 2022, 29, 472–486.e7. [Google Scholar] [CrossRef]
- Buta, C.; David, R.; Dressel, R.; Emgård, M.; Fuchs, C.; Gross, U.; Healy, L.; Hescheler, J.; Kolar, R.; Martin, U.; et al. Reconsidering Pluripotency Tests: Do We Still Need Teratoma Assays? Stem. Cell Res. 2013, 11, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Karanu, F.; Ott, L.; Webster, D.A.; Stehno-Bittel, L. Improved Harmonization of Critical Characterization Assays across Cell Therapies. Regen. Med. 2020, 15, 1661–1678. [Google Scholar] [CrossRef]
- Souralova, T.; Holubcova, Z.; Kyjovska, D.; Hampl, A.; Koutna, I. Xeno- and Feeder-Free Derivation of Two Sex-Discordant Sibling Lines of Human Embryonic Stem Cells. Stem Cell Res. 2021, 57, 102574. [Google Scholar] [CrossRef]


| Derivation No. | Number of Embryos 1 | Clinical-Grade hESC Lines |
|---|---|---|
| 1 | 8 | 2 |
| 2 | 10 | 1 |
| 3 | 10 | 0 |
| 4 | 10 | 0 |
| 4 | 38 | 3 |
| Conditions | |
|---|---|
| Embryo stage | MUCG01: hatching blastocyst |
| MUCG02: blastocyst | |
| MUCG03: hatching blastocyst | |
| Derivation conditions | 37 °C, 5% CO2, and 5% O2 |
| Derivation medium | NutriStem hPSC XF medium containing 20 mg/mL human serum albumin and 10 µM ROCK inhibitor |
| Derivation substrate | 16.6 µg/mL (2.6 µg/cm2) Biolaminin 521 CTG and 1.7 µg/mL (0.27 µg/cm2) E-cadherin |
| Conditions | |
|---|---|
| Culture conditions | 37 °C, 5% CO2 and 5% O2 |
| Culture medium | NutriStem hPSC XF Medium |
| Substrate | p0–p3: 16.6 µg/mL (1.9 µg/cm2) Biolaminin 521 CTG and 1.7 µg/mL (0.27 µg/cm2) E-cadherin >p3: 10.0 µg/mL (1.1 µg/cm2) Biolaminin 521 CTG |
| Passage | ROCK inhibitor 1 h before and 24 h after passage |
| p0–p3: mechanical passage >p3: 0.5mM EDTA passage, 1:10 | |
| Freezing medium | 65% NutriStem hPSC XF Medium, 25% CTS Knockout SR XenoFree Medium, 10% CryoSure-DMSO, and 10 µM ROCK inhibitor |
| Number of frozen cells | 0.5 × 106 cells/mL in cryotube |
| Title 1 | Expansion into PMCB | WCB |
|---|---|---|
| MUCG01 | 100.00% SSEA4+ | 99.97% SSEA4+ |
| 98.89% TRA-1-60+ | 93.37% TRA-1-60+ | |
| 96.75% TRA-1-81+ | 99.00% TRA-1-81+ | |
| MUCG02 | 100.00% SSEA4+ | 99.77% SSEA4+ |
| 97.79% TRA-1-60+ | 94.10% TRA-1-60+ | |
| 93.47% TRA-1-81+ | 98.81% TRA-1-81+ | |
| MUCG03 | 100.00% SSEA4+ | 99.96% SSEA4+ |
| 97.97% TRA-1-60+ | 77.13% TRA-1-60+ | |
| 90.90% TRA-1-81+ | 97.89% TRA-1-81+ |
| Test | Stage | Result 1 |
|---|---|---|
| HIV 1/2, hepatitis B, hepatitis C and syphilis | donor testing | negative |
| sterility | derivation, PMCB, MCB, WCB | negative |
| mycoplasma | derivation, WCB | negative |
| endotoxin | WCB | <1 EU/mL |
| environmental monitoring | during the manufacture | under the limit 2 |
| personnel monitoring | during the manufacture | under the limit 2 |
| hESC Line | HLA Profile |
|---|---|
| MUCG01 | A*02; B*15, *44; C*03, 07; DRB1*04, *16; DQB1*03, *05 |
| MUCG02 | A*02; *32; B*15, *40; C*02, 07; DRB1*04, *11; DQB1*03 |
| MUCG03 | A*01; *24; B*08, *58; C*07; DRB1*03, *08; DQB1*02, *04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souralova, T.; Rehakova, D.; Jeseta, M.; Tesarova, L.; Beranek, J.; Ventruba, P.; Hampl, A.; Koutna, I. The Manufacture of Xeno- and Feeder-Free Clinical-Grade Human Embryonic Stem Cell Lines: First Step for Cell Therapy. Int. J. Mol. Sci. 2022, 23, 12500. https://doi.org/10.3390/ijms232012500
Souralova T, Rehakova D, Jeseta M, Tesarova L, Beranek J, Ventruba P, Hampl A, Koutna I. The Manufacture of Xeno- and Feeder-Free Clinical-Grade Human Embryonic Stem Cell Lines: First Step for Cell Therapy. International Journal of Molecular Sciences. 2022; 23(20):12500. https://doi.org/10.3390/ijms232012500
Chicago/Turabian StyleSouralova, Tereza, Daniela Rehakova, Michal Jeseta, Lenka Tesarova, Jindrich Beranek, Pavel Ventruba, Ales Hampl, and Irena Koutna. 2022. "The Manufacture of Xeno- and Feeder-Free Clinical-Grade Human Embryonic Stem Cell Lines: First Step for Cell Therapy" International Journal of Molecular Sciences 23, no. 20: 12500. https://doi.org/10.3390/ijms232012500
APA StyleSouralova, T., Rehakova, D., Jeseta, M., Tesarova, L., Beranek, J., Ventruba, P., Hampl, A., & Koutna, I. (2022). The Manufacture of Xeno- and Feeder-Free Clinical-Grade Human Embryonic Stem Cell Lines: First Step for Cell Therapy. International Journal of Molecular Sciences, 23(20), 12500. https://doi.org/10.3390/ijms232012500






