Antifungal Effect of Nanoparticles against COVID-19 Linked Black Fungus: A Perspective on Biomedical Applications
Abstract
1. Introduction
2. Silver Nanoparticles
3. Zinc Oxide Nanoparticles
4. Gold Nanoparticles
5. Copper Oxide Nanoparticles
6. Titanium Dioxide Nanoparticles
7. Magnetic and Iron Oxide Nanoparticles
8. Carbon Nanoparticles
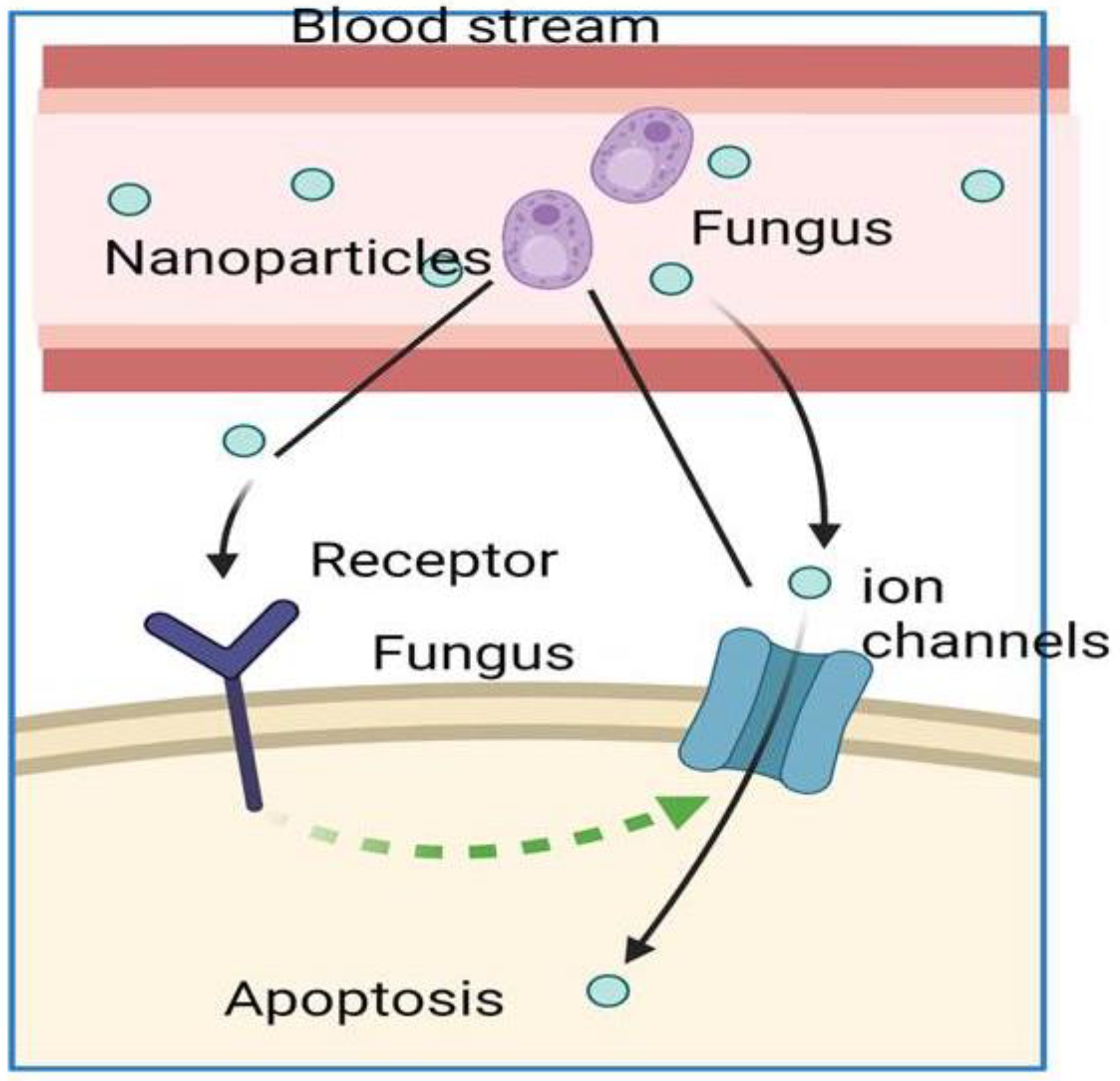
9. Impact of Nanoparticles on Black Fungus
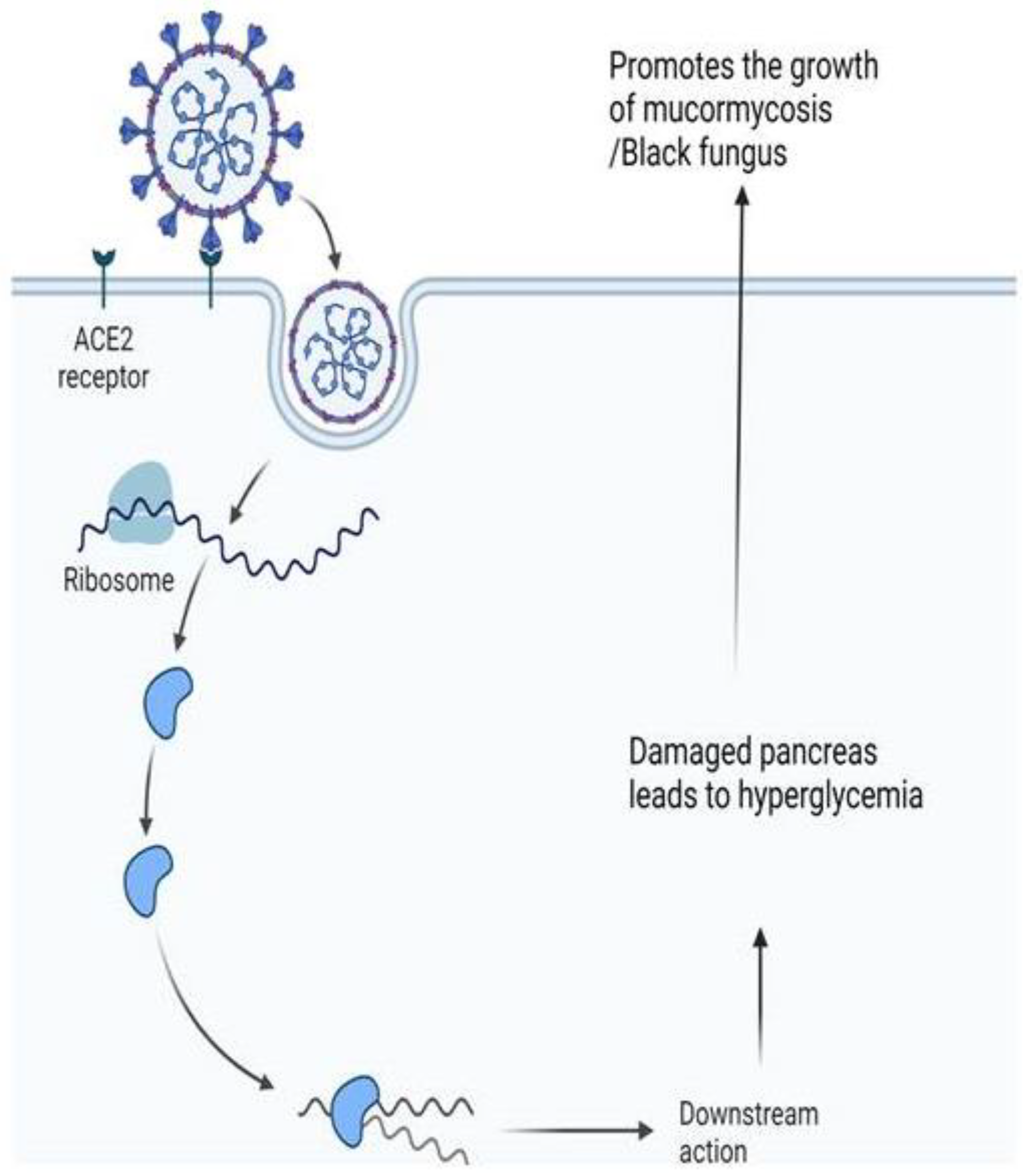
10. Black Fungus Co-Infection in the Context of the Global COVID-19 Outbreak
11. Management and Guidelines to Control COVID-19 Lead Black Fungus Infection
12. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Sahoo, S. Pandemic and mental health of the front-line healthcare workers: A review and implications in the Indian context amidst COVID-19. Gen. Psychiatry 2020, 33, e100284. [Google Scholar] [CrossRef]
- Baldin, C.; Ibrahim, A.S. Molecular mechanisms of mucormycosis—The bitter and the sweet. PLoS Pathog. 2017, 13, e1006408. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef]
- Gangneux, J.-P.; Bougnoux, M.-E.; Dannaoui, E.; Cornet, M.; Zahar, J. Invasive fungal diseases during COVID-19: We should be prepared. J. De Mycol. Med. 2020, 30, 100971. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2020, 27, 83–88. [Google Scholar] [CrossRef]
- Hoenigl, M. Fungal translocation: A driving force behind the occurrence of non-AIDS events. Clin. Infect. Dis. 2020, 70, 242–244. [Google Scholar] [CrossRef]
- Koehler, P.; Cornely, O.A.; Bottiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef]
- Dubey, S.; Mukherjee, D.; Sarkar, P.; Mukhopadhyay, P.; Barman, D.; Bandopadhyay, M.; Pandit, A.; Sengupta, A.; Das, S.; Ghosh, S.; et al. COVID-19 associated rhino-orbital-cerebral mucormycosis: An observational study from Eastern India, with special emphasis on neurological spectrum. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102267. [Google Scholar] [CrossRef]
- Mekki, S.O.; Hassan, A.A.; Falemban, A.; Alkotani, N.; Alsharif, S.M.; Haron, A.; Felemban, B.; Iqbal, M.S.; Tabassum, A. Pulmonary mucormycosis: A case report of a rare infection with potential diagnostic problems. Case Rep. Pathol. 2020, 2020, 4. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, J.; He, Y.; Su, Q.; Yang, J. Characteristics of COVID-19 clinical trials in China based on the registration data on ChiCTR and ClinicalTrials.gov. Drug Des. Dev. Ther. 2020, 14, 2159. [Google Scholar] [CrossRef]
- Ruan, P.-S.; Xu, H.-Q.; Wu, J.-H.; Song, Q.-F.; Qiu, H.-Y. COVID-19 in Children: Clinical Characteristics and Follow-Up Study. SN Compr. Clin. Med. 2020, 2, 1713–1716. [Google Scholar] [CrossRef]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef]
- Van Arkel, A.L.; Rijpstra, T.A.; Belderbos, H.N.; Van Wijngaarden, P.; Verweij, P.E.; Bentvelsen, R.G. COVID-19–associated pulmonary aspergillosis. Am. J. Respir. Crit. Care Med. 2020, 202, 132–135. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Baldin, C.; Gu, Y.; Singh, S.; Gebremariam, T.; Swidergall, M.; Alqarihi, A.; Youssef, E.G.; Alkhazraji, S.; Pikoulas, A.; et al. Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis. Nat. Microbiol. 2021, 6, 313–326. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef]
- Sardi, J.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.; Giannini, M.M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Su, S.-Y.; Chao, C.-M. Invasive Candida Infections in Critically Ill Patients. Crit. Care Med. 2015, 43, e322–e323. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Cowen, L.E.; Griffin, A.M.; Chan, L.; Köhler, J.R. The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. Proc. Natl. Acad. Sci. USA 2008, 105, 20918–20923. [Google Scholar] [CrossRef] [PubMed]
- Bilici, S.; Durna, Y.M.; Yigit, O.; Karatas, A.; Cimen, C.; Fincanci, M.; Gökduman, A.R. The Effect of Mupirocin- and Fusidic Acid-nasal Packings, Placed after Septoplasty, on the Nasal Bacterial Profile. Allergy Rhinol. 2016, 7, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Piktel, E.; Wilczewska, A.Z.; Markiewicz, K.H.; Durnaś, B.; Wątek, M.; Puszkarz, I.; Wróblewska, M.; Niklińska, W.; Savage, P.B.; et al. Core–shell magnetic nanoparticles display synergistic antibacterial effects against Pseudomonas aeruginosa and Staphylococcus aureus when combined with cathelicidin LL-37 or selected ceragenins. Int. J. Nanomed. 2016, 11, 5443–5455. [Google Scholar] [CrossRef]
- Voltan, A.R.; Quindos, G.; Alarcón, K.P.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715. [Google Scholar] [CrossRef]
- Brandelli, A. Nanostructures as Promising Tools for Delivery of Antimicrobial Peptides. Mini Rev. Med. Chem. 2012, 12, 731–741. [Google Scholar] [CrossRef]
- Ananias, H.; De Jong, I.; Dierckx, R.; De Wiele, C.V.; Helfrich, W.; Elsinga, P. Nuclear imaging of prostate cancer with gastrin-releasing-peptide-receptor targeted radiopharmaceuticals. Curr. Pharm. Des. 2008, 14, 3033–3047. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, M.; Wang, G.; Fang, W.; Ye, C.; Hu, H.; Fa, Z.; Yi, J.; Liao, W.-Q. Pd@Ag Nanosheets in Combination with Amphotericin B Exert a Potent Anti-Cryptococcal Fungicidal Effect. PLoS ONE 2016, 11, e0157000. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10, 95. [Google Scholar]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Sci. World J. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Robinson J, R.; Isikhuemhen, O.S.; Anike, F.N. Fungal-Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef]
- Gudikandula, K.; Vadapally, P.; Charya, M.S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Jamdagni, P.; Rana, J.; Khatri, P. Comparative study of antifungal effect of green and chemically synthesised silver nanoparticles in combination with carbendazim, mancozeb, and thiram. IET Nanobiotechnol. 2018, 12, 1102–1107. [Google Scholar] [CrossRef]
- Varshney, R.; Bhadauria, S.; Gaur, M.S. A Review: Biological Synthesis of Silver and Copper Nanoparticles. Nano Biomed. Eng. 2012, 4, 99–106. [Google Scholar] [CrossRef]
- Peters, V.; Tumkur, T.; Ma, J.; Kotov, N.A.; Noginov, M. Strong coupling of localized surface plasmons and ensembles of dye molecules. Optics Express 2016, 24, 25653–25664. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of Biologically Synthesized Silver Nanoparticles in MDA-MB-231 Human Breast Cancer Cells. Bio. Med. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, N.; Hu, Y.; Jia, C.; Chou, T.; Du, H.; Wang, H. Gold nanoparticle-enhanced photodynamic therapy: Effects of surface charge and mitochondrial targeting. Ther. Deliv. 2015, 6, 307–321. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Lin, Y.; Du, D. The vital function of Fe3O4@Au nanocomposites for hydrolase biosensor design and its application in detection of methyl parathion. Nanoscale 2013, 5, 1121–1126. [Google Scholar] [CrossRef]
- De Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018, 11, 915. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Bai, K.-J.; Chuang, K.-J.; Ma, C.-M.; Chang, T.-Y.; Chuang, H.-C. Human lung adenocarcinoma cells with an EGFR mutation are sensitive to non-autophagic cell death induced by zinc oxide and aluminium-doped zinc oxide nanoparticles. J. Toxicol. Sci. 2017, 42, 437–444. [Google Scholar] [CrossRef]
- Ravi, K.S.; Ranjana, G. Synthesis of zinc oxide nanoparticles by homogeneous precipitation method and its application in antifungal activity against Candida albicans. Ceram. Int. 2015, 41, 967–975. [Google Scholar]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Pulmonary toxicity and fibrogenic response of carbon nanotubes. Toxicol. Mech. Methods 2013, 23, 196–206. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012, 261, 121–133. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano. 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- YadavHMY, H.M.; Thorat, N.D.; Yallapu, M.M.; Tofail, S.A.; Kim, J.-S. Functional TiO2 nanocoral architecture for light-activated cancer chemotherapy. J. Mater. Chem. 2017, 5, 1461–1470. [Google Scholar]
- Zhao, S.; Wang, S.; Yan, J. Experimental study of hydroxyapatite (HA) granules filled around the titanium implant. Zhonghua Kou Qiang Yi Xue Za Zhi Zhonghua Kouqiang Yixue Zazhi = Chin. J. Stomatol. 1998, 33, 353–354. [Google Scholar]
- Diebold, U. Structure and properties of TiO2 surfaces: A brief review. Appl. Phys. A 2003, 76, 681–687. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, G.; Chawla-Sarkar, M.; Nayak, M.; Trivedi, K.; Rana, S.; Pandey, K.; Das, V.; Topno, R.; Das, P. Antiviral effect of Glycine coated Iron oxide nanoparticles iron against H1N1 influenza A virus. Int. J. Infect. Dis. 2016, 45, 281–282. [Google Scholar] [CrossRef][Green Version]
- Shukla, S.; Arora, V.; Jadaun, A.; Kumar, J.; Singh, N.; Jain, V.K. Magnetic removal of Entamoeba cysts from water using chitosan oligosaccharide-coated iron oxide nanoparticles. Int. J. Nanomed. 2015, 10, 4901–4917. [Google Scholar] [CrossRef]
- Taylor, E.N.; Webster, T.J. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int. J. Nanomed. 2009, 4, 145–152. [Google Scholar]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar] [CrossRef]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef]
- Tocco, M.P.; Ballardini, M.; Masala, M.; Perozzi, A. Post-sternotomy chronic osteomyelitis: Is sternal resection always necessary? Eur. J. Cardio-Thorac. Surg. 2013, 43, 715–721. [Google Scholar] [CrossRef]
- Barnes, R.J.; Riba, O.; Gardner, M.N.; Scott, T.B.; Jackman, S.A.; Thompson, I.P. Optimization of nano-scale nickel/iron particles for the reduction of high concentration chlorinated aliphatic hydrocarbon solutions. Chemosphere 2010, 79, 448–454. [Google Scholar] [CrossRef]
- Seabra, A.B.; Haddad, P.; Duran, N. Biogenic synthesis of nanostructured iron compounds: Applications and perspectives. IET Nanobiotechnol. 2013, 7, 90–99. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, A. The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 175–176. [Google Scholar] [CrossRef]
- Pasero, D.; Sanna, S.; Liperi, C.; Piredda, D.; Branca, G.P.; Casadio, L.; Simeo, R.; Buselli, A.; Rizzo, D.; Bussu, F. A challenging complication following SARS-CoV-2 infection: A case of pulmonary mucormycosis. Infection 2021, 49, 1055–1060. [Google Scholar] [CrossRef]
- Waizel-Haiat, S.; Guerrero-Paz, J.A.; Sanchez-Hurtado, L.; Calleja-Alarcon, S.; Romero-Gutierrez, L. A Case of Fatal Rhino-Orbital Mucormycosis Associated with New Onset Diabetic Ketoacidosis and COVID-19. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Narayanan, S.; Chua, J.V.; Baddley, J.W. Coronavirus disease 2019–associated mucormycosis: Risk factors and mechanisms of disease. Clin. Infect. Dis. 2022, 74, 1279–1283. [Google Scholar] [CrossRef]
- Muthu, V.; Rudramurthy, S.M.; Chakrabarti, A.; Agarwal, R. Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World. Mycopathologia 2021, 186, 739–754. [Google Scholar] [CrossRef]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus Disease (COVID-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef]
- Buil, J.B.; van Zanten, A.R.; Bentvelsen, R.G.; Rijpstra, T.A.; Goorhuis, B.; van der Voort, S.; Wammes, L.J.; Janson, J.A.; Melchers, M.; Heusinkveld, M. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Eurosurveillance 2021, 26, 2100510. [Google Scholar] [CrossRef]
- Mukherjee, B.; Raichura, N.D.; Alam, M.S. Fungal infections of the orbit. Indian J. Ophthalmol. 2016, 64, 337. [Google Scholar] [CrossRef]
- Millon, L.; Larosa, F.; Lepiller, Q.; Legrand, F.; Rocchi, S.; Daguindau, E.; Scherer, E.; Bellanger, A.-P.; Leroy, J.; Grenouillet, F. Quantitative Polymerase Chain Reaction Detection of Circulating DNA in Serum for Early Diagnosis of Mucormycosis in Immunocompromised Patients. Clin. Infect. Dis. 2013, 56, e95–e101. [Google Scholar] [CrossRef]
- Millon, L.; Herbrecht, R.; Grenouillet, F.; Morio, F.; Alanio, A.; Letscher-Bru, V.; Cassaing, S.; Chouaki, T.; Kauffmann-Lacroix, C.; Poirier, P.; et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: Retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 2016, 22, 810.e1–810.e8. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Niño, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef]
- Riaz, M.; Altaf, M.; Faisal, A.; Shekheli, M.A.; Miana, G.A.; Khan, M.Q.; Shah, M.A.; Ilyas, S.Z.; Khan, A.A. Biogenic Synthesis of AgNPs with Saussurea lappa C.B. Clarke and Studies on Their Biochemical Properties. J. Nanosci. Nanotechnol. 2018, 18, 8392–8398. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Dwivedi, S.P.; Siddiqui, M.H.; Prasad, T. In vitro studies on oxidative stress-independent, Ag nanoparticles-induced cell toxicity of Candida albicans, an opportunistic pathogen. Int. J. Nanomed. 2018, 13, 91–96. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; Shami, A.; Said-Galive, E.; Shtykova, E.V.; Naumkin, A.V. Silver/Chitosan nanocomposites: Preparation and characterization and their fungicidal activity against dairy cattle toxicosis Penicillium expansum. J. Fungi 2020, 6, 51. [Google Scholar] [CrossRef]
- Das, B.; Khan, M.; Jayabalan, R.; Behera, S.K.; Yun, S.-I.; Tripathy, S.K.; Mishra, A. Understanding the antifungal mechanism of Ag@ ZnO core-shell nanocomposites against Candida krusei. Sci. Rep. 2016, 6, 36403. [Google Scholar] [CrossRef]
- Auyeung, A.; Casillas-Santana, M.A.; Martinez-Castanon, G.A.; Slavin, Y.N.; Zhao, W.; Asnis, J.; Häfeli, U.O.; Bach, H. Effective control of molds using a combination of nanoparticles. PLoS ONE 2017, 12, e0169940. [Google Scholar] [CrossRef]
- Kim, K.-J.; Sung, W.-S.; Moon, S.-K.; Choi, J.-S.; Kim, J.-G.; Lee, D.-G. Antifungal effect of silver nanoparticles on dermatophytes. J. Microbiol. Biotechnol. 2008, 18, 1482–1484. [Google Scholar]
- Kim, J.; Kwon, S.; Ostler, E. Antimicrobial effect of silver-impregnated cellulose: Potential for antimicrobial therapy. J. Biol. Eng. 2009, 3, 20. [Google Scholar] [CrossRef]
- Nasrollahi, A.; Pourshamsian, K.; Mansourkiaee, P. Antifungal activity of silver nanoparticles on some of fungi. Int. J. Nanodimens. 2011, 1, 233–239. [Google Scholar]
- Gutiérrez, J.A.; Caballero, S.; Díaz, L.A.; Guerrero, M.A.; Ruiz, J.; Ortiz, C.C. High antifungal activity against candida species of monometallic and bimetallic nanoparticles synthesized in nanoreactors. ACS Biomater. Sci. Eng. 2018, 4, 647–653. [Google Scholar] [CrossRef]
- He, M.; Lu, L.; Zhang, J.; Li, D. Immobilized Silver Nanoparticles on Chitosan with Special Surface State-Enhanced Antimicrobial Efficacy and Reduced Cytotoxicity. J. Nanosci. Nanotechnol. 2015, 15, 6435–6443. [Google Scholar] [CrossRef]
- Pinto, R.J.; Almeida, A.; Fernandes, S.C.; Freire, C.S.; Silvestre, A.J.; Neto, C.P.; Trindade, T. Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surf. B Biointerfaces 2013, 103, 143–148. [Google Scholar] [CrossRef]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Mallmann, E.J.; Cunha, F.A.; Castro, B.N.; Maciel, A.M.; Menezes, E.A.; Fechine, P.B. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev. Inst. Med. Trop Sao Paulo 2015, 57, 165–167. [Google Scholar] [CrossRef]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521–522, 305–314. [Google Scholar] [CrossRef]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef]
- Qasim, M.; Singh, B.R.; Naqvi, A.; Paik, P.; Das, D. Silver nanoparticles embedded mesoporous SiO2 nanosphere: An effective anticandidal agent against Candida albicans 077. Nanotechnology 2015, 26, 285102. [Google Scholar] [CrossRef]
- Bajpai, R.; Yusuf, M.A.; Upreti, D.K.; Gupta, V.K.; Singh, B.N. Endolichenic fungus, Aspergillus quandricinctus of Usnea longissima inhibits quorum sensing and biofilm formation of Pseudomonas aeruginosa PAO1. Microb. Pathog. 2020, 140, 103933. [Google Scholar]
- Wunnoo, S.; Paosen, S.; Lethongkam, S.; Sukkurd, R.; Waen-ngoen, T.; Nuidate, T.; Phengmak, M.; Voravuthikunchai, S.P. Biologically rapid synthesized silver nanoparticles from aqueous Eucalyptus camaldulensis leaf extract: Effects on hyphal growth, hydrolytic enzymes, and biofilm formation in Candida albicans. Biotechnol. Bioeng. 2021, 118, 1578–1592. [Google Scholar] [CrossRef]
- El Sayed, M.T.; El-Sayed, A.S. Biocidal activity of metal nanoparticles synthesized by Fusarium solani against multidrug-resistant bacteria and mycotoxigenic fungi. J. Microbiol. Biotechnol. 2020, 30, 226–236. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Lock, A.L.; Shingfield, K.J.; Bauman, D.E. Biosynthesis of conjugated linoleic acid in ruminants and humans. Adv. Food Nutr. Res. 2005, 50, 179–217. [Google Scholar]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Gondal, M.A.; Alzahrani, A.J.; Randhawa, M.A.; Siddiqui, M.N. Morphology and antifungal effect of nano-ZnO and nano-Pd-doped nano-ZnO against Aspergillus and Candida. J. Environ. Sci. Health Part A 2012, 47, 1413–1418. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Ali, S.G.; Khan, H.M.; Rehman, S. Anticandidal activity of bioinspired ZnO NPs: Effect on growth, cell morphology and key virulence attributes of Candida species. Artif. Cells Nanomed. Biotechnol. 2018, 46, 912–925. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Mahmood, T.; Lee, C.-S. Contact lenses coated with hybrid multifunctional ternary nanocoatings (Phytomolecule-coated ZnO nanoparticles: Gallic Acid: Tobramycin) for the treatment of bacterial and fungal keratitis. Acta Biomater. 2021, 128, 262–276. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; De la Rosa-Garcia, S.C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, Photocatalytic, and Antifungal Properties of MgO, ZnO and Zn/Mg Oxide Nanoparticles for the Protection of Calcareous Stone Heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. [Google Scholar] [CrossRef]
- Jasim, D.A.; Ménard-Moyon, C.; Bégin, D.; Bianco, A.; Kostarelos, K. Tissue distribution and urinary excretion of intravenously administered chemically functionalized graphene oxide sheets. Chem. Sci. 2015, 6, 3952–3964. [Google Scholar] [CrossRef]
- Cierech, M.; Wojnarowicz, J.; Szmigiel, D.; Bączkowski, B.; Grudniak, A.M.; Wolska, K.I.; Łojkowski, W.; Mierzwińska-Nastalska, E. Preparation and characterization of ZnO-PMMA resin nanocomposites for denture bases. Acta Bioeng. Biomech. 2016, 18, 31–41. [Google Scholar]
- Lakshmeesha, T.R.; Kalagatur, N.K.; Mudili, V.; Mohan, C.D.; Rangappa, S.; Prasad, B.D.; Ashwini, B.S.; Hashem, A.; Alqarawi, A.A.; Malik, J.A. Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearum. Front. Microbiol. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Conde, J.; Bao, C.; Cui, D.; Baptista, P.V.; Tian, F. Antibody–drug gold nanoantennas with Raman spectroscopic fingerprints for in vivo tumour theranostics. J. Control. Release 2014, 183, 87–93. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, H.; Xie, Q.; Jia, X.; Zhang, Y.; Tan, L.; Yao, S. The preparation and characterization of poly (o-phenylenediamine)/gold nanoparticles interface for immunoassay by surface plasmon resonance and electrochemistry. Colloids Surf. B Biointerfaces 2008, 63, 254–261. [Google Scholar] [CrossRef]
- Brown, S.D.; Nativo, P.; Smith, J.-A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chang, H.-T.; Tan, W. Cancer cell targeting using multiple aptamers conjugated on nanorods. Anal. Chem. 2008, 80, 567–572. [Google Scholar] [CrossRef]
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; Guillén, A.d.J.P.; Tapia-Pérez, H.; Castañón, G.M. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Lima, E.; Guerra, R.; Lara, V.; Guzmán, A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem. Central J. 2013, 7, 11. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, S.; Azam, A.; Alam, F. Gold nanoparticles enhance methylene blue–induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomed. 2012, 7, 3245–3257. [Google Scholar] [CrossRef]
- Jebali, A.; Hajjar, F.H.E.; Pourdanesh, F.; Hekmatimoghaddam, S.; Kazemi, B.; Masoudi, A.; Daliri, K.; Sedighi, N. Silver and gold nanostructures: Antifungal property of different shapes of these nanostructures on Candida species. Med. Mycol. 2014, 52, 65–72. [Google Scholar]
- Seong, M.; Lee, D.G. Reactive oxygen species-independent apoptotic pathway by gold nanoparticles in Candida albicans. Microbiol. Res. 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Bajaj, M.; Pandey, S.K.; Nain, T.; Brar, S.K.; Singh, P.; Singh, S.; Wangoo, N.; Sharma, R.K. Stabilized cationic dipeptide capped gold/silver nanohybrids: Towards enhanced antibacterial and antifungal efficacy. Colloids Surf. B Biointerf. 2017, 158, 397–407. [Google Scholar] [CrossRef]
- Khatoon, N.; Alam, H.; Manzoor, N.; Sardar, M. Removal of toxic contaminants from water by sustainable green synthesised non-toxic silver nanoparticles. IET Nanobiotechnol. 2018, 12, 1090–1096. [Google Scholar] [CrossRef]
- Milanezi, F.G.; Meireles, L.M.; de Christo Scherer, M.M.; de Oliveira, J.P.; da Silva, A.R.; de Araujo, M.L.; Endringer, D.C.; Fronza, M.; Guimarães, M.C.C.; Scherer, R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm. J. 2019, 27, 968–974. [Google Scholar] [CrossRef]
- Rodriguez-Torres, M.D.P.; Díaz-Torres, L.A.; Millán-Chiu, B.E.; García-Contreras, R.; Hernández-Padrón, G.; Acosta-Torres, L.S. Antifungal and cytotoxic evaluation of photochemically synthesized heparin-coated gold and silver nanoparticles. Molecules 2020, 25, 2849. [Google Scholar] [CrossRef]
- Rahimi, H.; Roudbarmohammadi, S.; H, H.D.; Roudbary, M. Antifungal effects of indolicidin-conjugated gold nanoparticles against fluconazole-resistant strains of Candida albicans isolated from patients with burn infection. Int. J. Nanomed. 2019, 14, 5323–5338. [Google Scholar] [CrossRef]
- Chalandar, H.E.; Ghorbani, H.R.; Attar, H.; Alavi, S.A. Antifungal effect of copper and copper oxide nanoparticles against Penicillium on orange fruit. Biosci. Biotechnol. Res. Asia 2017, 14, 279–284. [Google Scholar] [CrossRef]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity. World, J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [CrossRef]
- Oussou-Azo, A.F.; Nakama, T.; Nakamura, M.; Futagami, T.; Vestergaard, M.D.C.M. Antifungal potential of nanostructured crystalline copper and its oxide forms. Nanomaterials 2020, 10, 1003. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Vig, S.; Thakar, R.; Sultan, A.H. Impact of copper compression stockings on venous insufficiency and lipodermatosclerosis: A randomised controlled trial. Phlebology 2019, 34, 224–230. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Noyce, J.O.; Michels, H.T.; Keevil, C.W. Potential action of copper surfaces on meticillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2010, 109, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.M.; Kalaivani, S.; Sabarathinam, S.; Vasuki, M.; Soundari, A.J.P.G.; Das, M.A.; Elfasakhany, A.; Pugazhendhi, A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environ. Res. 2021, 201, 111502. [Google Scholar] [CrossRef] [PubMed]
- Eremenko, A.M.; Petrik, I.S.; Smirnova, N.P.; Rudenko, A.V.; Marikvas, Y.S. Antibacterial and antimycotic activity of cotton fabrics, impregnated with silver and binary silver/copper nanoparticles. Nanoscale Res. Lett. 2016, 11, 28. [Google Scholar] [CrossRef]
- Viet, P.V.; Nguyen, H.T.; Cao, T.M.; Hieu, L.V. Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method. J. Nanomater. 2016, 2016, 1957612. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef]
- Devipriya, D.; Roopan, S.M. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Mater. Sci Eng C Mater. Biol. Appl. 2017, 80, 38–44. [Google Scholar] [CrossRef]
- Roopan, S.M.; Priya, D.D.; Shanavas, S.; Acevedo, R.; Al-Dhabi, N.A.; Arasu, M.V. CuO/C nanocomposite: Synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng. C 2019, 101, 404–414. [Google Scholar] [CrossRef]
- Ammar, H.Y.; Badran, H.M. Effect of CO adsorption on properties of transition metal doped porphyrin: A DFT and TD-DFT study. Heliyon 2019, 5, e2545. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Asghar, M.A.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 2018, 90, 98–107. [Google Scholar] [CrossRef]
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave synthesis of nanoparticles and their antifungal activities. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef]
- Hermida-Montero, L.A.; Pariona, N.; Mtz-Enriquez, A.I.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Aqueous-phase synthesis of nanoparticles of copper/copper oxides and their antifungal effect against Fusarium oxysporum. J. Hazard. Mater. 2019, 380, 120850. [Google Scholar] [CrossRef]
- Padmavathi, A.R.; Das, A.; Priya, A.; Sushmitha, T.J.; Pandian, S.K.; Toleti, S.R. Impediment to growth and yeast-to-hyphae transition in Candida albicans by copper oxide nanoparticles. Biofouling 2020, 36, 56–72. [Google Scholar] [CrossRef]
- Martínez, A.; Apip, C.; Meléndrez, M.; Domínguez, M.; Sánchez-Sanhueza, G.; Marzialetti, T.; Catalán, A. Dual antifungal activity against Candida albicans of copper metallic nanostructures and hierarchical copper oxide marigold-like nanostructures grown in situ in the culture medium. J. Appl. Microbiol. 2020, 130, 1883–1892. [Google Scholar] [CrossRef]
- Akturk, A.; Güler, F.K.; Taygun, M.E.; Goller, G.; Küçükbayrak, S. Synthesis and antifungal activity of soluble starch and sodium alginate capped copper nanoparticles. Mater. Res. Express 2020, 6, 1250g3. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO(2) nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Yuesuo, Y.; Ao, Q.; Adeel, M.; Hui, Z.Y.; Javed, R. Appraisal of Comparative Therapeutic Potential of Undoped and Nitrogen-Doped Titanium Dioxide Nanoparticles. Molecules 2019, 24, 3916. [Google Scholar] [CrossRef]
- Zacarias, S.M.; Marchetti, S.; Alfano, O.M.; de los Milagros Ballari, M. Photocatalytic paint for fungi growth control under different environmental conditions and irradiation sources. J. Photochem. Photobiol. A Chem. 2018, 364, 364. [Google Scholar] [CrossRef]
- Zuccheri, T.; Colonna, M.; Stefanini, I.; Santini, C.; Di Gioia, D. Bactericidal Activity of Aqueous Acrylic Paint Dispersion for Wooden Substrates Based on TiO2 Nanoparticles Activated by Fluorescent Light. Materials 2013, 6, 3270–3283. [Google Scholar] [CrossRef]
- Moradpoor, H.; Safaei, M.; Golshah, A.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Mobarakeh, M.S. Green synthesis and antifungal effect of titanium dioxide nanoparticles on oral Candida albicans pathogen. Inorg. Chem. Commun. 2021, 130, 108748. [Google Scholar] [CrossRef]
- Mukherjee, M.; Gangopadhyay, K.; Das, R.; Purkayastha, P. Development of Non-ionic Surfactant and Protein-Coated Ultrasmall Silver Nanoparticles: Increased Viscoelasticity Enables Potency in Biological Applications. ACS Omega 2020, 5, 8999–9006. [Google Scholar] [CrossRef]
- Kermani, F.; Sadeghian, M.; Shokohi, T.; Hashemi, S.; Moslemi, D.; Davodian, S.; Abastabar, M.; Bandalizadeh, Z.; Faeli, L.; Seifi, Z.; et al. Molecular identification and antifungal susceptibility testing of Candida species isolated from oral lesions in patients with head and neck cancer undergoing radiotherapy. Curr. Med. Mycol. 2021, 7, 44–50. [Google Scholar] [CrossRef]
- Ilkhechi, N.N.; Mozammel, M.; Khosroushahi, A.Y. Antifungal effects of ZnO, TiO2 and ZnO-TiO2 nanostructures on Aspergillus flavus. Pestic. Biochem. Physiol. 2021, 176, 104869. [Google Scholar] [CrossRef]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO(2) reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef]
- Singh, P. Biosynthesis of titanium dioxide nanoparticles and their antibacterial property. Int. J. Chem. Mol. Eng. 2016, 10, 275–278. [Google Scholar]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.-C.; Horvatovich, P.; Gies, J.-P.; Keller, V.; Keller, N.; Andre, P. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Kubacka, A.; Suarez-Diez, M.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Dos Santos, V.A.P.M.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef]
- Korshed, P.; Li, L.; Liu, Z.; Mironov, A.; Wang, T. Antibacterial mechanisms of a novel type picosecond laser-generated silver-titanium nanoparticles and their toxicity to human cells. Int. J. Nanomed. 2017, 13, 89–101. [Google Scholar] [CrossRef]
- Chawengkijwanich, C.; Hayata, Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int. J. Food Microbiol. 2008, 123, 288–292. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Food Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef]
- Jackson, S.L.; Heath, I.B. Roles of calcium ions in hyphal tip growth. Microbiol. Rev. 1993, 2, 367–382. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Effects of Ag doping on the photocatalytic disinfection of E. coli in bioaerosol by Ag-TiO(2)/GF under visible light. J. Colloid Interface Sci. 2014, 428, 24–31. [Google Scholar] [CrossRef]
- Nithya, A.; JeevaKumari, H.L.; Rokesh, K.; Ruckmani, K.; Jeganathan, K.; Jothivenkatachalam, K. A versatile effect of chitosan-silver nanocomposite for surface plasmonic photocatalytic and antibacterial activity. J. Photochem. Photobiol. B Biol. 2015, 153, 412–422. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Hamidinezhad, H.; Haddadi, H.; Mahmoudi, M. Double-doped TiO2 nanoparticles as an efficient visible-light-active photocatalyst and antibacterial agent under solar simulated light. Appl. Surf. Sci. 2014, 301, 338–345. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Wei, X.; Yu, J.; Ding, L.; Hu, J.; Jiang, W. Effect of oxide nanoparticles on the morphology and fluidity of phospholipid membranes and the role of hydrogen bonds. J. Environ. Sci. 2017, 57, 221–230. [Google Scholar] [CrossRef]
- Reddy, S.; Porter, D.; Patton, J.T.; Al-Nafussi, A.; Beggs, I. Endometriosis of the superior gluteal nerve. Skelet. Radiol. 2007, 36, 879–883. [Google Scholar] [CrossRef]
- Nehra, P.; Chauhan, R.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2017, 75, 13–18. [Google Scholar] [CrossRef]
- Alam, T.; Khan, R.A.A.; Ali, A.; Sher, H.; Ullah, Z.; Ali, M. Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Mater. Sci. Eng. C 2018, 98, 101–108. [Google Scholar] [CrossRef]
- Seddighi, N.S.; Salari, S.; Izadi, A.R. Evaluation of antifungal effect of iron-oxide nanoparticles against different Candida species. IET Nanobiotechnol. 2017, 11, 883–888. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Głuszek, K.; Wilczewska, A.; Misztalewska-Turkowicz, I.; Mystkowska, J.; Michalak, G.; Sodo, A.; Wątek, M.; et al. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2395–2404. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Piktel, E.; Michalak, G.; Gu, X.; Kułakowska, A.; Savage, P.B.; Bucki, R. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef]
- Hasany, S.F.; Abdurahman, N.H.; Sunarti, A.R.; Jose, R. Magnetic Iron Oxide Nanoparticles: Chemical Synthesis and Applications Review. Curr. Nanosci. 2013, 9, 561–575. [Google Scholar] [CrossRef]
- Abbas, H.S.; Krishnan, A. Magnetic Nanosystems as a Therapeutic Tool to Combat Pathogenic Fungi. Adv. Pharm. Bull. 2020, 10, 512–523. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- De Lima, T.M.; Arias, L.S.; Afanaci, L.F.; Ferraresse, R.F.; Neto, F.N.D.S.; De Lima, B.H.; Straioto, F.G.; De Camargo, E.R.; Pessan, J.P.; Monteiro, D.R. Assembly and antifungal effect of a new fluconazole-carrier nanosystem. Future Microbiol. 2020, 15, 273–285. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Harikrishnan, U.; Valand, N.N.; Modi, N.R.; Menon, S.K. Novel Cationic Quinazolin-4 (3H)-one Conjugated Fullerene Nanoparticles as Antimycobacterial and Antimicrobial Agents. Arch. Der Pharm. 2013, 346, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Diez-Orejas, R.; Feito, M.J.; Cicuéndez, M.; Casarrubios, L.; Rojo, J.M.; Portolés, M.T. Graphene oxide nanosheets increase Candida albicans killing by pro-inflammatory and reparative peritoneal macrophages. Colloids Surf. B Biointerfaces 2018, 171, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, R.; Tang, F.K.; Li, W.C.; Wong, S.S.W.; Leung, K.C.F.; Jin, L. Red-Emissive Guanylated Polyene-Functionalized Carbon Dots Arm Oral Epithelia against Invasive Fungal Infections. ACS Appl. Mater. Interfaces 2019, 11, 46591–46603. [Google Scholar] [CrossRef]
- Jhonsi, M.A.; Ananth, D.A.; Nambirajan, G.; Sivasudha, T.; Yamini, R.; Bera, S.; Kathiravan, A. Antimicrobial activity, cytotoxicity and DNA binding studies of carbon dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 295–302. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Rawat, K.; Prasad, T.; Bohidar, H. Antifungal efficacy of Au@ carbon dots nanoconjugates against opportunistic fungal pathogen, Candida albicans. Colloids Surf. B Biointerfaces 2018, 163, 355–361. [Google Scholar] [CrossRef]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Sangsri, T.; Siwayaprahm, P. Synthesis and antifungal activity of reduced graphene oxide nanosheets. Carbon 2012, 50, 5156–5161. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Han, H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf. B Biointerfaces 2013, 103, 136–142. [Google Scholar] [CrossRef]
- Xie, J.; Ming, Z.; Li, H.; Yang, H.; Yu, B.; Wu, R.; Liu, X.; Bai, Y.; Yang, S.-T. Toxicity of graphene oxide to white rot fungus Phanerochaete chrysosporium. Chemosphere 2016, 151, 324–331. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef]
- Miragoli, M.; Novak, P.; Ruenraroengsak, P.; Shevchuk, A.I.; Korchev, Y.E.; Lab, M.J.; Tetley, T.D.; Gorelik, J. Functional interaction between charged nanoparticles and cardiac tissue: A new paradigm for cardiac arrhythmia? Nanomedicine 2013, 8, 725–737. [Google Scholar] [CrossRef]
- Savi, M.; Rossi, S.; Bocchi, L.; Gennaccaro, L.; Cacciani, F.; Perotti, A.; Amidani, D.; Alinovi, R.; Goldoni, M.; Aliatis, I.; et al. Titanium dioxide nanoparticles promote arrhythmias via a direct interaction with rat cardiac tissue. Part. Fibre Toxicol. 2014, 11, 63. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Hoang Thi, T.T. Comparison of biogenic silver nanoparticles formed by Momordica charantia and Psidium guajava leaf extract and antifungal evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar] [CrossRef]
- Jebril, N.M.T. Evaluation of two fixation techniques for direct observation of biofilm formation of Bacillus subtilis in situ, on Congo red agar, using scanning electron microscopy. Vet. World 2020, 13, 1133–1137. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Jafari, A.A.; Tafti, A.F.; Hoseiny, S.M.; Kazemi, A. Antifungal Effect of Zataria multiflora Essence on Experimentally Contaminated Acryl Resin Plates with Candida albicans. Iran. Red Crescent Med. J. 2015, 17, e16552. [Google Scholar] [CrossRef]
- Huang, W.; Fang, X.; Wang, H.; Chen, F.; Duan, H.; Bi, Y.; Yu, H. Biosynthesis of AgNPs by B. maydis and its antifungal effect against Exserohilum turcicum. IET Nanobiotechnol. 2018, 12, 585–590. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Rajesh, S.; Rathi, J.A.M.; Kumar, V. Green preparation of seaweed-based silver nano-liquid for cotton pathogenic fungi management. IET Nanobiotechnol. 2018, 13, 219–225. [Google Scholar] [CrossRef]
- Ediz, C.; Tavukcu, H.H.; Akan, S.; Kizilkan, Y.E.; Alcin, A.; Oz, K.; Yilmaz, O. Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int. J. Clin. Pract. 2021, 75, e13753. [Google Scholar] [CrossRef]
- Soumdararajan, H.C.; Bullock, S.L. The influence of dynein processivity control, MAPs, and microtubule ends on directional movement of a localising mRNA. Elife 2014, 3, e01596. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef]
- Asghar, F.; Yan, H.; Jiang, L. The putative transcription factor CaMaf1 controls the sensitivity to lithium and rapamycin and represses RNA polymerase III transcription in Candida albicans. FEMS Yeast Res. 2018, 18, foy068. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Overy, D.P.; Lanteigne, M.; McQuillan, K.; Kerr, R.G. Antimicrobial Organometallic Dendrimers with Tunable Activity against Multidrug-Resistant Bacteria. Biomacromolecules 2015, 16, 3694–3703. [Google Scholar] [CrossRef]
- Bholay, A.D.; Nalawade, P.M.; Borkhataria, B.V. Fungicidal potential of biosynthesized silver nanoparticles against phytopatho gens and potentiation of fluconazole. World J. Pharm. Res. 2013, 1, 12–15. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.S.M.; Fouda, S.E.; El-Tahan, A.M.; et al. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef]
- Fernández, L.; Mediano, P.; García, R.; Rodríguez, J.M.; Marín, M. Risk Factors Predicting Infectious Lactational Mastitis: Decision Tree Approach versus Logistic Regression Analysis. Matern. Child Health J. 2016, 20, 1895–1903. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Rinitha, G. Nanostructural characterization of antimicrobial and antioxidant copper nanoparticles syn thesized using novel Persea americana seeds. OpenNano 2018, 3, 18–27. [Google Scholar] [CrossRef]
- Hasanin, M.; Elbahnasawy, M.A.; Shehabeldine, A.M.; Hashem, A.H. Ecofriendly preparation of silver nanoparticles-based nanocomposite stabilized by polysaccharides with antibacterial, antifungal and antiviral activities. Biometals 2021, 34, 1313–1328. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ranzoni, A.; Cooper, M.A. A nanoparticle-based method for culture-free bacterial DNA enrichment from whole blood. Biosens. Bioelectron. 2018, 99, 150–155. [Google Scholar] [CrossRef]
- Nemati, S.; Hassanzadeh, R.; Jahromi, S.K.; Abadi, A.D.N. Otomycosis in the north of Iran: Common pathogens and resistance to antifungal agents. Eur. Arch. Otorhinolaryngol. 2013, 271, 953–957. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Zinc Oxide Nanoparticles with Potential Synergistic Activity with Common Antifungal Agents against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- Kermani, S.A.; Salari, S.; Almani, P.G.N. Comparison of antifungal and cytotoxicity activities of titanium dioxide and zinc oxide nanoparticles with amphotericin B against different Candida species: In vitro evaluation. J. Clin. Lab. Anal. 2021, 35, e23577. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles with Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef]
- Rajendra, S.A.; Muddana, K.; Bakki, S.R. Fungal Infections of Oral Cavity: Diagnosis, Management, and Association with COVID-19. SN Compr. Clin. Med. 2021, 3, 1373–1384. [Google Scholar] [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiology and Diagnosis of Mucormycosis: An Update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef]
- Binder, U.; Maurer, E.; Lass-Florl, C. Mucormycosis—From the pathogens to the disease. Clin. Microbiol Infect. 2014, 20, 60–66. [Google Scholar] [CrossRef]
- Ibrahim, A.S. Host cell invasion in mucormycosis: Role of iron. Curr. Opin. Microbiol. 2011, 14, 406–411. [Google Scholar] [CrossRef]
- Ahmadikia, K.; Hashemi, S.J.; Khodavaisy, S.; Getso, M.I.; Alijani, N.; Badali, H.; Mirhendi, H.; Salehi, M.; Tabari, A.; Ardehali, M.M.; et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021, 64, 798–808. [Google Scholar] [CrossRef]
- Schoen, C.; Reichard, U.; Monod, M.; Kratzin, H.D.; Rüchel, R. Molecular cloning of an extracellular aspartic proteinase from Rhizopus microsporus and evidence for its expression during infection. Med. Mycol. 2002, 40, 61–71. [Google Scholar] [CrossRef]
- Spreer, A.; Rüchel, R.; Reichard, U. Characterization of an extracellular subtilisin protease of Rhizopus microsporus and evidence for its expression during invasive rhinoorbital mycosis. Med. Mycol. 2006, 44, 723–731. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Luna, M.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. A clinicopathological study of pulmonary mucormycosis in cancer patients: Extensive angioinvasion but limited inflammatory response. J. Infect. 2009, 59, 134–138. [Google Scholar] [CrossRef]
- Jose, A.; Singh, S.; Roychoudhury, A.; Kholakiya, Y.; Arya, S.; Roychoudhury, S. Current Understanding in the Pathophysiology of SARS-CoV-2-Associated Rhino-Orbito-Cerebral Mucormycosis: A Comprehensive Review. J. Maxillofac. Oral Surg. 2021, 20, 373–380. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Bowman, J.C.; Avanessian, V.; Brown, K.; Spellberg, B.; Edwards, J.E., Jr.; Douglas, C.M. Caspofungin inhibits Rhizopus oryzae 1,3-beta-D-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 2005, 49, 721–727. [Google Scholar] [CrossRef]
- Alqarihi, A.; Gebremariam, T.; Gu, Y.; Swidergall, M.; Alkhazraji, S.; Soliman, S.S.M.; Bruno, V.M.; Edwards, J.E.; Filler, S.G.; Uppuluri, P.; et al. GRP78 and Integrins Play Different Roles in Host Cell Invasion during Mucormycosis. mBio 2020, 11, e01087-20. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Del Castillo, R.G.; Sanchez-Gonzalez, M. High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef]
- Deb, A.K.; Sarkar, S.; Gokhale, T.; Choudhury, S.S. COVID-19 and orbital mucormycosis. Indian J. Ophthalmol. 2021, 69, 1002–1004. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Sany, D.; El Shahawy, Y.; Awdallah, A. Effect of activated vitamin D on glucoparameters in HCV seropositive and seronegative patients on chronic hemodialysis. Ren. Fail. 2012, 34, 1188–1194. [Google Scholar] [CrossRef][Green Version]
- Stanford, F.A.; Voigt, K. Iron Assimilation during Emerging Infections Caused by Opportunistic Fungi with emphasis on Mucorales and the Development of Antifungal Resistance. Genes 2020, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Lecube, A.; Hernández, C.; Genescà, J.; Esteban, J.I.; Jardí, R.; García, L.; Simó, R. Diabetes Is the Main Factor Accounting for the High Ferritin Levels Detected in Chronic Hepatitis C Virus Infection. Diabetes Care 2004, 27, 2669–2675. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021, 136, 111228. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Y.; Ji, Y.; He, X.; Xue, D. Increased Serum Levels of Hepcidin and Ferritin Are Associated with Severity of COVID-19. Med. Sci. Monit. 2020, 26, e926178-1. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Weigand, M.; Kim, J.; Wu, X.; Strayer, J.; Palmer, A.F.; Zborowski, M.; Yazer, M.; Chalmers, J.J. Hyperferritinemia in critically ill COVID-19 patients—Is ferritin the product of inflammation or a pathogenic mediator? Clin. Chim. Acta 2020, 509, 249–251. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Deng, L.S.; Yuan, J.; Ding, L.; Chen, Y.L.; Zhao, C.H.; Chen, G.Q.; Li, X.H.; Li, X.H.; Luo, W.T.; Lan, J.F.; et al. Comparison of patients hospitalized with COVID-19, H7N9 and H1N1. Infect. Dis. Poverty 2020, 9, 163. [Google Scholar] [CrossRef]
- Han, Y.J.; Ren, Z.G.; Li, X.X.; Yan, J.L.; Ma, C.Y.; Wu, D.D.; Ji, X.Y. Advances and challenges in the prevention and treatment of COVID-19. Int. J. Med. Sci. 2020, 17, 1803–1810. [Google Scholar] [CrossRef]
- Chan, K.H.; Sridhar, S.; Zhan, R.R.; Chu, H.; Fung, A.F.; Chan, G.; Chan, J.W.; To, K.W.; Hung, I.N.; Cheng, V.C.; et al. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020, 106, 226–231. [Google Scholar] [CrossRef]
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Ou, R.; Mo, L.; Tang, H.; Leng, S.; Zhu, H.; Zhao, L.; Ren, Y.; Xu, Y. circRNA-AKT1 Sequesters miR-942-5p to Upregulate AKT1 and Promote Cervical Cancer Progression. Mol. Ther. Nucleic Acids 2020, 20, 308–322. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Liu, J.; Li, M.; Xie, J.; Lv, Q.; Deng, W.; Zhou, N.; Zhou, Y.; Song, J.; et al. Mucus production stimulated by IFN-AhR signaling triggers hypoxia of COVID-19. Cell Res. 2020, 30, 1078–1087. [Google Scholar] [CrossRef]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wang, S.H.; Wu, C.H.; Yen, L.C.; Lai, H.F.; Ho, C.L.; Chiu, Y.L. In silico immune infiltration profiling combined with functional enrichment analysis reveals a potential role for naive B cells as a trigger for severe immune responses in the lungs of COVID-19 patients. PLoS ONE 2020, 15, e242900. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Liu, Z. Expression of ACE2 in airways: Implication for COVID-19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin. Exp. Allergy 2020, 50, 1313–1324. [Google Scholar] [CrossRef]
- Mehta, N.S.; Mytton, O.T.; Mullins, E.W.; Fowler, T.A.; Falconer, C.L.; Murphy, O.B.; Langenberg, C.; Jayatunga, W.J.; Eddy, D.H.; Nguyen-Van-Tam, J.S. SARS-CoV-2 (COVID-19): What Do We Know about Children? A Systematic Review. Clin. Infect. Dis. 2020, 71, 2469–2479. [Google Scholar] [CrossRef]
- Farmakiotis, D.; Kontoyiannis, D.P. Mucormycoses. Infect Dis. Clin. North Am. 2016, 30, 143–163. [Google Scholar] [CrossRef]
- Nasseri, M.; Golmohammadzadeh, S.; Arouiee, H.; Jaafari, M.R.; Neamati, H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran. J. Basic Med. Sci. 2016, 19, 1231. [Google Scholar]
- Jose, P.A.; Raja, J.D.; Sankarganesh, M.; Rajesh, J. Evaluation of antioxidant, DNA targeting, antimicrobial and cytotoxic studies of imine capped copper and nickel nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 178, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Assessing the efficacy of zinc oxide nanoparticles against Penicillium expansum by automated turbidimetric analysis. Mycology 2018, 9, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kolahalam, L.A.; Prasad, K.R.S.; Krishna, P.M.; Supraja, N. Saussurea lappa plant rhizome extract-based zinc oxide nanoparticles: Synthesis, characterization and its antibacterial, antifungal activities and cytotoxic studies against Chinese Hamster Ovary (CHO) cell lines. Heliyon 2021, 7, e07265. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Pandey, R.; Barman, I.; Prasad, R. Leveraging the Attributes of Mucor hiemalis-Derived Silver Nanoparticles for a Synergistic Broad-Spectrum Antimicrobial Platform. Front. Microbiol. 2016, 7, 1984. [Google Scholar] [CrossRef]
- Dilshad, E.; Bibi, M.; Sheikh, N.A.; Tamrin, K.F.; Mansoor, Q.; Maqbool, Q.; Nawaz, M. Synthesis of Functional Silver Nanoparticles and Microparticles with Modifiers and Evaluation of Their Antimicrobial, Anticancer, and Antioxidant Activity. J. Funct. Biomater. 2020, 11, 76. [Google Scholar] [CrossRef]
- Kubin, C.J.; McConville, T.H.; Dietz, D.; Zucker, J.; May, M.; Nelson, B.; Istorico, E.; Bartram, L.; Small-Saunders, J.; Sobieszczyk, M.E.; et al. Characterization of Bacterial and Fungal Infections in Hospitalized Patients with Coronavirus Disease 2019 and Factors Associated with Health Care-Associated Infections. Open Forum Infect. Dis. 2021, 8, ofab201. [Google Scholar] [CrossRef]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.A.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson III, G.R. Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Salehi, M.R.; Shahi, F.; Rizvi, F.S.; Ghaderkhani, S.; Zainaldain, H.; Khodavaysi, S.; Jamali-Moghaddam, S.R.; Manshadi, S.A.D.; Rezahosseini, O. Combination antifungal therapy without craniotomy in an immunocompromised patient with rhino-orbito-cerebral mucormycosis: A case report. Caspian J. Intern. Med. 2020, 11, 227–230. [Google Scholar] [CrossRef]
- Soffritti, I.; D’Accolti, M.; Fabbri, C.; Passaro, A.; Manfredini, R.; Zuliani, G.; Libanore, M.; Franchi, M.; Contini, C.; Caselli, E. Oral Microbiome Dysbiosis Is Associated with Symptoms Severity and Local Immune/Inflammatory Response in COVID-19 Patients: A Cross-Sectional Study. Front. Microbiol. 2021, 12, 687513. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, Y.; Shi, Z.; Zhang, L.; Ren, H.; He, W.; Zhang, Z.; Zhu, A.; Zhao, J.; Xiao, F.; et al. Characterization of respiratory microbial dysbiosis in hospitalized COVID-19 patients. Cell Discov. 2021, 7, 23. [Google Scholar] [CrossRef]
- Fan, G.; Cheng, L.; Fu, Z.; Sun, B.; Teng, C.; Jiang, X.; Li, X. Screening of yeasts isolated from Baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech 2020, 10, 275. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, M.; Li, X.; Zhang, Y.; Wang, Z.; Zheng, J. Enantioselective Resolution of (R, S)-2-Phenoxy-Propionic Acid Methyl Ester by Covalent Immobilized Lipase from Aspergillus oryzae. Appl. Biochem. Biotechnol. 2019, 190, 1049–1059. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.Y.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.; Chan, P.K.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.; Lui, G.C.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.; et al. Alterations in Fecal Fungal Microbiome of Patients with COVID-19 during Time of Hospitalization until Discharge. Gastroenterology 2020, 159, 1302–1310. [Google Scholar] [CrossRef]
- Buil, J.B.; Meijer, E.F.; Denning, D.W.; Verweij, P.E.; Meis, J.F. Burden of serious fungal infections in the Netherlands. Mycoses 2020, 63, 625–631. [Google Scholar] [CrossRef]
- Mekonnen, Z.K.; Ashraf, D.C.; Jankowski, T.; Grob, S.R.; Vagefi, M.R.; Kersten, R.C.; Simko, J.P.; Winn, B.J. Acute Invasive Rhino-Orbital Mucormycosis in a Patient with COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e40–e80. [Google Scholar] [CrossRef]
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Rezaie, Y.; Rajaeih, S.; Alijani, N.; Barac, A.; et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses 2021, 64, 1238–1252. [Google Scholar] [CrossRef]
- Placik, D.A.; Taylor, W.L.; Wnuk, N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 2020, 15, 2378–2381. [Google Scholar] [CrossRef]
- Goldman, N.; Fink, D.; Cai, J.; Lee, Y.-N.; Davies, Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res. Clin. Pr. 2020, 166, 108291. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. 2021, 15, 102146. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, S.; Shastri, S.; Sudershan, A.; Mansotra, V. Black fungus immunosuppressive epidemic with COVID-19 associated mucormycosis (zygomycosis): A clinical and diagnostic perspective from India. Immunogenetics 2021, 74, 197–206. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Drissi, C. Black fungus, the darker side of COVID-19. J. Neuroradiol. 2021, 48, 317–318. [Google Scholar] [CrossRef]
- Barron, M.A.; Lay, M.; Madinger, N.E. Surgery and treatment with high-dose liposomal amphotericin B for eradication of craniofacial zygomycosis in a patient with Hodgkin’s disease who had undergone allogeneic hematopoietic stem cell transplantation. J. Clin. Microbiol. 2005, 43, 2012–2014. [Google Scholar] [CrossRef]
- Çagatay, A.A.; Öncü, S.S.; Çalangu, S.S.; Yildirmak, T.T.; Özsüt, H.H.; Eraksoy, H.H. Rhinocerebral mucormycosis treated with 32 gram liposomal amphotericin B and incomplete surgery: A case report. BMC Infect. Dis. 2001, 1, 22. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J., Jr.; Ibrahim, A. Novel Perspectives on Mucormycosis: Pathophysiology, Presentation, and Management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Van Cutsem, J.; de Locht, M.; Schneider, Y.J.; Crichton, R.R. Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney Int. 1994, 45, 667–671. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mitchell, T.G.; Moon, R.; Camporesi, E.M.; Farmer, J. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev. Infect. Dis. 1988, 10, 551–559. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19-Associated Pulmonary Aspergillosis, March–August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Type of Nanoparticles | Size | Target Fungi | References |
|---|---|---|---|---|
| (nm) | ||||
| 1 | AgNPs | 17.0 | A. niger, A. flavus, and F. oxysporum | [193] |
| 2 | AgNPs | 23.0 | V. dahliae | [194] |
| 3 | AgNPs | 10–50 | A. alternata, S. sclerotiorum, M. phaseolina, R. solani, B. cinerea, and C. lunata | [195] |
| 4 | AgNPs | 50 | A. flavus and P. chrysogenum | [196] |
| 5 | AgNPs | 20 | B. maydis | [197] |
| 6 | AgNPs | 29 | R. nigricans | [198] |
| 7 | AgNPs | 10–20 | ||
| Colletotrichum sp., F. oxysporum, F. acuminatum, F. tricinctum, F. graminearum, F. incarnatum, R. solani, S. sclerotiorum, and A. alternate | [199] | |||
| 8 | AgNPs | 75 | [200] | |
| 9 | AgNPs | 50 | A. alternata and C. lunata. | |
| [201] | ||||
| H. rostratum, F. solani, F. oxysporum, and A. alternate | ||||
| 10 | AgNPs | 10–20 | [202] | |
| A. flavus and A. parasiticus | ||||
| 11 | AgNPs | 5–30 | ||
| F. oxysporum, F. moniliform, F. solani, F. verticillioides, F. semitectum, A. flavus, A. terreus, A. niger, A. ficuum, P. citrinum, P. islandicum, P. chrysogenum, R. stolonifer, Phoma, A. alternata, and A. chlamydospora | [203] | |||
| 12 | AgNPs | 16–20 | A. alternate, A. niger, A. nidulans, C. herbarum, F. moniliforme, Fusarium spp., | [204] |
| 13 | AgNPs | 25–40 | F. oxysporum, and T. harzianum | [205] |
| 14 | AgNPs | 10 | A. flavus, A. niger, A. tereus, P. notatum, R. olina, F. solani, F. oxysporum, T. viride, V. dahlia, and P. spinosum | [206] |
| 15 | AgNPs | 15–400 | Alternaria sp., F. oxysporum, F. moniliforme, and F. tricinctum. | [207] |
| 40–90 | B. cinerea, P. expansum, A. niger, Alternaria sp., and Rhizopus sp. | [208] | ||
| 16 | CuNPs | |||
| 20–40 | ||||
| 17 | CuNPs | |||
| A. flavus, A. fumigates, and F. oxysporum. | ||||
| 200–500 | [202] | |||
| 18 | CuNPs | A. flavus and A. parasiticus | ||
| 10 | [136] | |||
| 19 | CuNPs | F. solani, Neofusicoccum sp., | ||
| 3–60 | and F. oxysporum. | |||
| [209] | ||||
| 20 | CuNPs | |||
| 30–300 | ||||
| A. niger, A terreus, and | [210] | |||
| 21 | CuNPs | A. fumigatus | ||
| 22 | ||||
| Alternaria spp., A. niger, | [211] | |||
| Pythium spp., and | ||||
| 22 | ZnONPs | 10–25 | Fusarium spp. | |
| A. alternata, A solani, | ||||
| F. expansum, and | [212] | |||
| 23 | ZnONPs | 10–25 | Penicilliun sp. | |
| B. albicans, C. glabrata, C. tropicalis | [213] | |||
| 24 | TiO2 NPs | |||
| 70–300 | ||||
| Candida sps | [213] | |||
| 25 | SeNPs | |||
| Candida sps | ||||
| [214] | ||||
| Candida albicans |
| S. No. | Place of Organ | Name of Microorganisms | Reference |
|---|---|---|---|
| 1 | Oral | Streptococcus, Porphyromonas, Abiotrophia, Enterobacter, Neisseria mucosa, Veillonella parvula, Lactobacillus fermentum, Enterococcus faecalis, Atopobium parvulum, Acinetobacter baumannii, Prevotella melaninogenica, jejuni, denticola, and oris; Eikenella corrodens; Capnocytophaga sputigena and gingivalis; and Aggregatibacter aphrophilus), Aspergillus sp., Nakaseomyces sp., and Malassezia sp., Candida sp., Saccharomyces sp., Epstein–Barr virus, Staphylococcus phage ROSA, Streptococcus phage EJ-1, phage PH10, Lactobacillus phage phiadh. | [259] |
| 2 | Lung | Burkholderiacepacia complex (BCC), Staphylococcus epidermidis, Mycoplasma spp. (including M. hominis and M. orale) | [260] |
| Cutaneotricosporon, Issatchenkia, Wallemia, Cladosporium, Alternaria, Dipodascus, Mortierella, Aspergillus, Naganishia, Diutina, and Candida | [261] | ||
| 3 | Lung | Lung alphaherpesvirus 1, rhinovirus B, human orthopneumovirus | [262] |
| 4 | Lung | Acinetobacter, Chryseobacterium, Burkholderia, Brevundimonas, Sphingobium, Enterobacteriaceae | [261] |
| 5 | Gut | Streptococcus, Rothia, Actinomyces, Vellionella, Collinsella aerofaciens, Collinsella tanakaei, Streptococcus infantis, Morganella morganii, Candida albicans, Candida auris, Aspergillus flavus, Coprobacillus sp., Clostridium ramosum, Clostridium hathewayi | [263,264,265] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurunathan, S.; Lee, A.R.; Kim, J.H. Antifungal Effect of Nanoparticles against COVID-19 Linked Black Fungus: A Perspective on Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 12526. https://doi.org/10.3390/ijms232012526
Gurunathan S, Lee AR, Kim JH. Antifungal Effect of Nanoparticles against COVID-19 Linked Black Fungus: A Perspective on Biomedical Applications. International Journal of Molecular Sciences. 2022; 23(20):12526. https://doi.org/10.3390/ijms232012526
Chicago/Turabian StyleGurunathan, Sangiliyandi, Ah Reum Lee, and Jin Hoi Kim. 2022. "Antifungal Effect of Nanoparticles against COVID-19 Linked Black Fungus: A Perspective on Biomedical Applications" International Journal of Molecular Sciences 23, no. 20: 12526. https://doi.org/10.3390/ijms232012526
APA StyleGurunathan, S., Lee, A. R., & Kim, J. H. (2022). Antifungal Effect of Nanoparticles against COVID-19 Linked Black Fungus: A Perspective on Biomedical Applications. International Journal of Molecular Sciences, 23(20), 12526. https://doi.org/10.3390/ijms232012526






