Annexin A2 Improves the Osteogenic Differentiation of Mesenchymal Stem Cells Exposed to High-Glucose Conditions through Lessening the Senescence
Abstract
:1. Introduction
2. Results
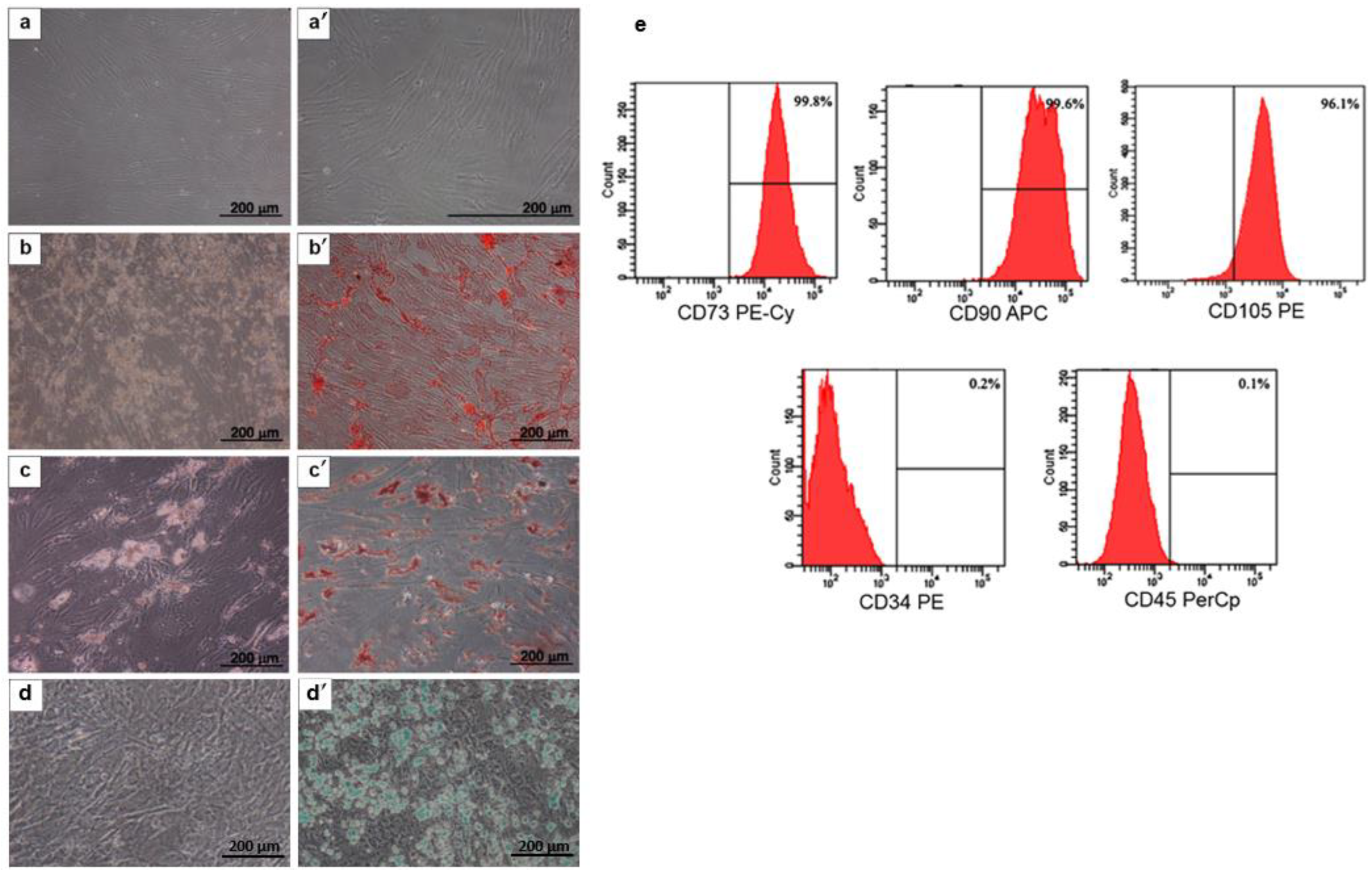
2.1. MSC Characterization
2.2. The Effect of High Levels of Glucose on Osteogenic Differentiation of MSCs
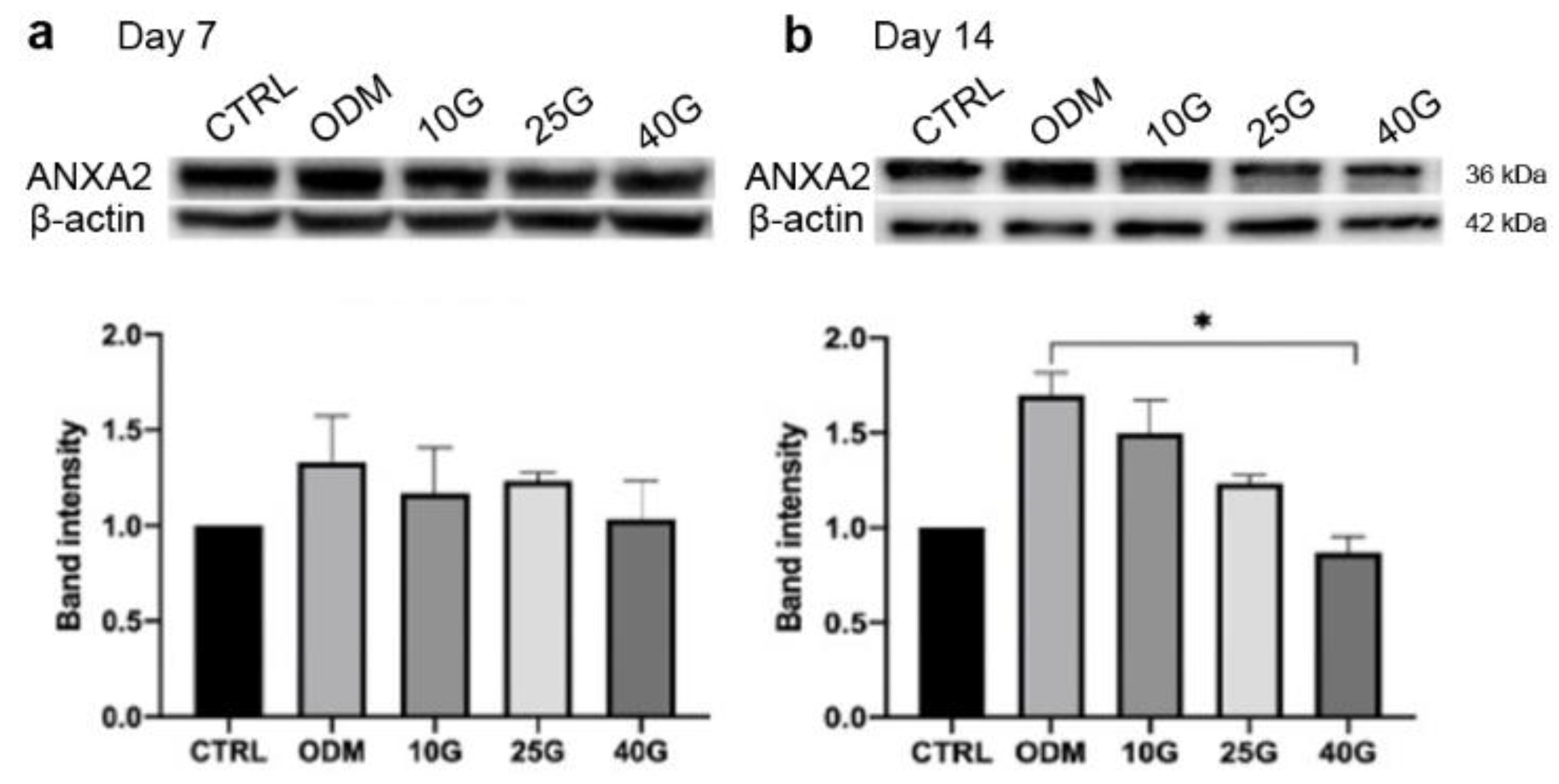
2.3. Alteration of ANXA2 Expression in MSC Osteoblastic Differentiation under High-Glucose Conditions
2.4. The Improvement of In Vitro Osteogenic Differentiation of MSCs by Recombinant Annexin A2 (rANXA2)
2.5. rANXA2 Attenuated High Glucose-Induced Cellular Senescence of MSCs during Osteogenic Differentiation
3. Discussion
4. Material and Methods
4.1. MSC Isolation and Expansion
4.2. MSC Characterization
4.3. Cell Surface Markers
4.4. Osteogenic, Adipogenic, and Chondrogenic Differentiation
4.5. The Effects of High Glucose on MSC Osteoblastic Differentiation
4.6. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.7. Western Blot Analysis
4.8. Senescence-Associated Beta-Galactosidase (SA-β-Gal) Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Föger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [PubMed]
- Wongdee, K.; Charoenphandhu, N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Qi, M.; Wang, Y.; Feng, X.; Liu, H. Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem. Biophys. Res. Commun. 2018, 505, 1195–1202. [Google Scholar] [CrossRef]
- Reni, C.; Mangialardi, G.; Meloni, M.; Madeddu, P. Diabetes stimulates osteoclastogenesis by acidosis-induced activation of transient receptor potential cation channels. Sci. Rep. 2016, 6, 30639. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.-C.; Chuang, P.-Y.; Yang, T.-Y.; Huang, T.-W.; Chang, S.-F. Hyperglycemia inhibits osteoblastogenesis of rat bone marrow stromal cells via activation of the Notch2 signaling pathway. Int. J. Med. Sci. 2019, 16, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Aswamenakul, K.; Klabklai, P.; Pannengpetch, S.; Tawonsawatruk, T.; Isarankura-Na-Ayudhya, C.; Roytrakul, S.; Nantasenamat, C.; Supokawej, A. Proteomic study of in vitro osteogenic differentiation of mesenchymal stem cells in high glucose condition. Mol. Biol. Rep. 2020, 47, 7505–7516. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, C.; Zhang, M. High glucose inhibits osteogenic differentiation and proliferation of MC3T3-E1 cells by regulating P2X7. Mol. Med. Rep. 2019, 20, 5084–5090. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Chen, W.; Hu, Q.; Wang, X.; Zhao, Q.; Tuerxunyiming, M. High glucose inhibits osteogenic differentiation of bone marrow mesenchymal stem cells via regulating miR-493-5p/ZEB2 signalling. J. Biochem. 2020, 167, 613–621. [Google Scholar] [CrossRef]
- Chang, T.-C.; Hsu, M.-F.; Wu, K.K. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS ONE 2015, 10, e0126537. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Hajjar, K.A. Annexin A2 system in human biology: Cell surface and beyond. Semin. Thromb. Hemost. 2013, 39, 338–346. [Google Scholar] [CrossRef]
- Lokman, N.A.; Ween, M.P.; Oehler, M.K.; Ricciardelli, C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. Off. J. Int. Cancer Microenviron. 2011, 4, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genetos, D.; Wong, A.; Watari, S.; Yellowley, C.E. Hypoxia increases Annexin A2 expression in osteoblastic cells via VEGF and ERK. Bone 2010, 47, 1013–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.; Hajjar, K. Annexin A2 in fibrinolysis, inflammation and fibrosis. Int. J. Mol. Sci. 2021, 22, 6836. [Google Scholar] [CrossRef] [PubMed]
- Menaa, C.; Devlin, R.D.; Reddy, S.V.; Gazitt, Y.; Choi, S.J.; Roodman, G.D. Annexin II increases osteoclast formation by stimulating the proliferation of osteoclast precursors in human marrow cultures. J. Clin. Investig. 1999, 103, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, T.; Harrison, G.; Golub, E.E.; Nah, H.-D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J. Biol. Chem. 2000, 275, 35577–35583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Reddy, S.V.; Chirgwin, J.M.; Devlin, R.; Haipek, C.; Anderson, J.; Roodman, G.D. Cloning and identification of annexin II as an autocrine/paracrine factor that increases osteoclast formation and bone resorption. J. Biol. Chem. 1994, 269, 28696–28701. [Google Scholar] [CrossRef]
- Genetos, D.C.; Wong, A.; Weber, T.J.; Karin, N.J.; Yellowley, C.E. Impaired osteoblast differentiation in annexin A2- and -A5-deficient cells. PLoS ONE 2014, 9, e107482. [Google Scholar]
- Xiang, L.; Zheng, J.; Zhang, M.; Ai, T.; Cai, B. FOXQ1 promotes the osteogenic differentiation of bone mesenchymal stem cells via Wnt/β-catenin signaling by binding with ANXA2. Stem Cell Res. Ther. 2020, 11, 403. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Li, Y.; Wang, D.; Lingling, E.; Bai, Y.; Liu, H. High glucose inhibits osteogenic differentiation through the BMP signaling pathway in bone mesenchymal stem cells in mice. EXCLI J. 2013, 12, 584–597. [Google Scholar]
- Junaid, R.; Wahid, M.; Waseem, F.S.; Habib, R.; Hasan, A. Effect of glucose mediated oxidative stress on apoptotic gene expression in gingival mesenchymal stem cells. BMC Oral Health 2021, 21, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, H.; Chen, Z.; Wang, Y.; Lin, J.; Xu, S.; Zhang, C.; Wang, B.; Yuan, Z.; Feng, X.; et al. High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol. Med. Rep. 2017, 16, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Dallacasagrande, V.; Hajjar, K.A. Annexin A2 in Inflammation and Host Defense. Cells 2020, 9, 1499. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; O’Neill, K.D.; Chen, X.; Moe, S.M. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J. Bone Miner. Res. 2008, 23, 1798–1805. [Google Scholar] [CrossRef] [Green Version]
- Galazis, N.; Afxentiou, T.; Xenophontos, M.; Diamanti-Kandarakis, E.; Atiomo, W. Proteomic biomarkers of type 2 diabetes mellitus risk in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2013, 168, R33–R43. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, X.; Liu, N.; Yan, M.; Chen, Z.; Lo, E.H.; Hajjar, K.A.; Dai, H.; Wang, X. Dysfunction of annexin A2 contributes to hyperglycaemia-induced loss of human endothelial cell surface fibrinolytic activity. Thromb. Haemost. 2013, 109, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Gillette, J.M.; Nielsen-Preiss, S.M. The role of annexin 2 in osteoblastic mineralization. J. Cell Sci. 2004, 117, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Davies, O.G.; Cox, S.C.; Williams, R.L.; Tsaroucha, D.; Dorrepaal, R.M.; Lewis, M.P.; Grover, L.M. Annexin-enriched osteoblast-derived vesicles act as an extracellular site of mineral nucleation within developing stem cell cultures. Sci. Rep. 2017, 7, 12639. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, M.; Kubbutat, M.H.G.; Vousden, K.H. Regulation of p53 function and stability by phosphorylation. Mol. Cell. Biol. 1999, 19, 1751–1758. [Google Scholar] [CrossRef] [Green Version]
- Macip, S.; Igarashi, M.; Berggren, P.; Yu, J.; Lee, S.W.; Aaronson, S.A. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol. Cell. Biol. 2003, 23, 8576–8585. [Google Scholar] [CrossRef] [Green Version]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the regulation of cellular senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Sun, Y.; Xu, F.; Liang, Y.; Liu, H.; Yi, Y. Annexin A2 silencing inhibits proliferation and epithelial-to-mesenchymal transition through p53-dependent pathway in NSCLCs. J. Cancer 2019, 10, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chen, C.-L.; Tseng, Y.-L.; Fang, Y.-T.; Lin, Y.-S.; Su, W.-C.; Chen, C.-C.; Chang, K.-C.; Wang, Y.-C.; Lin, C.-F. Annexin A2 silencing induces G2 arrest of non-small cell lung cancer cells through p53-dependent and -independent mechanisms. J. Biol. Chem. 2012, 287, 32512–32524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Li, X.; Li, R.; Fang, L.; Sun, L.; Wang, Y.; Wu, M. Annexin A2 Modulates ROS and Impacts Inflammatory Response via IL-17 Signaling in Polymicrobial Sepsis Mice. PLoS Pathog. 2016, 12, e1005743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Xu, N.; Xu, D.; Dong, M.; Shi, X.; Wang, Y.; Hao, Z.; Zhu, S.; Zhao, D.; Jin, H.; et al. Comparison of senescence-related changes between three- and two-dimensional cultured adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 226. [Google Scholar] [CrossRef]
- Despars, G.; Carbonneau, C.L.; Bardeau, P.; Coutu, D.L.; Beauséjour, C.M. Loss of the osteogenic differentiation potential during senescence is limited to bone progenitor cells and is dependent on p53. PLoS ONE 2013, 8, e73206. [Google Scholar] [CrossRef]
- Tezuka, K.; Suzuki, M.; Sato, R.; Kawarada, S.; Terasaki, T.; Uchida, Y. Activation of Annexin A2 signaling at the blood–brain barrier in a mouse model of multiple sclerosis. J. Neurochem. 2022, 160, 662–674. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Zhang, H.; Wang, F.; Shao, L.; Wang, X. Effect and mechanism of action of SLP-2 on the apoptosis and autophagy of gastric cancer cells. Oncol. Lett. 2021, 22, 707. [Google Scholar] [CrossRef]



| Target Gene | Nucleotide Sequence | Product Size (bp) | |
|---|---|---|---|
| Forward | RUNX2 | 5′-AACCGAGAAGGCACAGACAG-3′ | 192 |
| Reverse | RUNX2 | 5′-GCCTGGGGTCTGTTATCTGA-3′ | |
| Forward | Osterix | 5′-TGCTTGAGGACGAAGTTCAC-3′ | 114 |
| Reverse | Osterix | 5′-GTGCTTTGCCCAGAGTTGTT-3′ | |
| Forward | BGLAP | 5′-TTTGTCCAAACCATCCGCAC-3′ | 154 |
| Reverse | BGLAP | 5′-GCATCAACTTCGATACCGGC-3′ | |
| Forward | p16 | 5′-TGAGGGTTTTCGTGGTTCAC-3′ | 190 |
| Reverse | p16 | 5′-TGGTCTTCTAGGAAGCGGC-3′ | |
| Forward | p21 | 5′-GATGAGTTGGGAGGAGGCAG-3′ | 210 |
| Reverse | p21 | 5′-CTGAGAGTCTCCAGGTCCAC -3′ | |
| Forward | p53 | 5′-ATGATTTGATGCTGTCCCCG-3′ | 175 |
| Reverse | p53 | 5′-CAAGAAGCCCAGACGGAAAC-3′ | |
| Forward | GAPDH | 5′-CAACTACATGGTTTACATGTTCGAA-3′ | 206 |
| Reverse | GAPDH | 5′-CAGCCTTCTCCATGCTGGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klabklai, P.; Phetfong, J.; Tangporncharoen, R.; Isarankura-Na-Ayudhya, C.; Tawonsawatruk, T.; Supokawej, A. Annexin A2 Improves the Osteogenic Differentiation of Mesenchymal Stem Cells Exposed to High-Glucose Conditions through Lessening the Senescence. Int. J. Mol. Sci. 2022, 23, 12521. https://doi.org/10.3390/ijms232012521
Klabklai P, Phetfong J, Tangporncharoen R, Isarankura-Na-Ayudhya C, Tawonsawatruk T, Supokawej A. Annexin A2 Improves the Osteogenic Differentiation of Mesenchymal Stem Cells Exposed to High-Glucose Conditions through Lessening the Senescence. International Journal of Molecular Sciences. 2022; 23(20):12521. https://doi.org/10.3390/ijms232012521
Chicago/Turabian StyleKlabklai, Parin, Jitrada Phetfong, Rattanawan Tangporncharoen, Chartchalerm Isarankura-Na-Ayudhya, Tulyapruek Tawonsawatruk, and Aungkura Supokawej. 2022. "Annexin A2 Improves the Osteogenic Differentiation of Mesenchymal Stem Cells Exposed to High-Glucose Conditions through Lessening the Senescence" International Journal of Molecular Sciences 23, no. 20: 12521. https://doi.org/10.3390/ijms232012521






