Reversible Platelet Integrin αIIbβ3 Activation and Thrombus Instability
Abstract
:1. Molecular Concept of Integrin Activation
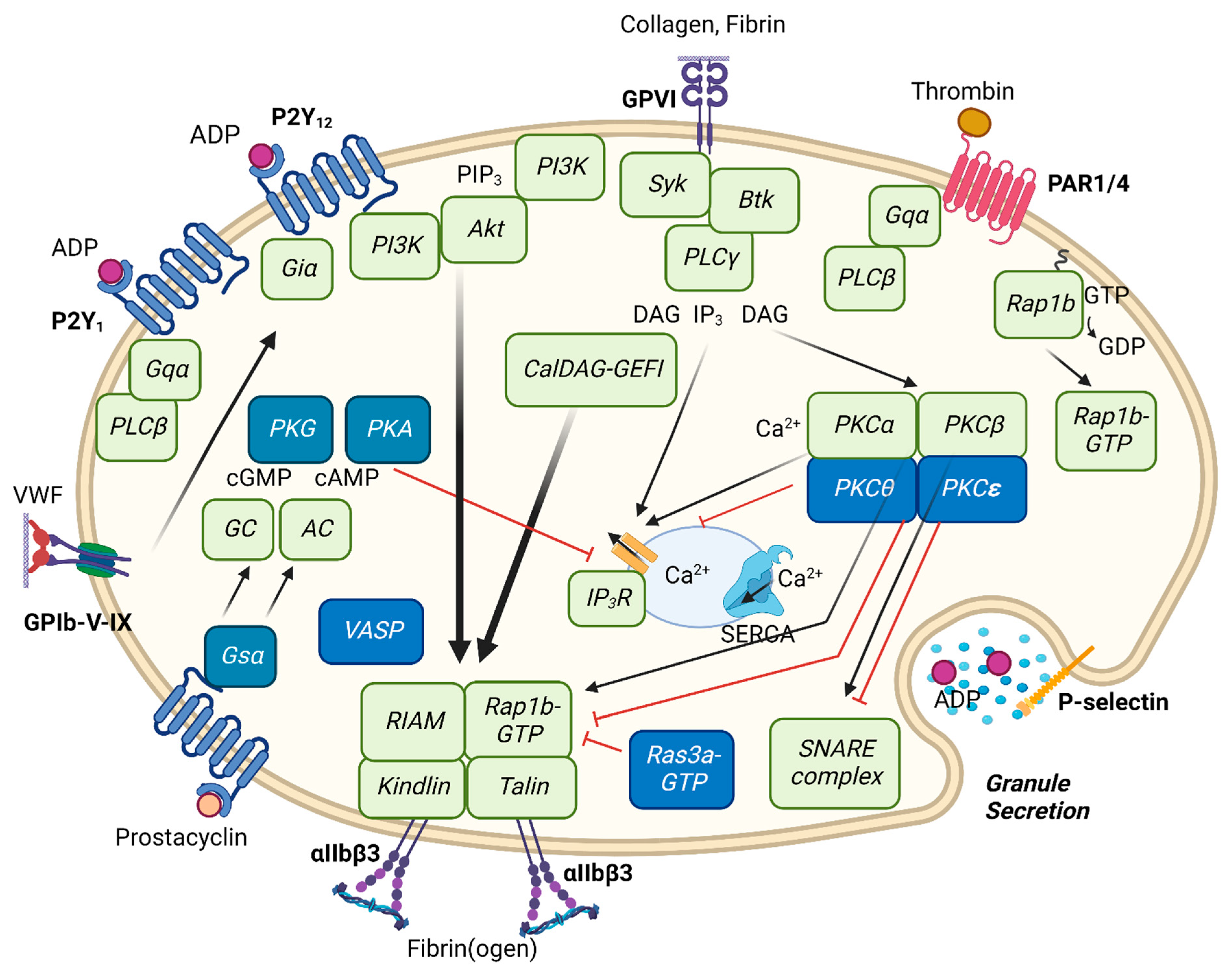
2. Reversible Integrin αIIbβ3 Activation and Inside-Out Signaling
3. ADP Receptor Stimulation
4. Collagen GPVI Receptor Stimulation
5. Thrombin PAR1 and PAR4 Receptor Stimulation
6. Integrin αIIbβ3 Regulation by Other Extracellular Proteases
7. Integrin αIIbβ3 Regulation via Protease-Dependent Receptor Cleavage
8. Reversible Integrin Activation and Thrombus Instability
9. Integrin αIIbβ3 Regulation by Intracellular Signaling Molecules
9.1. PLC and PKC Isoforms
9.2. PI3K Isoforms
9.3. Akt Isoforms
9.4. Small GTPases and Integrin Regulation
10. Platelet Inhibition by Protein Kinases A and G
11. Concluding Remarks and Relevance
| Agonist | Reversing Inhibitor | Reversing Pathway | Reference |
|---|---|---|---|
| ADP | tirofiban, abciximab | αIIbβ3 antagonism | [30] |
| ADP | SR121566 | αIIbβ3 antagonism | [115] |
| ADP | Gas6 depletion | TAM antagonism | [116] |
| ADP | citrated PRP plus CaCl2 | Ca2+/Mg2+ replacement | [117] |
| ADP, shear | lamifiban | αIIbβ3 antagonism | [118] |
| ADP, collagen | ticagrelor | P2Y12 antagonism | [31,119] |
| ADP, collagen | TGX-221, wortmannin | PI3K antagonism | [31] |
| ADP, collagen | iloprost | cAMP elevation | [31] |
| ADP, TRAP6 | αCD62P antibody | P-selectin blockage | [120] |
| TRAP6 | iloprost (+tirofiban) | cAMP elevation | [110] |
| PAR1p | wortmannin | PI3K antagonism | [121] |
| PAR4p | 2-MeSADP | P2Y antagonism | [86] |
| Gene Defect | Protein Defect | Thrombus Formation | Disaggregation or Embolization | References |
|---|---|---|---|---|
| Akt1 | protein kinase Akt1 | ↓ | no | [77,80,81,122] |
| Akt2 | protein kinase Akt2 | ↓ | yes | [78] |
| Akt3 | protein kinase Akt3 | ↓ | yes | [79] |
| Arhgef10 | GEF Rho-GEF10 | ↓↓ | yes | [96] |
| Cd18 | integrin β2 (CD18) | ↓ | no | [123] |
| Gp6 | GPVI receptor | ↓ | no (human yes) | [42] |
| Happ2 | factor VII activating (FSAP) | ↓ | yes | [45] |
| Itga2 | integrin α2 | 0 or ↓ | no | [124,125,126] |
| Itga2b | integrin αIIb | ↓↓ | no | [127] |
| Itga6 | integrin α6 | ↓↓ | no | [128] |
| Itgb1 | integrin β1 | ↓ | yes | [129,130,131] |
| Itgb3 | integrin β3 | ↓↓ | yes | [43] |
| P2ry1 | P2Y1 receptor | ↓↓ | yes | [62,63] |
| P2ry12 | P2Y12 receptor | ↓↓ | yes | [62,64,66,67,132] |
| Pik3ca | PI3K alpha | ↓ | no | [133] |
| Pik3cb | PI3K beta | ↓↓ | yes (U46619) | [134] |
| Pik3cg | PI3K gamma | ↓↓ | yes (ADP) | [31,135] |
| Prkca | PKC alpha | ↓↓ | no | [70] |
| Prkcd | PKC delta | 0 | no | [73,136] |
| Prkce | PKC epsilon | ↑ | no | [71] |
| Prkcq | PKC theta | ↓ or ↑ | no | [72,73,137,138] |
| Rasa3 | GAP Rasa3 | ↑ | no | [95] |
| Rasgrp2 | GEF CalDAG-GEFI | ↓↓ | no | [132,139] |
| Rap1b | GTPase Rap1b | ↓↓ | no | [90] |
| Rhoa | GTPase RhoA | ↓↓ | no | [98] |
| Rras2 | TC21/RRas | ↓ | yes | [99] |
| Tln1 | talin 1 | ↓↓ | no | [11,84] |
| Treml1 | TLT-1 | ↓ | no | [140] |
| Vasp | VASP protein | 0 | no | [103] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Plow, E.F.; Pesho, M.M.; Ma, Y.Q. Integrin αIIbβ3. Platelets; Michelson, A.D., Ed.; Academic Press: Amsterdam, The Netherlands, 2007; pp. 165–178. [Google Scholar]
- Coller, B.S.; Shattil, S.J. The GPIIb/IIIa (integrin αIIbβ3) odyssey: A technology-driven saga of a receptor with twists, turns, and even a bend. Blood 2008, 112, 3011–3025. [Google Scholar] [CrossRef] [Green Version]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev Immunol. 2007, 25, 619. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.-P.; Stehle, T.; Diefenbach, B.; Zhang, R.; Dunker, R.; Scott, D.L.; Joachimiak, A.; Goodman, S.L.; Arnaout, M.A. Crystal structure of the extracellular segment of integrin αvβ3. Science 2001, 294, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Adair, B.D.; Yeager, M. Three-dimensional model of the human platelet integrin αIIbβ3 based on electron cryomicroscopy and x-ray crystallography. Proc. Natl. Acad. Sci. USA 2002, 99, 14059–14064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, K.-E. A coiled-coil structure of the αIIbβ3 integrin transmembrane and cytoplasmic domains in its resting state. Structure 2005, 13, 703–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinogradova, O.; Velyvis, A.; Velyviene, A.; Hu, B.; Haas, T.A.; Plow, E.F.; Qin, J. A structural mechanism of integrin αIIbβ3 inside-out activation as regulated by its cytoplasmic face. Cell 2002, 110, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Lim, C.J.; Watanabe, N.; Soriani, A.; Ratnikov, B.; Calderwood, D.A.; Puzon-McLaughlin, W.; Lafuente, E.M.; Boussiotis, V.A.; Shattil, S.J. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr. Biol. 2006, 16, 1796–1806. [Google Scholar] [CrossRef] [Green Version]
- Arnaout, M.A.; Goodman, S.L.; Xiong, J.P. Structure and mechanics of integrin-based cell adhesion. Curr. Opin. Cell Biol. 2007, 19, 495–507. [Google Scholar] [CrossRef] [Green Version]
- Petrich, B.G.; Marchese, P.; Ruggeri, Z.M.; Spiess, S.; Weichert, R.A.; Ye, F.; Tiedt, R.; Skoda, R.C.; Monkley, S.J.; Critchley, D.R. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J. Exp. Med. 2007, 204, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, F.; Kim, C.; Ginsberg, M.H. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood 2016, 128, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural basis of collagen recognition by integrin α2β1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Nuyttens, B.P.; Thijs, T.; Deckmyn, H.; Broos, K. Platelet adhesion to collagen. Thromb. Res. 2011, 127, S26–S29. [Google Scholar] [CrossRef]
- Sun, Z.; Costell, M.; Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell. Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Swieringa, F.; Solari, F.A.; Provenzale, I.; Grassi, L.; De Simone, I.; Baaten, C.C.; Cavill, R.; Sickmann, A.; Frontini, M.; et al. Assessment of a complete and classified platelet proteome from genome-wide transcripts of human platelets and megakaryocytes covering platelet functions. Sci. Rep. 2021, 11, 12358. [Google Scholar] [CrossRef]
- Durrant, T.N.; van den Bosch, M.T.; Hers, I. Integrin αIIbβ3 outside-in signaling. Blood 2017, 130, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.D.; Stalker, T.J.; Voronov, R.; Muthard, R.W.; Tomaiuolo, M.; Diamond, S.L.; Brass, L.F. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood 2014, 124, 1808–1815. [Google Scholar] [CrossRef]
- Hrdinova, J.; Fernández, D.I.; Ercig, B.; Tullemans, B.M.; Suylen, D.P.; Agten, S.M.; Jurk, K.; Hackeng, T.M.; Vanhoorelbeke, K.; Voorberg, J.; et al. Structure-based cyclic glycoprotein Ibα-derived peptides interfering with von Willebrand factor binding affecting platelet aggregation under shear. Int. J. Mol. Sci. 2022, 23, 2046. [Google Scholar] [CrossRef]
- Kasirer-Friede, A.; Cozzi, M.R.; Mazzucato, M.; De Marco, L.; Ruggeri, Z.M.; Shattil, S.J. Signaling through GP Ib-IX-V activates αIIbβ3 independently of other receptors. Blood 2004, 103, 3403–3411. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.S. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, D.I.; Kuijpers, M.J.; Heemskerk, J.W. Platelet calcium signalling by G-protein coupled and ITAM-linked receptors regulating anoctamin-6 and procoagulant activity. Platelets 2021, 32, 863–871. [Google Scholar] [CrossRef]
- Van der Meijden, P.E.; Feijge, M.A.; Swieringa, F.; Gilio, K.; Nergiz-Unal, R.; Hamulyak, K.; Heemskerk, J.W. Key role of integrin αIIbβ3 signaling to Syk kinase in tissue factor-induced thrombin generation. Cell. Mol. Life Sci. 2012, 69, 3481–3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, B.; Wu, P.; Xue, F.; Yang, R.; Yu, Z.; Dai, K.; Ruan, C.; Liu, G.; Newman, P.J.; Gao, C. Integrin-αIIbβ3-mediated outside-in signalling activates a negative feedback pathway to suppress platelet activation. Thromb. Haemost. 2016, 116, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckly, A.; Gendrault, J.L.; Hechler, B.; Ceazenave, J.P.; Gachet, C. Differential involvement of the P2Y1 and P2Yt receptors in the morphological changes of platelet aggregation. Thromb. Haemost. 2001, 85, 694–701. [Google Scholar] [CrossRef]
- Cattaneo, M. The platelet P2Y₁₂ receptor for adenosine diphosphate: Congenital and drug-induced defects. Blood 2011, 117, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Van der Meijden, P.E.; Heemskerk, J.W. Platelet biology and functions: New concepts and future clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar]
- Moser, M.; Bertram, U.; Peter, K.; Bode, C.; Ruef, J. Abciximab, eptifibatide, and tirofiban exhibit dose-dependent potencies to dissolve platelet aggregates. J. Cardiovasc. Pharmacol. 2003, 41, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Cosemans, J.M.; Munnix, I.C.; Wetzker, R.; Heller, R.; Jackson, S.P.; Heemskerk, J.W. Continuous signaling via PI3K isoforms β and γ is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood 2006, 108, 3045–3052. [Google Scholar] [CrossRef]
- Li, Y.F.; Spencer, F.A.; Becker, R.C. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am. Heart J. 2002, 143, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Bärnthaler, T.; Mahla, E.; Toth, G.G.; Schuligoi, R.; Prüller, F.; Buschmann, E.; Heinemann, A. Supplemental fibrinogen restores platelet inhibitor-induced reduction in thrombus formation without altering platelet function: An in vitro study. Thromb. Haemost. 2020, 120, 1548–1556. [Google Scholar] [CrossRef]
- Cosemans, J.M.; Iserbyt, B.F.; Deckmyn, H.; Heemskerk, J.W. Multiple ways to switch platelet integrins on and off. J. Thromb. Haemost. 2008, 6, 1253–1261. [Google Scholar] [CrossRef]
- Hechler, B.; Nonne, C.; Roh, E.J.; Cattaneo, M.; Cazenave, J.P.; Lanza, F.; Jacobson, K.A.; Gachet, C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J. Pharmacol. Exp. Ther. 2006, 316, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, B.; Dwyer, K.; Enjyoji, K.; Robson, S.C. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: Potential as therapeutic targets. Blood Cells Mol. Diseas. 2006, 36, 217–222. [Google Scholar] [CrossRef]
- Hohmann, J.D.; Wang, X.; Krajewski, S.; Selan, C.; Haller, C.A.; Straub, A.; Chaikof, E.L.; Nandurkar, H.H.; Hagemeyer, C.E.; Peter, K. Delayed targeting of CD39 to activated platelet GPIIb/IIIa via a single-chain antibody: Breaking the link between antithrombotic potency and bleeding? Blood 2013, 121, 3067–3075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, M.; Hohmann, J.D.; Searle, A.K.; Abraham, M.K.; Nandurkar, H.H.; Wang, X.; Peter, K. A single-chain antibody-CD39 fusion protein targeting activated platelets protects from cardiac ischaemia/reperfusion injury. Eur. Heart J. 2018, 39, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki-Inoue, K.; Inoue, O.; Frampton, J.; Watson, S.P. Murine GPVI stimulates weak integrin activation in PLCγ2-/- platelets: Involvement of PLCγ1 and PI3-kinase. Blood 2003, 102, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Gilio, K.; Munnix, I.C.; Mangin, P.; Cosemans, J.M.; Feijge, M.A.; van der Meijden, P.E.; Olieslagers, S.; Chrzanowska-Wodnicka, M.B.; Lillian, R.; Schoenwaelder, S.; et al. Non-redundant roles of phosphoinositide 3-kinase isoforms α and β in glycoprotein VI-induced platelet signaling and thrombus formation. J. Biol. Chem. 2009, 285, 33750–33762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herfs, L.; Swieringa, F.; Jooss, N.; Kozlowski, M.; Heubel-Moenen, F.C.; van Oerle, R.; Machiels, P.; Henskens, Y.; Heemskerk, J.W. Multiparameter microfluidics assay of thrombus formation reveals increased sensitivity to contraction and antiplatelet agents at physiological temperature. Thromb. Res. 2021, 203, 46–56. [Google Scholar] [CrossRef]
- Janus-Bell, E.; Ahmed, M.U.; Receveur, N.; Mouriaux, C.; Nieswandt, B.; Gardiner, E.E.; Gachet, C.; Jandrot-Perrus, M.; Mangin, P.H. Differential role of glycoprotein VI in mouse and human thrombus progression and stability. Thromb. Haemost. 2021, 121, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Valiyaveettil, M.; Dudiki, T.; Mahabeleshwar, G.H.; André, P.; Podrez, E.A.; Byzova, T.V. β3 phosphorylation of platelet αIIbβ3 is crucial for stability of arterial thrombus and microparticle formation in vivo. Thromb. J. 2017, 15, 22. [Google Scholar] [CrossRef]
- Navarro, S.; Stegner, D.; Nieswandt, B.; Heemskerk, J.W.; Kuijpers, M.E. Temporal roles of platelet and coagulation pathways in collagen and tissue factor induced thrombus formation. Int. J. Mol. Sci. 2021, 23, 358. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Thielmann, I.; Morowski, M.; Pragst, I.; Sandset, P.M.; Nieswandt, B.; Etscheid, M.; Kanse, S.M. Defective thrombus formation in mice lacking endogenous factor VII activating protease (FSAP). Thromb. Haemost. 2015, 113, 870–880. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Heemskerk, J.W.; Baaten, C.C. Platelet membrane receptor proteolysis: Implications for platelet function. Front. Cardiovasc. Med. 2021, 7, 608391. [Google Scholar] [CrossRef]
- Bäck, M.; Ketelhuth, D.F.; Agewall, S. Matrix metalloproteinases in atherothrombosis. Progr. Cardiovasc. Dis. 2010, 52, 410–428. [Google Scholar] [CrossRef]
- Fernandez-Patron, C.; Martinez-Cuesta, M.A.; Salas, E.; Sawicki, G.; Wozniak, M.; Radomski, M.W.; Davidge, S.T. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and-2. Thromb. Haemost. 1999, 82, 1730–1735. [Google Scholar] [CrossRef]
- Mastenbroek, T.G.; Feijge, M.A.; Kremers, R.M.; van den Bosch, M.T.; Swieringa, F.; De Groef, L.; Moons, L.; Bennett, C.; Ghevaert, C.; Johnson, J.L. Platelet-associated matrix metalloproteinases regulate thrombus formation and exert local collagenolytic activity. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2554–2561. [Google Scholar] [CrossRef] [Green Version]
- Falcinelli, E.; Guglielmini, G.; Torti, M.; Gresele, P. Intraplatelet signaling mechanisms of the priming effect of matrix metalloproteinase-2 on platelet aggregation. J. Thromb. Haemost. 2005, 3, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, Y.; Wei, G.; Hu, W.; Li, L.; Lu, S.; Meng, Z. Matrix metalloproteinase12 facilitated platelet activation by shedding carcinoembryonic antigen related cell adhesion molecule1. Biochem. Biophys. Res. Commun. 2017, 486, 1103–1109. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Piffath, C.L.; Cheng, G.; Dole, V.S.; Zhang, Y.; von Andrian, U.H.; Wagner, D.D. Tumor necrosis factor-α–converting enzyme (ADAM17) mediates GPIbα shedding from platelets in vitro and in vivo. Circ. Res. 2004, 95, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montague, S.J.; Andrews, R.K.; Gardiner, E.E. Mechanisms of receptor shedding in platelets. Blood 2018, 132, 2535–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baaten, C.C.; Swieringa, F.; Misztal, T.; Mastenbroek, T.G.; Feijge, M.A.; Bock, P.E.; Donners, M.M.; Collins, P.W.; Li, R.; van der Meijden, P.E.; et al. Platelet heterogeneity in activation-induced glycoprotein shedding: Functional effects. Blood Adv. 2018, 2, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Mattheij, N.J.; Gilio, K.; van Kruchten, R.; Jobe, S.M.; Wieschhaus, A.J.; Chishti, A.H.; Collins, P.; Heemskerk, J.W.; Cosemans, J.M. Dual mechanism of integrin αIIbβ3 closure in procoagulant platelets. J. Biol. Chem. 2013, 288, 13325–13336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solari, F.A.; Mattheij, N.J.; Burkhart, J.M.; Swieringa, F.; Collins, P.W.; Cosemans, J.M.; Sickmann, A.; Heemskerk, J.W.; Zahedi, R.P. Combined quantification of the global proteome, phosphoproteome, and proteolytic cleavage to characterize altered platelet functions in the human Scott syndrome. Mol. Cell. Proteom. 2016, 15, 3154–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heemskerk, J.W.; Mattheij, N.J.; Cosemans, J.M. Platelet-based coagulation: Different populations, different functions. J. Thromb. Haemost. 2013, 11, 2–16. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Stalker, T.J.; Welsh, J.D.; Diamond, S.L.; Sinno, T.; Brass, L.F. A systems approach to hemostasis: 2. Computational analysis of molecular transport in the thrombus microenvironment. Blood 2014, 124, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Munnix, I.C.; Cosemans, J.M.; Auger, J.M.; Heemskerk, J.W. Platelet response heterogeneity in thrombus formation. Thromb. Haemost. 2009, 102, 1149–1156. [Google Scholar]
- Erhardt, J.A.; Toomey, J.R.; Douglas, S.A.; Johns, D.G. P2X1 stimulation promotes thrombin receptor-mediated platelet aggregation. J. Thromb. Haemost. 2006, 4, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Kahner, B.N.; Dorsam, R.T.; Mada, S.R.; Kim, S.; Stalker, T.J.; Brass, L.F.; Daniel, J.L.; Kitamura, D.; Kunapuli, S.P. Hematopoietic lineage cell–specific protein 1 is a functionally important signaling molecule in platelet activation. Blood 2007, 110, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, I.; Palmer, D.; David, T.; Wilsbacher, L.; Concengco, C.; Conley, P.; Pandey, A.; Coughlin, S.R. Roles and interactions among protease-activated receptors and P2ry12 in hemostasis and thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 18605–18610. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.B.; Jackman, L.E.; Francis, S.E.; Judge, H.M.; Nylander, S.; Storey, R.F. Ticagrelor effectively and reversibly blocks murine platelet P2Y12-mediated thrombosis and demonstrates a requirement for sustained P2Y12 inhibition to prevent subsequent neointima. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2385–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nergiz-Unal, R.; Cosemans, J.M.; Feijge, M.A.; van der Meijden, P.E.; Storey, R.F.; van Giezen, J.J.; oude Egbrink, M.G.; Heemskerk, J.W.; Kuijpers, M.J. Stabilizing role of platelet P2Y12 receptors in shear-dependent thrombus formation on ruptured plaques. PLoS ONE 2010, 5, e10130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescence, L.; Kramberg, M.; Baumann, M.; Rey, M.; Roux, S.; Panicot-Dubois, L.; Dubois, C.; Riederer, M.A. The P2Y12 receptor antagonist selatogrel dissolves preformed platelet thrombi in vivo. J. Clin. Med. 2021, 10, 5349. [Google Scholar] [CrossRef]
- Buensuceso, C.S.; Obergfell, A.; Soriani, A.; Eto, K.i.; Kiosses, W.B.; Arias-Salgado, E.G.; Kawakami, T.; Shattil, S.J. Regulation of outside-in signaling in platelets by integrin-associated protein kinase Cβ. J. Biol. Chem. 2005, 280, 644–653. [Google Scholar] [CrossRef] [Green Version]
- Harper, M.T.; Poole, A.W. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J. Thromb. Haemost. 2010, 8, 454–462. [Google Scholar] [CrossRef]
- Konopatskaya, O.; Gilio, K.; Harper, M.T.; Zhao, Y.; Cosemans, J.M.; Karim, Z.A.; Whiteheart, S.W.; Molkentin, J.D.; Verkade, P.; Watson, S.P.; et al. PKCα regulates platelet granule secretion and thrombus formation in mice. J. Clin. Investig. 2009, 119, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Bynagari-Settipalli, Y.S.; Lakhani, P.; Jin, J.; Bhavaraju, K.; Rico, M.C.; Kim, S.; Woulfe, D.; Kunapuli, S.P. Protein kinase C isoform ε negatively regulates ADP-induced calcium mobilization and thromboxane generation in platelets. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Nagy, B.; Bhavaraju, K.; Getz, T.; Bynagari, Y.S.; Kim, S.; Kunapuli, S.P. Impaired activation of platelets lacking protein kinase C-θ isoform. Blood 2009, 113, 2557–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilio, K.; Harper, M.T.; Cosemans, J.M.; Konopatskaya, O.; Munnix, I.C.; Prinzen, L.; Leitges, M.; Liu, Q.; Molkentin, J.D.; Heemskerk, J.W.; et al. Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. J. Biol. Chem. 2010, 285, 23410–23419. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.F.; Canobbio, I.; Torti, M. PI3K/Akt in platelet integrin signaling and implications in thrombosis. Adv. Biol. Regul. 2015, 59, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Rauch, U. PI3K inhibitors in cardiovascular disease. Cardiovasc. Therapeut. 2011, 29, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Li, Q.; Shen, J.; Ren, L.; Liu, X.; Wang, Q.; He, S.; Wu, Q.; Hu, H.; Mao, X. Modulation of platelet activation and thrombus formation using a pan-PI3K inhibitor S14161. PLoS ONE 2014, 9, e102394. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; De, S.; Damron, D.S.; Chen, W.S.; Hay, N.; Byzova, T.V. Impaired platelet responses to thrombin and collagen in Akt1-deficient mice. Blood 2004, 104, 1703–1710. [Google Scholar] [CrossRef] [Green Version]
- Woulfe, D.S.; Jiang, H.; Morgans, A.; Monks, R.; Birnbaum, M.; Brass, L.F. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J. Clin. Investig. 2004, 113, 441–450. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Stojanovic-Terpo, A.; Hay, N.; Du, X. An important role for Akt3 in platelet activation and thrombosis. Blood 2011, 118, 4215–4223. [Google Scholar] [CrossRef] [Green Version]
- Kroner, C.; Eybrechts, K.; Akkerman, J.W. Dual regulation of platelet protein kinase B. J. Biol. Chem. 2000, 275, 27790–27798. [Google Scholar] [CrossRef]
- Yin, H.; Stojanovic, A.; Hay, N.; Du, X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood 2008, 111, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Franke, B.; Akkerman, J.W.; Bos, J.L. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 1997, 16, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidetti, G.F.; Torti, M. The small GTPase Rap1b: A bidirectional regulator of platelet adhesion receptors. J. Signal Transduct. 2012, 2012, 412089. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, L.; Ye, F.; Snider, A.K.; Sarabakhsh, K.; Piatt, R.; Paul, D.S.; Bergmeier, W.; Petrich, B.G. A talin mutant that impairs talin-integrin binding in platelets decelerates αIIbβ3 activation without pathological bleeding. Blood 2014, 123, 2722–2731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crittenden, J.R.; Bergmeier, W.; Zhang, Y.; Piffath, C.L.; Liang, Y.; Wagner, D.D.; Housman, D.E.; Graybiel, A.M. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat. Med. 2004, 10, 982–986. [Google Scholar] [CrossRef]
- Cifuni, S.M.; Wagner, D.D.; Bergmeier, W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin αIIbβ3 in platelets. Blood 2008, 112, 1696–1703. [Google Scholar] [CrossRef] [Green Version]
- Stefanini, L.; Bergmeier, W. RAP1-GTPase signaling and platelet function. J. Mol. Med. 2016, 94, 13–19. [Google Scholar] [CrossRef]
- Lova, P.; Paganini, S.; Sinigaglia, F.; Balduini, C.; Torti, M. A Gi-dependent pathway is required for activation of the small GTPase Rap1b in human platelets. J. Biol. Chem. 2002, 277, 12009–12015. [Google Scholar] [CrossRef] [Green Version]
- Woulfe, D.S. Akt signaling in platelets and thrombosis. Exp.Rev. Hematol. 2010, 3, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowska-Wodnicka, M.; Smyth, S.S.; Schoenwaelder, S.M.; Fischer, T.H.; White, G.C. Rap1b is required for normal platelet function and hemostasis in mice. J. Clin. Investig. 2005, 115, 680–687. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Xiang, B.; Ye, S.; Chrzanowska-Wodnicka, M.; Morris, A.J.; Gartner, T.K.; Whiteheart, S.W.; White, G.C.; Smyth, S.S.; Li, Z. Distinct roles for Rap1b protein in platelet secretion and integrin αIIbβ3 outside-in signaling. J. Biol. Chem. 2011, 286, 39466–39477. [Google Scholar] [CrossRef] [Green Version]
- Stefanini, L.; Boulaftali, Y.; Ouellette, T.D.; Holinstat, M.; Désiré, L.; Leblond, B.; André, P.; Conley, P.B.; Bergmeier, W. Rap1-Rac1 circuits potentiate platelet activation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czikora, A.; Lundberg, D.J.; Abramovitz, A.; Lewin, N.E.; Kedei, N.; Peach, M.L.; Zhou, X.; Merritt, R.C.; Craft, E.A.; Braun, D.C.; et al. Structural basis for the failure of the C1 domain of Ras guanine nucleotide releasing protein 2 (RasGRP2) to bind phorbol ester with high affinity. J. Biol. Chem. 2016, 291, 11133–11147. [Google Scholar] [CrossRef] [PubMed]
- Stolla, M.; Stefanini, L.; Roden, R.C.; Chavez, M.; Hirsch, J.; Greene, T.; Ouellette, T.D.; Maloney, S.F.; Diamond, S.L.; Poncz, M.; et al. The kinetics of αIIbβ3 activation determines the size and stability of thrombi in mice: Implications for antiplatelet therapy. Blood 2011, 117, 1005–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanini, L.; Paul, D.S.; Robledo, R.F.; Chan, E.R.; Getz, T.M.; Campbell, R.A.; Kechele, D.O.; Casari, C.; Piatt, R.; Caron, K.M.; et al. Rasa3 is a critical inhibitor of Rap1-dependent platelet activation. J. Clin. Investig. 2015, 125, 1419–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.H.; Hsu, C.C.; Huang, S.W.; Tu, H.J.; Huang, T.F.; Liou, H.C.; Liao, H.M.; Chen, C.H.; Fu, W.M.; Gau, S.S. ARHGEF 10 knockout inhibits platelet aggregation and protects mice from thrombus formation. J. Thromb. Haemost. 2017, 15, 2053–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shattil, S.J.; Kashiwagi, H.; Pampori, N. Integrin signaling: The platelet paradigm. Blood 1998, 91, 2645–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleines, I.; Hagedorn, I.; Gupta, S.; May, F.; Chakarova, L.; van Hengel, J.; Offermanns, S.; Krohne, G.; Kleinschnitz, C.; Brakebusch, C.; et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 2012, 119, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janapati, S.; Wurtzel, J.; Dangelmaier, C.; Manne, B.K.; Bhavanasi, D.; Kostyak, J.C.; Kim, S.; Holinstat, M.; Kunapuli, S.P.; Goldfinger, L.E. TC21/RRas2 regulates glycoprotein VI-FcRγ-mediated platelet activation and thrombus stability. J. Thromb. Haemost. 2018, 16, 1632–1645. [Google Scholar] [CrossRef] [Green Version]
- Swieringa, F.; Solari, F.A.; Pagel, O.; Beck, B.; Faber, J.; Feijge, M.A.; Jurk, K.; Körver-Keularts, I.M.; Mattheij, N.J.; Pohlenz, J.; et al. Impaired iloprost-induced platelet inhibition and phosphoproteome changes in patients with confirmed pseudohypo-parathyroidism type Ia, linked to genetic mutations in GNAS. Sci. Rep. 2020, 10, 11389. [Google Scholar] [CrossRef]
- Beck, F.; Geiger, J.; Gambaryan, S.; Solari, F.A.; Dell’Aica, M.; Loroch, S.; Mattheij, N.; Mindukshev, I.; Pötz, O.; Jurk, K.; et al. Temporal quantitative phosphoproteomics of ADP stimulation reveals novel central nodes in platelet activation and inhibition. Blood 2017, 129, e1–e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhard, M.; Jarchau, T.; Walter, U. Actin-based motility: Stop and go with Ena/VASP proteins. Trends Biochem. Sci. 2001, 26, 243–249. [Google Scholar] [CrossRef]
- Aszódi, A.; Pfeifer, A.; Ahmad, M.; Glauner, M.; Zhou, X.H.; Ny, L.; Andersson, K.E.; Kehrel, B.; Offermanns, S.; Fässler, R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP-and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999, 18, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Benz, P.M.; Laban, H.; Zink, J.; Günther, L.; Walter, U.; Gambaryan, S.; Dib, K. Vasodilator-stimulated phosphoprotein (VASP)-dependent and -independent pathways regulate thrombin-induced activation of Rap1b in platelets. Cell. Commun. Signal. 2016, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, U.R.; Geiger, J.; Walter, U.; Eigenthaler, M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thromb. Haemost. 1999, 82, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Danielewski, O.; Schultess, J.; Smolenski, A. The NO/cGMP pathway inhibits Rap 1 activation in human platelets via cGMP-dependent protein kinase I. Thromb. Haemost. 2005, 93, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslam, R.J.; Dickinson, N.T.; Jang, E.K. Cyclic nucleotides and phosphodiesterases in platelets. Thromb. Haemost. 1999, 82, 412–423. [Google Scholar] [PubMed]
- Yang, J.; Wu, J.; Jiang, H.; Mortensen, R.; Austin, S.; Manning, D.R.; Woulfe, D.; Brass, L.F. Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J. Biol. Chem. 2002, 277, 46035–46042. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Colman, R.W. Thrombin regulates intracellular cyclic AMP concentration in human platelets through phosphorylation/activation of phosphodiesterase 3A. Blood 2007, 110, 1475–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Wu, J.; Roest, M.; Heemskerk, J.W. Long-term platelet priming after glycoprotein VI stimulation in comparison to protease-activating receptor (PAR) stimulation. PLoS ONE 2021, 16, e0247425. [Google Scholar] [CrossRef]
- Van Geet, C.; Izzi, B.; Labarque, V.; Freson, K. Human platelet pathology related to defects in the G-protein signaling cascade. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 282–286. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, L.; Thomson, G.J.; Adams, R.C.M.; Nell, T.A.; Laubscher, W.A.; Pretorius, E. Platelet activity and hypercoagulation in type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiodorenko-Dumas, Z.; Dumas, I.; Mastej, K.; Jakobsche-Policht, U.; Bittner, J.; Adamiec, R. Receptor GPIIb/IIIa as an indicator of risk in vascular events. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619845056. [Google Scholar] [CrossRef]
- Ju, L.; McFadyen, J.D.; Al-Daher, S.; Alwis, I.; Chen, Y.; Tønnesen, L.L.; Maiocchi, S.; Coulter, B.; Calkin, A.C.; Felner, E.I. Compression force sensing regulates integrin αIIbβ3 adhesive function on diabetic platelets. Nat. Commun. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savi, P.; Bernat, A.; Lalu, A.; Roque, C.; Zamboni, G.; Herbert, J.M. Effect of aspirin on platelet desaggregation induced by SR121566, a potent GPIIb/IIIa antagonist. Platelets 2000, 11, 43–48. [Google Scholar] [PubMed]
- Cosemans, J.M.; van Kruchten, R.; Olieslagers, S.; Schurgers, L.J.; Verheyen, F.K.; Munnix, I.C.; Waltenberger, J.; Angelillo-Scherrer, A.; Hoylaerts, M.F.; Carmeliet, P.; et al. Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J. Thromb. Haemost. 2010, 8, 1797–1808. [Google Scholar] [CrossRef]
- Filkova, A.A.; Martyanov, A.A.; Garzon Dasgupta, A.K.; Panteleev, M.A.; Sveshnikova, A.N. Quantitative dynamics of reversible platelet aggregation: Mathematical modelling and experiments. Sci. Rep. 2019, 9, 6217. [Google Scholar] [CrossRef] [Green Version]
- Frojmovic, M.; Labarthe, B.; Legrand, C. Inhibition and reversal of platelet aggregation by αIIbβ3 antagonists depends on the anticoagulant and flow conditions: Differential effects of abciximab and lamifiban. Br. J. Haematol. 2005, 131, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Choi, B.G.; Zafar, M.U.; Viles-Gonzalez, J.F.; Vorchheimer, D.A.; Fuster, V.; Badimon, J.J. Normalization of platelet reactivity in clopidogrel-treated subjects. J. Thromb. Haemost. 2007, 5, 82–90. [Google Scholar] [CrossRef]
- Merten, M.; Thiagarajan, P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 2000, 102, 1931–1936. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, S.Y.; Liao, C.Y.; Teng, C.M.; Wu, Y.C.; Kuo, S.C. The roles and mechanisms of PAR4 and P2Y12/phosphatidylinositol 3-kinase pathway in maintaining thrombin-induced platelet aggregation. Br. J. Pharmacol. 2010, 161, 643–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, M.; Borst, O.; Walker, B.; Fotinos, A.; Vogel, S.; Seizer, P.; Mack, A.; Alampour-Rajabi, S.; Rath, D.; Geisler, T. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ. Res. 2014, 115, 939–949. [Google Scholar] [CrossRef]
- Rumbaut, R.E.; Randhawa, J.K.; Smith, C.W.; Burns, A.R. Mouse cremaster venules are predisposed to light/dye-induced thrombosis independent of wall shear rate, CD18, ICAM-1, or P-selectin. Microcirculation 2004, 11, 239–247. [Google Scholar] [CrossRef]
- Grüner, S.; Prostredna, M.; Schulte, V.; Krieg, T.; Eckes, B.; Brakebusch, C.; Nieswandt, B. Multiple integrin-ligand interactions synergize in shear-resistant platelet adhesion at sites of arterial injury in vivo. Blood 2003, 102, 4021–4027. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pappan, L.K.; Grenache, D.G.; Li, Z.; Tollefsen, D.M.; Santoro, S.A.; Zutter, M.M. The contributions of the α2β1 integrin to vascular thrombosis in vivo. Blood 2003, 102, 3652–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjoram, R.J.; Li, Z.; He, L.; Tollefsen, D.M.; Kunicki, T.J.; Dickeson, S.K.; Santoro, S.A.; Zutter, M.M. α2β1 integrin, GPVI receptor, and common FcRγ chain on mouse platelets mediate distinct responses to collagen in models of thrombosis. PLoS ONE 2014, 9, e114035. [Google Scholar] [CrossRef] [Green Version]
- Roux, D.; Roullot, V.; Poujol, C.; Kortulewski, T.; Nurden, P.; Marguerie, G. Thrombasthenic mice generated by replacement of the integrin αIIb gene: Demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood 2000, 96, 1399–1408. [Google Scholar] [CrossRef]
- Schaff, M.; Tang, C.J.; Maurer, E.; Bourdon, C.; Receveur, N.; Eckly, A.; Hechler, B.; Arnold, C.; de Arcangelis, A.; Nieswandt, B.; et al. Integrin α6β1 is the main receptor for vascular laminins and plays a role in platelet adhesion, activation, and arterial thrombosis. Circulation 2013, 128, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Kuijpers, M.J.; Schulte, V.; Bergmeier, W.; Lindhout, T.; Brakebusch, C.; Offermanns, S.; Fässler, R.; Heemskerk, J.W.; Nieswandt, B. Complementary roles of glycoprotein VI and α2β1 integrin in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003, 17, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Eckly, A.; Hechler, B.; Freund, M.; Zerr, M.; Cazenave, J.P.; Lanza, F.; Mangin, P.H.; Gachet, C. Mechanisms underlying FeCl3-induced arterial thrombosis. J. Thromb. Haemost. 2011, 9, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Petzold, T.; Ruppert, R.; Pandey, D.; Barocke, V.; Meyer, H.; Lorenz, M.; Zhang, L.; Siess, W.; Massberg, S.; Moser, M. β1 integrin-mediated signals are required for platelet granule secretion and hemostasis in mouse. Blood 2013, 122, 2723–2731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolla, M.; Stefanini, L.; André, P.; Ouellette, T.D.; Reilly, M.P.; McKenzie, S.E.; Bergmeier, W. CalDAG-GEFI deficiency protects mice in a novel model of Fcγ RIIA-mediated thrombosis and thrombocytopenia. Blood 2011, 118, 1113–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holy, E.W.; Jakob, P.; Eickner, T.; Camici, G.G.; Beer, J.H.; Akhmedov, A.; Sternberg, K.; Schmitz, K.P.; Lüscher, T.F.; Tanner, F.C. PI3K/p110α inhibition selectively interferes with arterial thrombosis and neointima formation, but not re-endothelialization: Potential implications for drug-eluting stent design. Eur. Heart J. 2014, 35, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Guillermet-Guibert, J.; Chicanne, G.; Cabou, C.; Jandrot-Perrus, M.; Plantavid, M.; Vanhaesebroeck, B.; Payrastre, B.; Gratacap, M.P. Deletion of the p110β isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood 2010, 115, 2008–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, L.; Wang, Y.; Draznin, J.; Eslin, D.; Bennett, J.S.; Poncz, M.; Wu, D.; Abrams, C.S. The relative role of PLCβ and PI3Kγ in platelet activation. Blood 2005, 106, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chari, R.; Getz, T.; Nagy, B.; Bhavaraju, K.; Mao, Y.; Bynagari, Y.S.; Murugappan, S.; Nakayama, K.; Kunapuli, S.P. Protein kinase Cδ differentially regulates platelet functional responses. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 699–705. [Google Scholar] [CrossRef]
- Unsworth, A.J.; Finney, B.A.; Navarro-Nunez, L.; Severin, S.; Watson, S.P.; Pears, C.J. Protein kinase Cε and protein kinase Cθ double-deficient mice have a bleeding diathesis. J. Thromb. Haemost. 2012, 10, 1887–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, K.J.; Harper, M.T.; Gilio, K.; Cosemans, J.M.; Heemskerk, J.W.; Poole, A.W. Genetic analysis of the role of protein kinase Cθ in platelet function and thrombus formation. PLoS ONE 2008, 3, e3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piatt, R.; Paul, D.S.; Lee, R.H.; McKenzie, S.E.; Parise, L.V.; Cowley, D.O.; Cooley, B.C.; Bergmeier, W. Mice expressing low levels of CalDAG-GEFI exhibit markedly impaired platelet activation with minor impact on hemostasis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1838–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessica, M.O.; Fiorella, R.; Ocatavio, S.; Linnette, R.; Nahomy, L.; Kanth, M.B.; Bismarck, M.; Rondina, M.T.; Valance, W.A. Tlt-1 controls early thrombus formation and stability by facilitating αIIbβ3 outside-in signaling in mice. Int. J. Adv. Res. 2018, 6, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Swieringa, F.; de Laat, B.; de Groot, P.G.; Roest, M.; Heemskerk, J.W.M. Reversible Platelet Integrin αIIbβ3 Activation and Thrombus Instability. Int. J. Mol. Sci. 2022, 23, 12512. https://doi.org/10.3390/ijms232012512
Zou J, Swieringa F, de Laat B, de Groot PG, Roest M, Heemskerk JWM. Reversible Platelet Integrin αIIbβ3 Activation and Thrombus Instability. International Journal of Molecular Sciences. 2022; 23(20):12512. https://doi.org/10.3390/ijms232012512
Chicago/Turabian StyleZou, Jinmi, Frauke Swieringa, Bas de Laat, Philip G. de Groot, Mark Roest, and Johan W. M. Heemskerk. 2022. "Reversible Platelet Integrin αIIbβ3 Activation and Thrombus Instability" International Journal of Molecular Sciences 23, no. 20: 12512. https://doi.org/10.3390/ijms232012512
APA StyleZou, J., Swieringa, F., de Laat, B., de Groot, P. G., Roest, M., & Heemskerk, J. W. M. (2022). Reversible Platelet Integrin αIIbβ3 Activation and Thrombus Instability. International Journal of Molecular Sciences, 23(20), 12512. https://doi.org/10.3390/ijms232012512







