Upgrading Monocytes Therapy for Critical Limb Ischemia Patient Treatment: Pre-Clinical and GMP-Validation Aspects
Abstract
:1. Introduction
2. Results
2.1. Pre-Clinical Experiments
2.1.1. Characterization of PBMC by Flow Cytometry Immunophenotyping
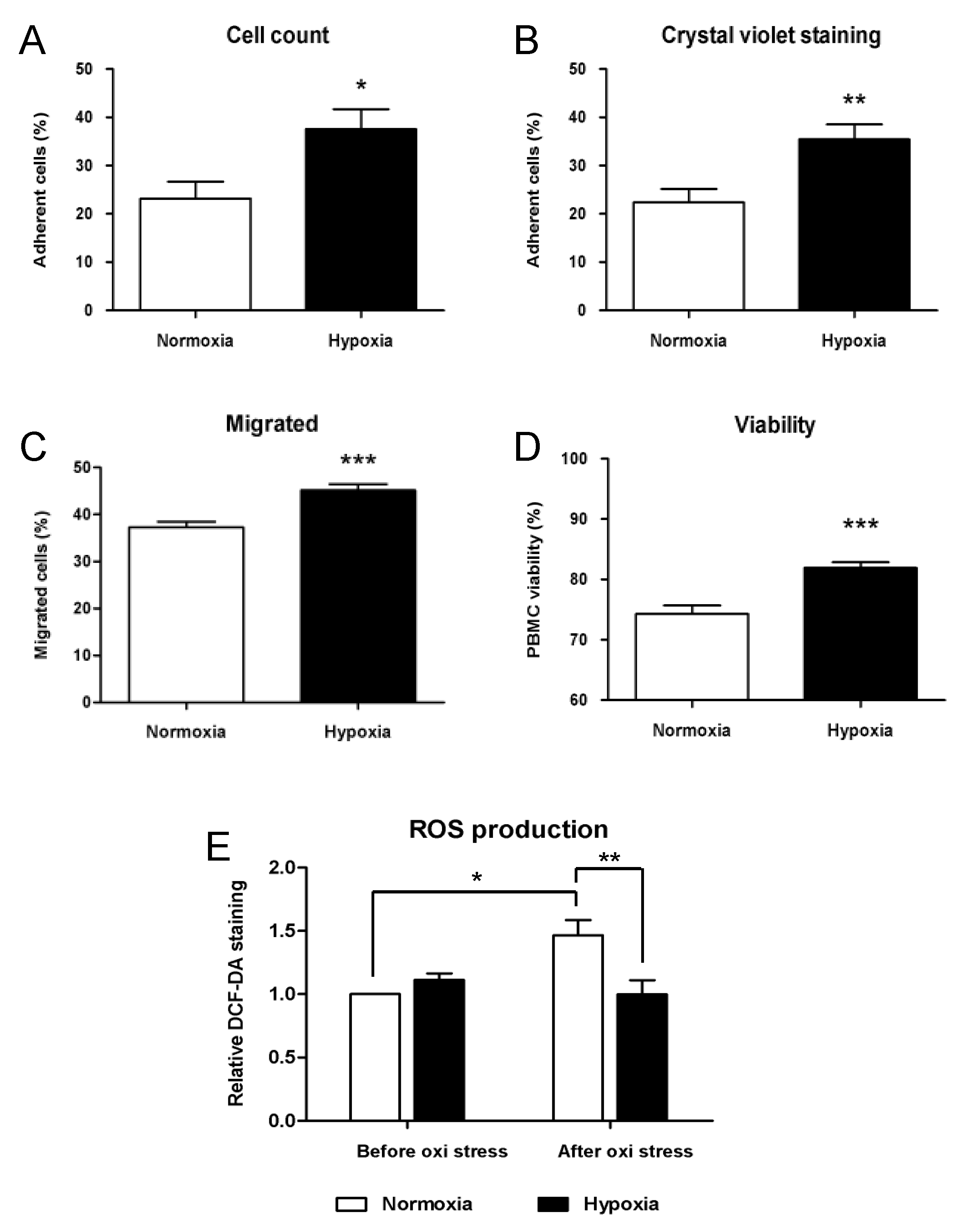
2.1.2. Functional Analysis of Cryopreserved PBMCs under Hypoxic Conditions
2.2. GMP-Compliant Validation
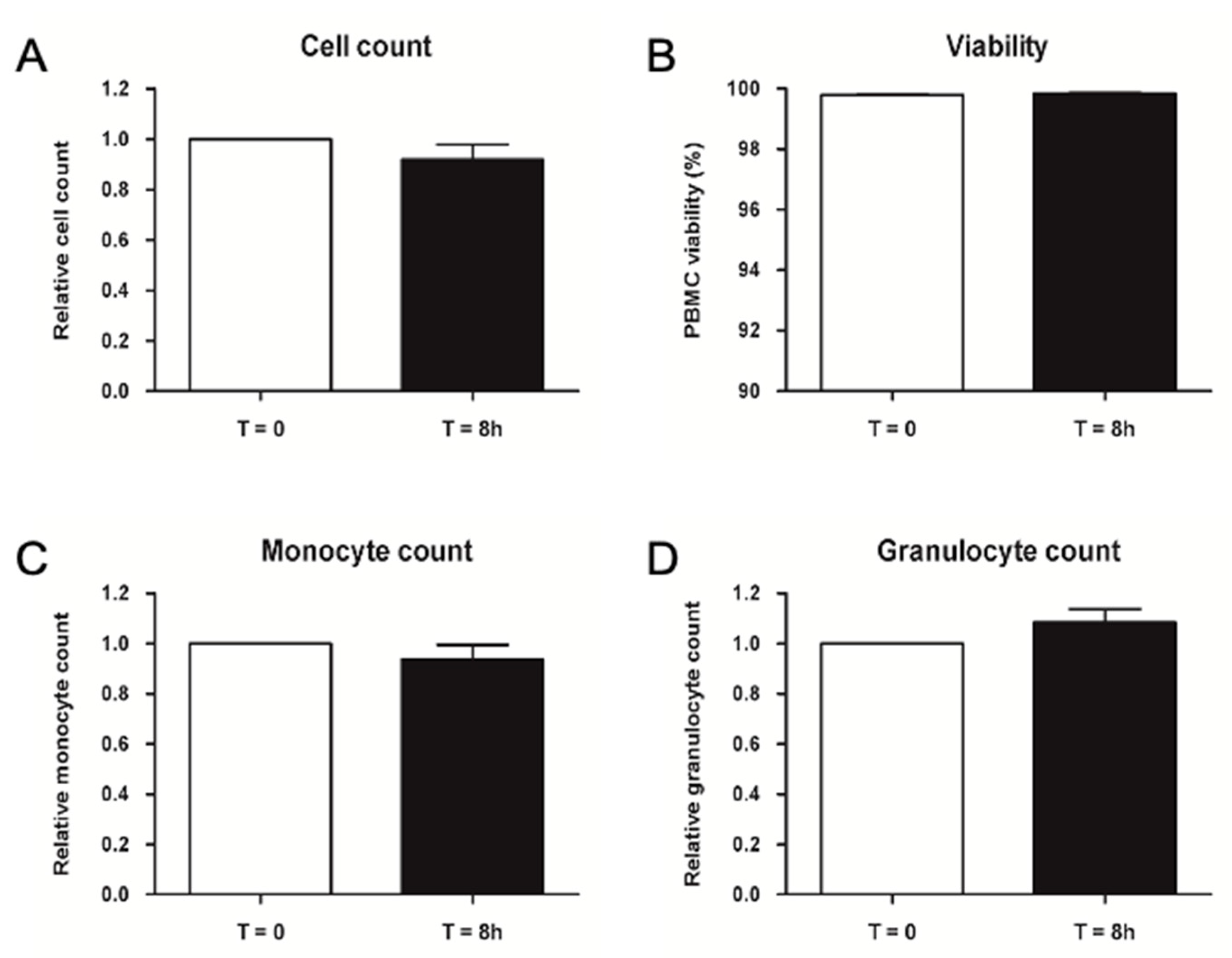
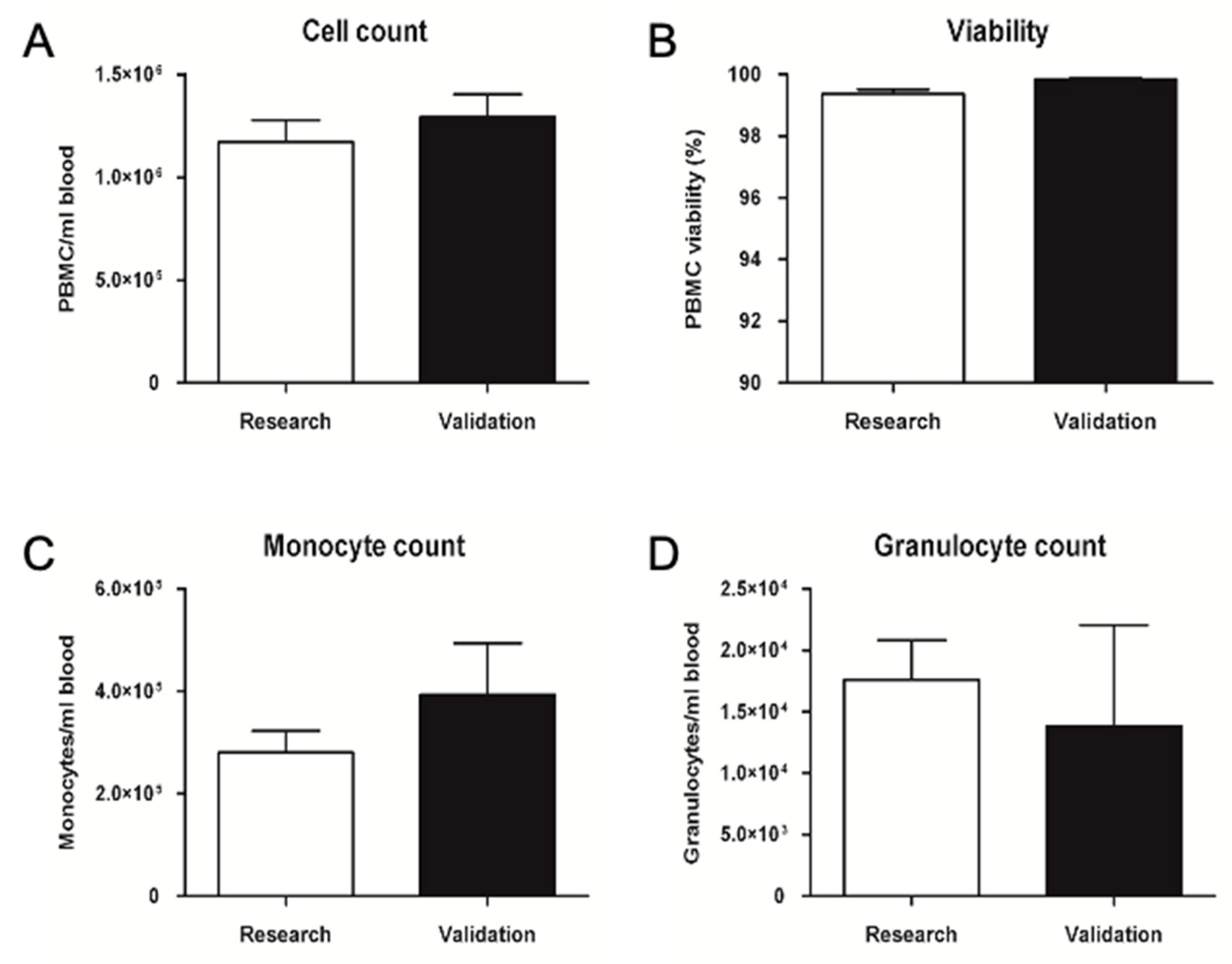
2.2.1. Validation of Pre-Processing Time and Temperature
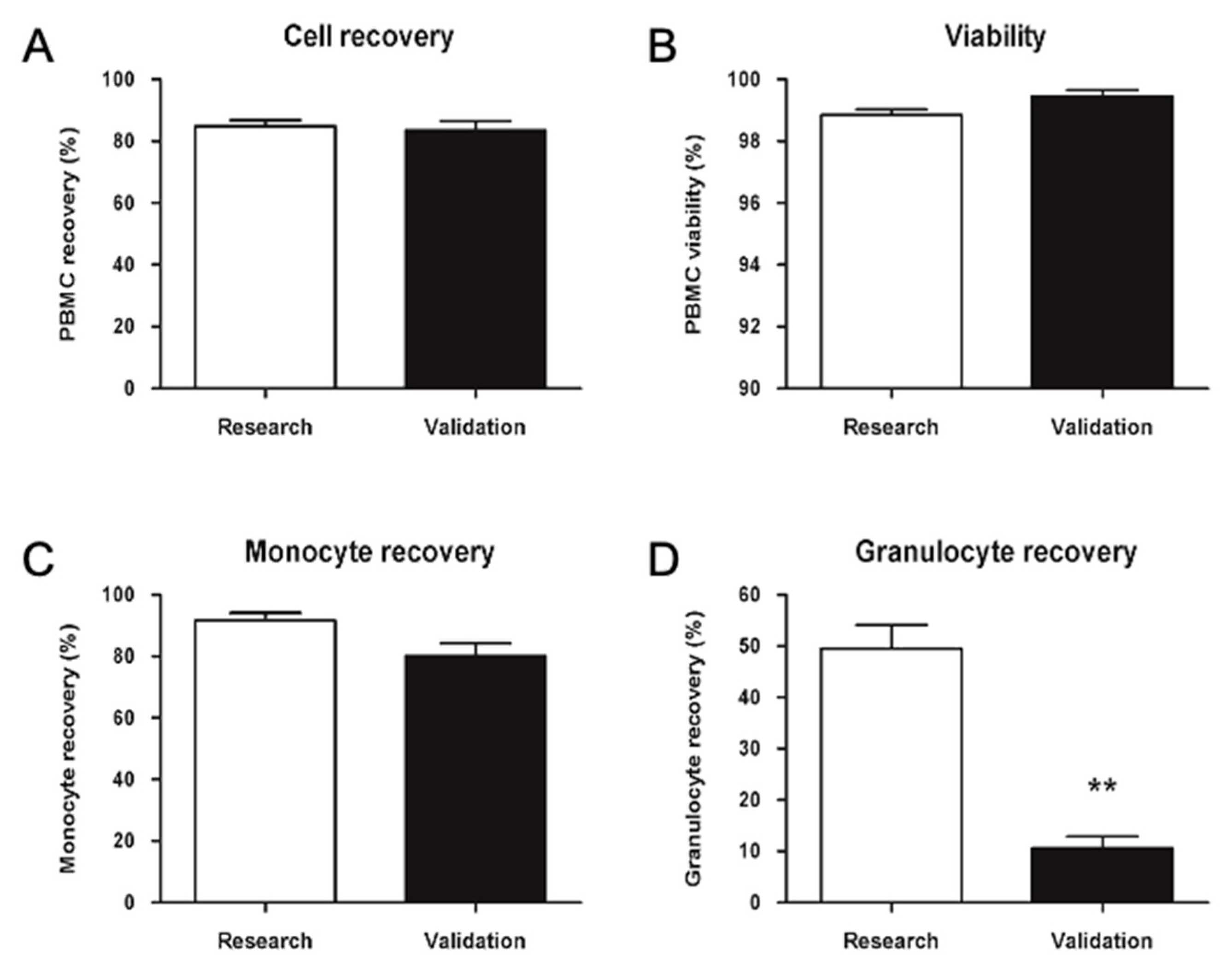
2.2.2. GMP Upgrade of PBMC Isolation, Cryopreservation, and Thawing
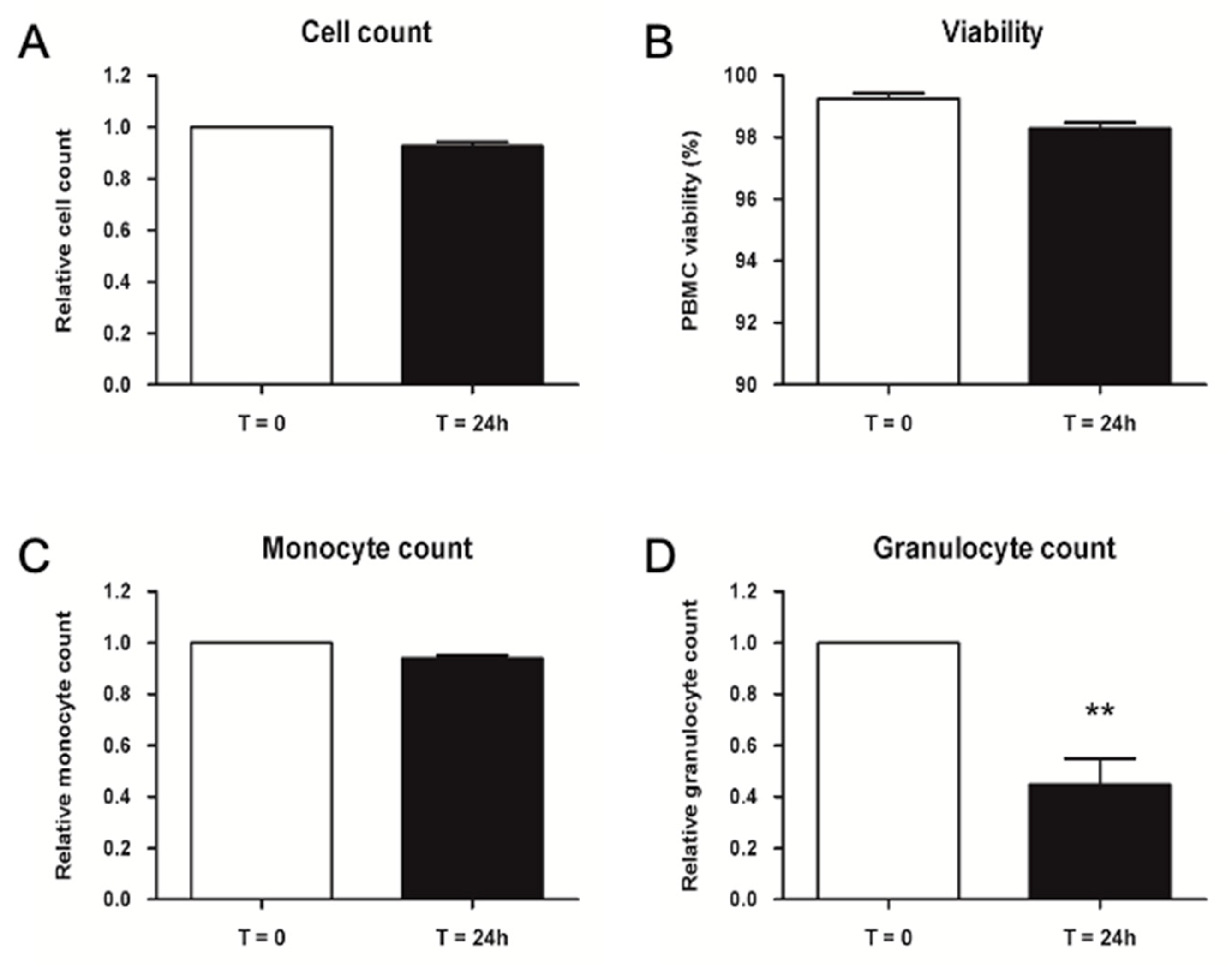
2.2.3. Validation of Storage Time for Thawed PBMCs before Injection
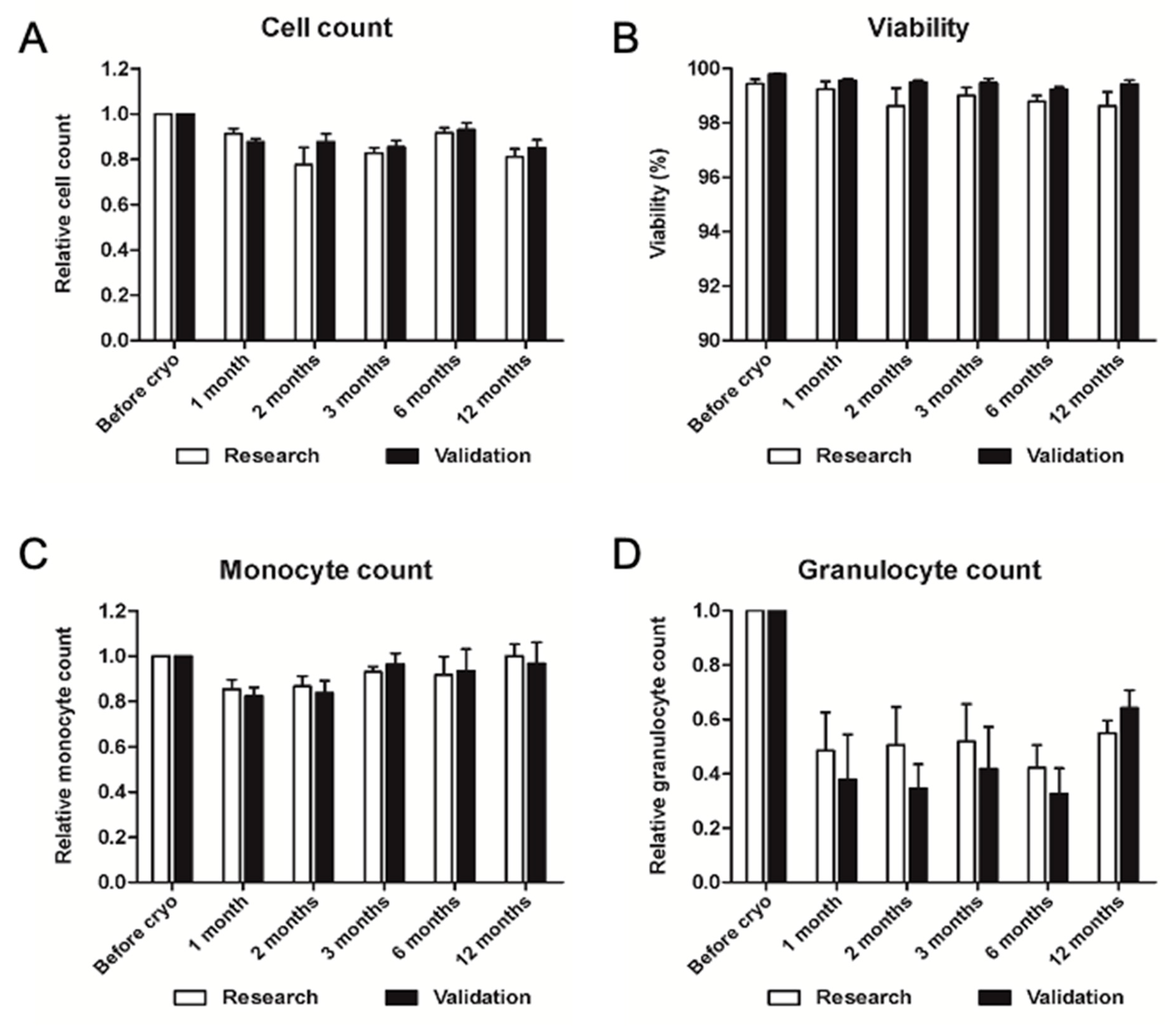
2.2.4. Validation of Long-Term Storage of Cryopreserved PBMC
3. Discussion
3.1. Pre-Clinical Experiments
Functional Analysis of Cryopreserved PBMCs under Hypoxic Conditions
3.2. GMP-Compliant Validation
4. Materials and Methods
4.1. Pre-Clinical Validation
4.1.1. Patient Selection and Informed Consent
4.1.2. Blood Collection
4.1.3. PBCM Isolation by Density Gradient Centrifugation
4.1.4. Cell Counting and Viability Measurement
4.1.5. Phenotypic Characterization of PBMC by Flow Cytometry
4.1.6. PBMC Cryopreservation
4.1.7. PBMC Thawing
4.1.8. Cell Culture
4.1.9. Hypoxic Conditioning
4.1.10. Adhesion Potential Assay
4.1.11. Migration Potential Assay
4.1.12. Oxidative Stress Resistance Assay
4.1.13. Measurement of Reactive Oxygen Species (ROS)
4.1.14. RNA Extraction and Reverse Transcription
4.1.15. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.1.16. Statistical Analysis
4.2. GMP-Compliant Validation
4.2.1. Validation of Pre-Processing Time and Temperature
4.2.2. Validation of PBMC Isolation, Cryopreservation, and Thawing
4.2.3. Validation of Storage Time for Thawed PBMC before Injection
4.2.4. Validation of Long-Term Storage of Cryopreserved PBMC
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goula, A.; Gkioka, V.; Michalopoulos, E.; Katsimpoulas, M.; Noutsias, M.; Sarri, E.F.; Stavropoulos, C.; Kostakis, A. Advanced Therapy Medicinal Products Challenges and Perspectives in Regenerative Medicine. J. Clin. Med. Res. 2020, 12, 780–786. [Google Scholar] [CrossRef]
- Gouveia, B.G.; Rijo, P.; Gonçalo, T.S.; Reis, C.P. Good manufacturing practices for medicinal products for human use. J. Pharm. Bioallied Sci. 2015, 7, 87–96. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Human Cell-Based Medicinal Products; EMA: London, UK, 2008. [Google Scholar]
- Kleiveland, C.R. Peripheral Blood Mononuclear Cells; Springer Open: Berlin/Heidelberg, Germany, 2015; pp. 161–167. [Google Scholar]
- Carrick, J.B.; Begg, A.P. Peripheral Blood Leukocytes. Vet. Clin. N. Am. Equine Pract. 2008, 24, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Martinez-Pomares, L.; Stacey, M.; Lin, H.-H.; Brown, G.; Gordon, S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005, 23, 901–944. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Barron, L. Macrophages: Master Regulators of Inflammation and Fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Fung, E.; Helisch, A. Macrophages in Collateral Arteriogenesis. Front. Physiol. 2012, 3, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, Q.; Li, C.; Wang, Y.; Chen, X. VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget 2017, 8, 77020–77027. [Google Scholar] [CrossRef]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A Review of the Pathophysiology and Potential Biomarkers for Peripheral Artery Disease. Int. J. Mol. Sci. 2015, 16, 11294–11322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teraa, M.; Conte, M.S.; Moll, F.L.; Verhaar, M.C. Critical Limb Ischemia: Current Trends and Future Directions. J. Am. Heart Assoc. 2016, 5, e002938. [Google Scholar] [CrossRef] [Green Version]
- Scholz, D.; Ito, W.; Fleming, I.; Deindl, E.; Sauer, A.; Wiesnet, M.; Busse, R.; Schaper, J. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch. 2000, 436, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Ziegelhoeffer, T.; Fernandez, B.; Kostin, S.; Heil, M.; Voswinckel, R.; Helisch, A.; Schaper, W. Bone Marrow-Derived Cells Do Not Incorporate into the Adult Growing Vasculature. Circ. Res. 2004, 94, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harraz, M.; Jiao, C.; Hanlon, H.D.; Hartley, R.S.; Schatteman, G.C. CD34—Blood-Derived Human Endothelial Cell Progenitors. Stem Cells 2001, 19, 304–312. [Google Scholar] [CrossRef]
- Schmeisser, A.; Garlichs, C.D.; Zhang, H.; Eskafi, S.; Graffy, C.; Ludwig, J.; Strasser, R.H.; Daniel, W.G. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel® under angiogenic conditions. Cardiovasc. Res. 2001, 49, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Jaipersad, A.S.; Lip, G.Y.; Silverman, S.; Shantsila, E. The Role of Monocytes in Angiogenesis and Atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Abe, H.; Semba, H.; Takeda, N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J. Atheroscler. Thromb. 2017, 24, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.C.; Puppo, M.; Blengio, F.; Fraone, T.; Cappello, P.; Giovarelli, M.; Varesio, L. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology 2008, 213, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Li, T.-S.; Suzuki, R.; Shirasawa, B.; Morikage, N.; Ohshima, M.; Qin, S.-L.; Hamano, K. Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am. J. Physiol. Circ. Physiol. 2008, 294, H590–H595. [Google Scholar] [CrossRef] [Green Version]
- Kubo, M.; Li, T.-S.; Kamota, T.; Ohshima, M.; Qin, S.-L.; Hamano, K. Increased expression of CXCR4 and integrin αM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J. Cell. Physiol. 2009, 220, 508–514. [Google Scholar] [CrossRef]
- Kudo, T.; Hosoyama, T.; Samura, M.; Katsura, S.; Nishimoto, A.; Kugimiya, N.; Fujii, Y.; Li, T.-S.; Hamano, K. Hypoxic preconditioning reinforces cellular functions of autologous peripheral blood-derived cells in rabbit hindlimb ischemia model. Biochem. Biophys. Res. Commun. 2014, 444, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kubo, M.; Katsura, S.; Nishimoto, A.; Ueno, K.; Samura, M.; Fujii, Y.; Hosoyama, T.; Hamano, K. Hypoxically preconditioned human peripheral blood mononuclear cells improve blood flow in hindlimb ischemia xenograft model. Am. J. Transl. Res. 2014, 6, 570–579. [Google Scholar] [PubMed]
- The Committee for Advanced Therapies (CAT); Schneider, C.K.; Salmikangas, P.; Jilma, B.; Flamion, B.; Todorova, L.R.; Paphitou, A.; Haunerova, I.; Maimets, T.; Trouvin, J.-H.; et al. Challenges with advanced therapy medicinal products and how to meet them. Nat. Rev. Drug Discov. 2010, 9, 195–201. [Google Scholar] [CrossRef]
- Kinlay, S. Management of Critical Limb Ischemia. Circ. Cardiovasc. Interv. 2016, 9, e001946. [Google Scholar] [CrossRef] [Green Version]
- Blum, A.; Balkan, W.; Hare, J.M. Advances in cell-based therapy for peripheral vascular disease. Atherosclerosis 2012, 223, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Benoit, E.; O’Donnell, J.T.F.; Patel, A.N. Safety and Efficacy of Autologous Cell Therapy in Critical Limb Ischemia: A Systematic Review. Cell Transplant. 2013, 22, 545–562. [Google Scholar] [CrossRef]
- Samura, M.; Hosoyama, T.; Takeuchi, Y.; Ueno, K.; Morikage, N. Therapeutic strategies for cell-based neovascularization in critical limb ischemia. J. Transl. Med. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Grisendi, G.; Annerén, C.; Cafarelli, L.; Sternieri, R.; Veronesi, E.; Cervo, G.L.; Luminari, S.; Maur, M.; Frassoldati, A.; Palazzi, G.; et al. GMP-manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion. Cytotherapy 2010, 12, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Ueno, Y.; Kimura, T.; Ogawa, R. Early and Long-term Effects of the Autologous Peripheral Stem Cell Implantation for Critical Limb Ischemia. Ann. Vasc. Dis. 2011, 4, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaltro, G.; Straino, S.; Gambini, E.; Bassetti, B.; Persico, L.; Zoli, S.; Zanobini, M.; Capogrossi, M.C.; Spirito, R.; Quarti, C.; et al. Characterization of the Pall Celeris system as a point-of-care device for therapeutic angiogenesis. Cytotherapy 2015, 17, 1302–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-N.; Sohn, J.Y.; Kong, J.H.; Eom, H.S.; Lee, H.; Kong, S.-Y. Comparison of Two Apheresis Systems of COBE and Optia for Autologous Peripheral Blood Stem Cell Collection. Ann. Lab. Med. 2017, 37, 327–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigma-Aldrich. Histopaque. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar]
- Guimarães-Costa, A.B.; Rochael, N.C.; Oliveira, F.; Echevarria-Lima, J.; Saraiva, E.M. Neutrophil extracellular traps reprogram IL-4/GM-CSF-induced monocyte differentiation to anti-inflammatory macrophages. Front. Immunol. 2017, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Streitz, M.; Miloud, T.; Kapinsky, M.; Reed, M.R.; Magari, R.; Geissler, E.K.; A Hutchinson, J.; Vogt, K.; Schlickeiser, S.; Kverneland, A.H.; et al. Standardization of whole blood immune phenotype monitoring for clinical trials: Panels and methods from the ONE study. Transplant. Res. 2013, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Finak, G.; Langweiler, M.; Jaimes, M.; Malek, M.; Taghiyar, J.; Korin, Y.; Raddassi, K.; Devine, L.; Obermoser, G.; Pekalski, M.L.; et al. Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci. Rep. 2016, 6, 20686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Shantsila, E.; Wrigley, B.; Tapp, L.; Apostolakis, S.; Montoro-Garcia, S.; Drayson, M.T.; Lip, G.Y.H. Immunophenotypic characterization of human monocyte subsets: Possible implications for cardiovascular disease pathophysiology. J. Thromb. Haemost. 2011, 9, 1056–1066. [Google Scholar] [CrossRef]
- Van Velzen, J.F.; Laros-van Gorkom, B.A.; Pop, G.A.; van Heerde, W.L. Multicolor flow cytometry for evaluation of platelet surface antigens and activation markers. Thromb. Res. 2012, 130, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp. Cell Res. 2016, 342, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, M.; Li, T.-S.; Kurazumi, H.; Takemoto, Y.; Ohshima, M.; Murata, T.; Katsura, S.; Morikage, N.; Furutani, A.; Hamano, K. Hypoxic Preconditioning Enhances Angiogenic Potential of Bone Marrow Cells with Aging-Related Functional Impairment. Circ. J. 2012, 76, 986–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS signaling and ER stress in cardiovascular disease. Mol. Asp. Med. 2018, 63, 18–29. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [Green Version]
- Birk, D.M.; Barbato, J.; Mureebe, L.; Chaer, R.A. Basic Science Review: Current Insights on the Biology and Clinical Aspects of VEGF Regulation. Vasc. Endovasc. Surg. 2009, 42, 517–530. [Google Scholar] [CrossRef]
- França, C.N.; Izar, M.C.; Hortêncio, M.N.; Amaral, J.B.D.; Ferreira, C.E.; Tuleta, I.D.; Fonseca, F.A. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin. Sci. 2017, 131, 1215–1224. [Google Scholar] [CrossRef] [Green Version]
- Pozzobon, T.; Goldoni, G.; Viola, A.; Molon, B. CXCR4 signaling in health and disease. Immunol. Lett. 2016, 177, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bleul, C.C.; Fuhlbrigge, R.C.; Casasnovas, J.M.; Aiuti, A.; Springer, T.A. A Highly Efficacious Lymphocyte Chemoattractant, Stromal Cell-Derived Factor 1 (SDF-1). J. Exp. Med. 1996, 184, 1101–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staller, P.; Sulitkova, J.; Lisztwan, J.; Moch, H.; Oakeley, E.J.; Krek, W. Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature 2003, 425, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.; Lee, D.; Stucky, J.; Chiu, Y.-L.; Rubin, A.; Horton, H.; McElrath, M.J. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 2007, 322, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Afonso, G.; Scotto, M.; Renand, A.; Arvastsson, J.; Vassilieff, D.; Cilio, C.M.; Mallone, R. Critical parameters in blood processing for T-cell assays: Validation on ELISpot and tetramer platforms. J. Immunol. Methods 2010, 359, 28–36. [Google Scholar] [CrossRef]
- McKenna, K.C.; Beatty, K.M.; Vicetti Miguel, R.; Bilonick, R.A. Delayed processing of blood increases the frequency of activated CD11b+ CD15+ granulocytes which inhibit T cell function. J. Immunol. Methods 2009, 341, 68–75. [Google Scholar] [CrossRef]
- Olson, W.C.; Smolkin, M.E.; Farris, E.M.; Fink, R.J.; Czarkowski, A.R.; Fink, J.H.; Chianese-Bullock, K.A.; Slingluff, C.L., Jr. Shipping blood to a central laboratory in multicenter clinical trials: Effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J. Transl. Med. 2011, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Naranbhai, V.; Bartman, P.; Ndlovu, D.; Ramkalawon, P.; Ndung’U, T.; Wilson, D.; Altfeld, M.; Carr, W.H. Impact of blood processing variations on natural killer cell frequency, activation, chemokine receptor expression and function. J. Immunol. Methods 2011, 366, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Peng, X.L.; Hai, F.L.; Zhi, H.F.; Zhi, B.H.; Ren, H.; Poon, M.-C.; Han, Z.C. Enhancement of neovascularization with mobilized blood cell transplantation: Supply of angioblasts and angiogenic cytokines. J. Cell. Biochem. 2007, 102, 183–195. [Google Scholar] [CrossRef]
- Li, S.; Zhou, B.; Han, Z.C. Therapeutic neovascularization by transplantation of mobilized peripheral blood mononuclear cells for limb ischemia. Thromb. Haemost. 2006, 95, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Mallone, R.; I Mannering, S.; Brooks-Worrell, B.M.; Durinovic-Belló, I.; Cilio, C.M.; Wong, F.S.; Schloot, N.C. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: Position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin. Exp. Immunol. 2011, 163, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.C.; Germann, A.; Kemp-Kamke, B.; Mazzotta, A.; von Briesen, H.; Zimmermann, H. Towards a xeno-free and fully chemically defined cryopreservation medium for maintaining viability, recovery, and antigen-specific functionality of PBMC during long-term storage. J. Immunol. Methods 2012, 382, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jin, F.; Zhong, H. A novel experimental hypoxia chamber for cell culture. Am. J. Cancer Res. 2014, 4, 53–60. [Google Scholar]
- Bakmiwewa, S.M.; Heng, B.; Guillemin, G.J.; Ball, H.J.; Hunt, N.H. An effective, low-cost method for achieving and maintaining hypoxia during cell culture studies. BioTechniques 2015, 59, 223–229. [Google Scholar] [CrossRef]
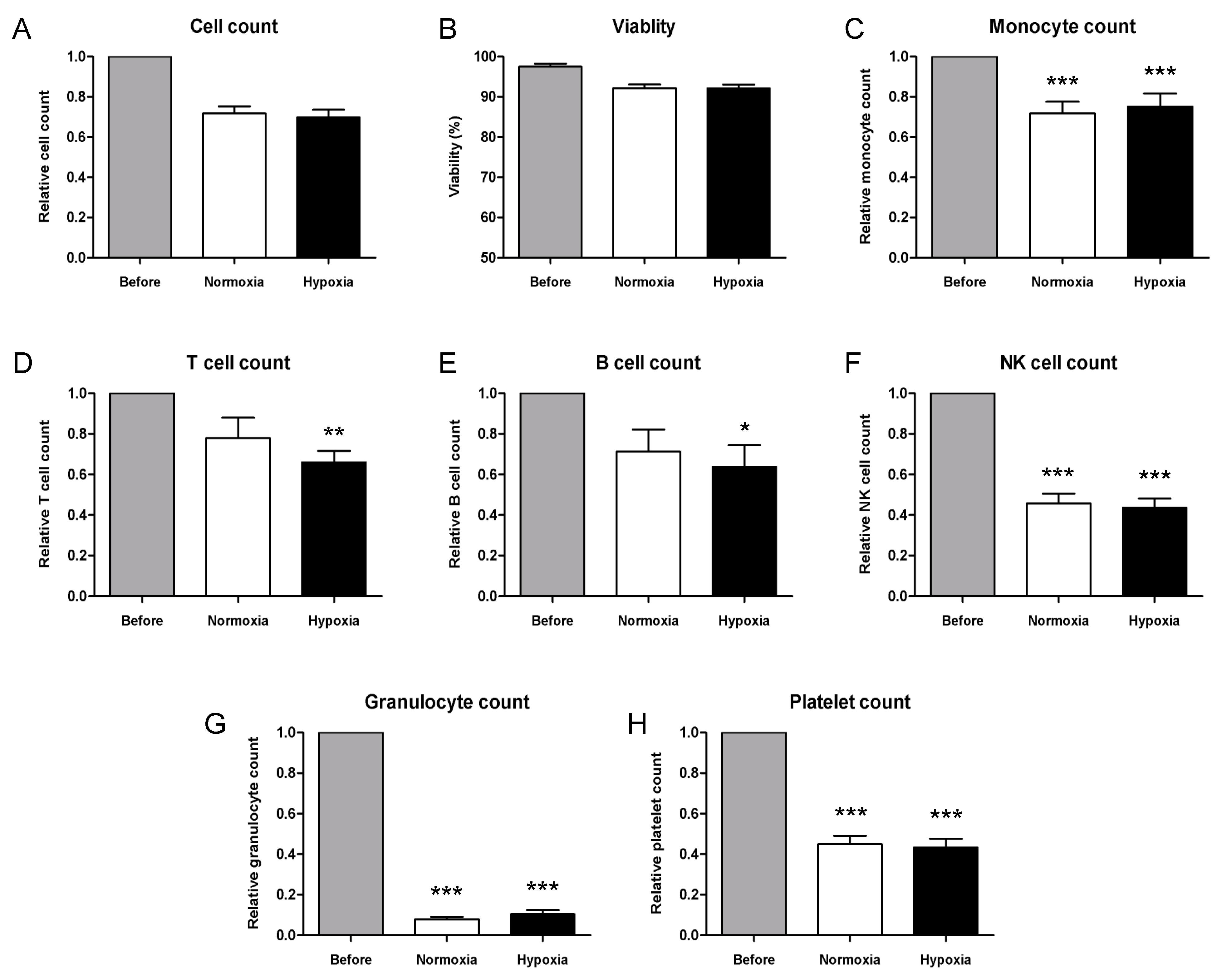

| Before Freezing | After Thawing | |||
|---|---|---|---|---|
| % | Cells/mL Blood | % | Cells/mL Blood | |
| Monocytes | 11.36 | 2.67 × 105 | 17.25 | 2.72 × 105 |
| T lymphocytes | 37.79 | 6.69 × 105 | 35.04 | 4.17 × 105 |
| B lymphocytes | 10.69 | 1.73 × 105 | 12.59 | 1.63 × 105 |
| NK lymphocytes | 10.29 | 1.93 × 105 | 15.86 | 1.98 × 105 |
| Platelets | 24.27 | 6.03 × 105 | 23.42 | 3.99 × 105 |
| Granulocytes | 0.47 | 1.10 × 104 | 0.45 | 7.23 × 103 |
| STEP | Temperature (°C) | Time (min) | Heat | Hold |
|---|---|---|---|---|
| 1 | 4.0 | 2 | Off | Stop |
| 2 | 4.0 | 5 | Off | |
| 3 | −6.0 | 10 | Off | |
| 4 | −45.0 | 1.5 | Off | |
| 5 | −20.0 | 2.5 | On | |
| 6 | −45.0 | 25 | Off | |
| 7 | −120.0 | 5.5 | Off | |
| 8 | −120.0 | 5 | Off |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusconi, G.; Cusumano, G.; Mariotta, L.; Canevascini, R.; Gola, M.; Gornati, R.; Soldati, G. Upgrading Monocytes Therapy for Critical Limb Ischemia Patient Treatment: Pre-Clinical and GMP-Validation Aspects. Int. J. Mol. Sci. 2022, 23, 12669. https://doi.org/10.3390/ijms232012669
Rusconi G, Cusumano G, Mariotta L, Canevascini R, Gola M, Gornati R, Soldati G. Upgrading Monocytes Therapy for Critical Limb Ischemia Patient Treatment: Pre-Clinical and GMP-Validation Aspects. International Journal of Molecular Sciences. 2022; 23(20):12669. https://doi.org/10.3390/ijms232012669
Chicago/Turabian StyleRusconi, Giulio, Giuseppe Cusumano, Luca Mariotta, Reto Canevascini, Mauro Gola, Rosalba Gornati, and Gianni Soldati. 2022. "Upgrading Monocytes Therapy for Critical Limb Ischemia Patient Treatment: Pre-Clinical and GMP-Validation Aspects" International Journal of Molecular Sciences 23, no. 20: 12669. https://doi.org/10.3390/ijms232012669






