Impact of Oxidative Stress on Molecular Mechanisms of Cervical Ripening in Pregnant Women
Abstract
:1. Introduction
2. Anatomy and Physiology of Cervix
3. Oxidative Stress
4. Oxidative Stress and Apoptosis
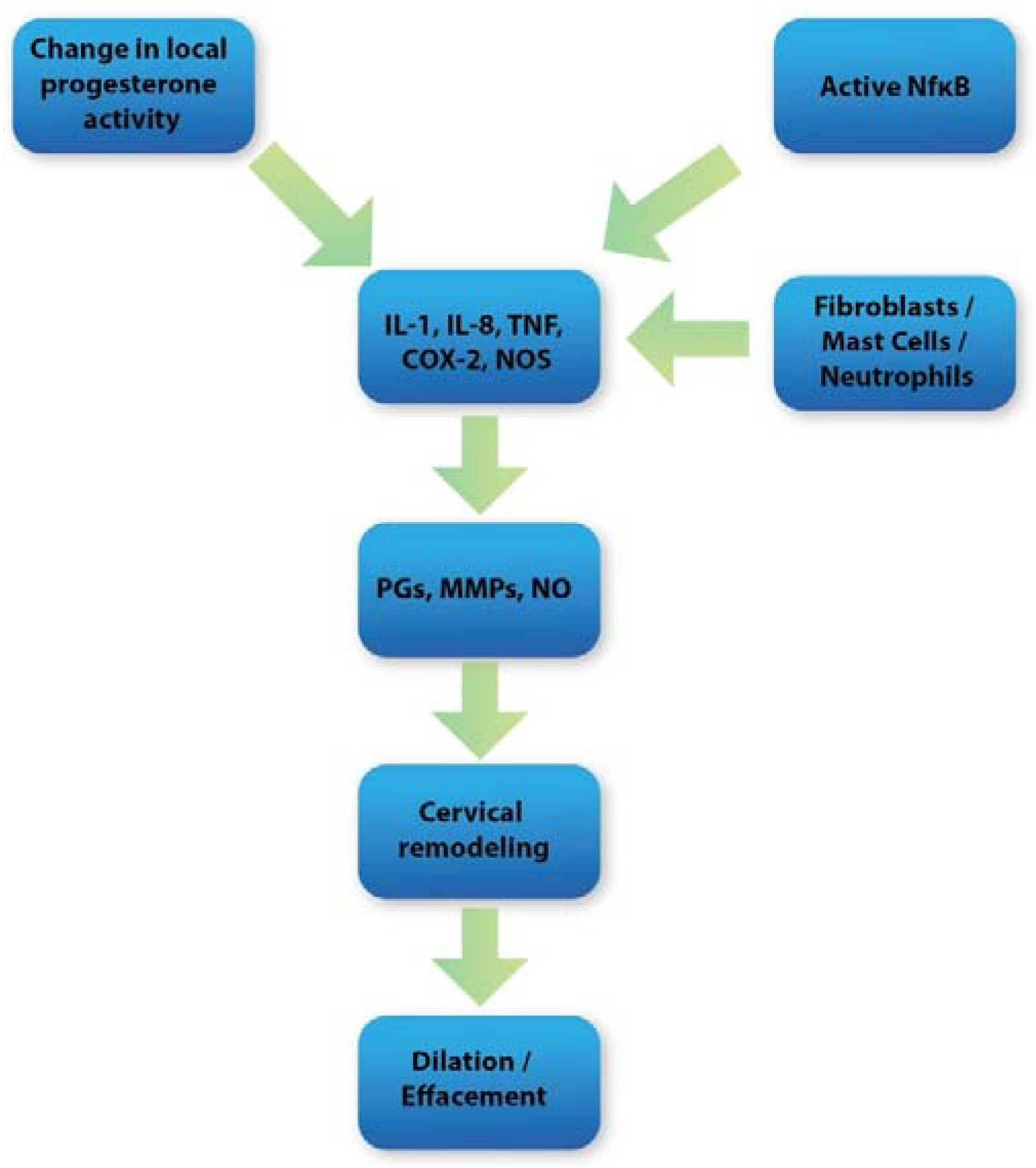
5. Oxidative Stress and Inflammation in Cervical Tissue
6. Reactive Nitrogen Species and Cervical Ripening
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polettini, J.; Richardson, L.S.; Menon, R. Oxidative stress induces senescence and sterile inflammation in murine amniotic cavity. Placenta 2018, 63, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, T.E.; Hayashi, K.; Hu, J.; Carpenter, K.D. Comparative developmental biology of the mammalian uterus. Curr. Top. Dev. Biol. 2005, 68, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Schlembach, D.; Mackay, L.; Shi, L.; Maner, W.L.; Garfield, R.E.; Maul, H. Cervical ripening and insufficiency: From biochemical and molecular studies to in vivo clinical examination. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144 (Suppl. 1). [Google Scholar] [CrossRef]
- Singer, A.; Jordan, J.A. The Functional Anatomy of the Cervix, the Cervical Epithelium and the Stroma. Cervix Second Ed. 2009, 13–37. [Google Scholar] [CrossRef]
- Leppert, P.C. Anatomy and physiology of cervical ripening. Clin. Obstet. Gynecol. 1995, 38, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Jayyosi, C.; Lee, N.; Mahendroo, M.; Myers, K.M. Mechanics of cervical remodelling: Insights from rodent models of pregnancy. Interface Focus 2019, 9, 20190026. [Google Scholar] [CrossRef]
- Leppert, P.C.; Keller, S.; Cerreta, J.; Hosannah, Y.; Mandl, I. The content of elastin in the uterine cervix. Arch. Biochem. Biophys. 1983, 222, 53–58. [Google Scholar] [CrossRef]
- Vink, J.Y.; Qin, S.; Brock, C.O.; Zork, N.M.; Feltovich, H.M.; Chen, X.; Urie, P.; Myers, K.M.; Hall, T.J.; Wapner, R.; et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am. J. Obstet. Gynecol. 2016, 215, 478.e1–478.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoll, A. The Physiology of Cervical Ripening and the Induction of Labour: A Potential Role for the Nitric Oxide Donor Isosorbide Mononitrate; University of Glasgow: Glasgow, UK, 2001. [Google Scholar]
- Akgul, Y.; Holt, R.; Mummert, M.; Word, A.; Mahendroo, M. Dynamic Changes in Cervical Glycosaminoglycan Composition during Normal Pregnancy and Preterm Birth. Endocrinology 2012, 153, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Nallasamy, S.; Yoshida, K.; Akins, M.; Myers, K.; Iozzo, R.; Mahendroo, M. Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagen and Elastic Fibers. Endocrinology 2017, 158, 950–962. [Google Scholar] [CrossRef]
- Uldbjerg, N.; Ekman, G.; Malmström, A.; Olsson, K.; Ulmsten, U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am. J. Obstet. Gynecol. 1983, 147, 662–666. [Google Scholar] [CrossRef]
- Ruscheinsky, M.; De la Motte, C.; Mahendroo, M. Hyaluronan and its binding proteins during cervical ripening and parturition: Dynamic changes in size, distribution and temporal sequence. Matrix Biol. J. Int. Soc. Matrix Biol. 2008, 27, 487–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Maradny, E.M.; Kanayama, N.; Kobayashi, H.; Hossain, B.; Khatun, S.; Liping, S.; Kobayashi, T.; Terao, T. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum. Reprod. Oxf. Engl. 1997, 12, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Osmers, R.; Rath, W.; Adelmann-Grill, B.C.; Fittkow, C.; Kuloczik, M.; Szeverenyi, M.; Tschesche, H.; Kuhn, W. Origin of cervical collagenase during parturition. Am. J. Obstet. Gynecol. 1992, 166, 1455–1460. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaset, H.; Woessner, J.F.; Nagase, H.; Woessner, J.F. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [Green Version]
- Ledingham, M.-A.A.; Denison, F.C.; Riley, S.C.; Norman, J.E. Matrix metalloproteinases-2 and -9 and their inhibitors are produced by the human uterine cervix but their secretion is not regulated by nitric oxide donors. Hum. Reprod. 1999, 14, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Bollopragada, S.; Youssef, R.; Jordan, F.; Greer, I.; Norman, J.; Nelson, S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am. J. Obstet. Gynecol. 2009, 200, 104.e1–104.e11. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Guilbert, L.J.; Olson, D.M. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J. Leukoc. Biol. 2010, 88, 625–633. [Google Scholar] [CrossRef]
- Pavlov, O.; Pavlova, O.; Ailamazyan, E.; Selkov, S. Characterization of cytokine production by human term placenta macrophages in vitro. Am. J. Reprod. Immunol. 2008, 60, 556–567. [Google Scholar] [CrossRef]
- Menzies, F.M.; Shepherd, M.C.; Nibbs, R.J.; Nelson, S.M. The role of mast cells and their mediators in reproduction, pregnancy and labour. Hum. Reprod. Update 2011, 17, 383–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell. Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willets, J.M.; Taylor, A.H.; Shaw, H.; Konje, J.C.; Challiss, R.A.J.J. Selective Regulation of H1 Histamine Receptor Signaling by G Protein-Coupled Receptor Kinase 2 in Uterine Smooth Muscle Cells. Mol. Endocrinol. 2008, 22, 1893–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sennström, M.B.; Ekman, G.; Westergren-Thorsson, G.; Malmström, A.; Byström, B.; Endrésen, U.; Mlambo, N.; Norman, M.; Ståbi, B.; Brauner, A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol. Hum. Reprod. 2000, 6, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.D.; Edwin, S.S.; Lundin-Schiller, S.; Silver, R.M.; Smotkin, D.; Trautman, M.S. Mechanism of interleukin-1 beta stimulation of human amnion prostaglandin biosynthesis: Mediation via a novel inducible cyclooxygenase. Placenta 1993, 14, 615–625. [Google Scholar] [CrossRef]
- Christiaens, I.; Zaragoza, D.B.; Guilbert, L.; Robertson, S.A.; Mitchell, B.F.; Olson, D.M. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 2008, 79, 50–57. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S.; Allen, R.G.; Nations, C. Oxidative stress and cellular differentiation. Ann. N. Y. Acad. Sci. 1988, 551, 59–73. [Google Scholar] [CrossRef]
- Lavu, N.; Richardson, L.; Radnaa, E.; Kechichian, T.; Urrabaz-Garza, R.; Sheller-Miller, S.; Bonney, E.; Menon, R. Oxidative stress-induced downregulation of glycogen synthase kinase 3 beta in fetal membranes promotes cellular senescence†. Biol. Reprod. 2019, 101, 1018–1030. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. RBE 2005, 3, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raijmakers, M.T.M.; Burton, G.J.; Jauniaux, E.; Seed, P.T.; Peters, W.H.M.; Steegers, E.A.P.; Poston, L. Placental NAD(P)H Oxidase Mediated Superoxide Generation in Early Pregnancy. Placenta 2006, 27, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J.A. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Yung, H.W.; Johns, J.; Spasic-Boskovic, O.; Korolchuk, S.; Jauniaux, E.; Burton, G.J.; Charnock-Jones, D.S. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am. J. Pathol. 2007, 171, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.G.; Moncada, S. Nitric oxide synthases in mammals. Biochem. J. 1994, 298 Pt 2, 249–258. [Google Scholar] [CrossRef]
- Murad, F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 2004, 24, 452–474. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 2006, 13, 126–134. [Google Scholar] [CrossRef]
- Webster, R.P.; Roberts, V.H.J.; Myatt, L. Protein nitration in placenta—Functional significance. Placenta 2008, 29, 985–994. [Google Scholar] [CrossRef] [Green Version]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef]
- Orsi, N.M.; Tribe, R.M. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J. Neuroendocrinol. 2008, 20, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2014, 22, 526–539. [Google Scholar] [CrossRef] [Green Version]
- Ricci, J.E.; Muñoz-Pinedo, C.; Fitzgerald, P.; Bailly-Maitre, B.; Perkins, G.A.; Yadava, N.; Scheffler, I.E.; Ellisman, M.H.; Green, D.R. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 2004, 117, 773–786. [Google Scholar] [CrossRef] [Green Version]
- Crawford, E.D.; Wells, J.A. Caspase substrates and cellular remodeling. Annu. Rev. Biochem. 2011, 80, 1055–1087. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Walle, L.V.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010 119 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Landes, T.; Martinou, J.C. Mitochondrial outer membrane permeabilization during apoptosis: The role of mitochondrial fission. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 540–545. [Google Scholar] [CrossRef]
- Youle, R.J.; Bliek, A.M. Van Der Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.G.; Pathan, N.; Ethell, I.M.; Krajewski, S.; Yamaguchi, Y.; Shibasaki, F.; McKeon, F.; Bobo, T.; Franke, T.F.; Reed, J.C. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999, 284, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; Strasser, A.; Jost, P.J. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012, 19, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, Z.; Shukla, Y. Death receptors: Targets for cancer therapy. Exp. Cell Res. 2010, 316, 887–899. [Google Scholar] [CrossRef]
- Holoch, P.A.; Griffith, T.S. TNF-related apoptosis-inducing ligand (TRAIL): A new path to anti-cancer therapies. Eur. J. Pharmacol. 2009, 625, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Leibowitz, B.; Yu, J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol. Ther. 2010, 9, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Leppert, P.C.; Yu, S.Y. Apoptosis in the cervix of pregnant rats in association with cervical softening. Gynecol. Obstet. Investig. 1994, 37, 150–154. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; Vink, J.; Medina, P.M.B.; Menon, R. Oxidative stress promotes cellular damages in the cervix: Implications for normal and pathologic cervical function in human pregnancy†. Biol. Reprod. 2021, 105, 204–216. [Google Scholar] [CrossRef]
- Sahlin, L.; Wang, H.; Stjernholm, Y.; Lundberg, M.; Ekman, G.; Holmgren, A.; Eriksson, H. The expression of glutaredoxin is increased in the human cervix in term pregnancy and immediately post-partum, particularly after prostaglandin-induced delivery. Mol. Hum. Reprod. 2000, 6, 1147–1153. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, S.F.; Hutchinson, J.L.; Rossi, A.G.; Norman, J.E. Anti-inflammatory mediators as physiological and pharmacological regulators of parturition. Expert Rev. Clin. Immunol. 2011, 7, 675–696. [Google Scholar] [CrossRef]
- El Maradny, E.; Kanayama, N.; Halim, A.; Maehara, K.; Sumimoto, K.; Terao, T. The effect of interleukin-1 in rabbit cervical ripening. Eur. J. Obstet. Gynecol. 1995, 60, 75–80. [Google Scholar] [CrossRef]
- Kniss, D.A.; Zimmerman, P.D.; Garver, C.L.; Fertel, R.H.; DA, K.; PD, Z.; CL, G.; RH, F. Interleukin-1 receptor antagonist blocks interleukin-1-induced expression of cyclooxygenase-2 in endometrium. Am. J. Obstet. Gynecol. 1997, 177, 559–567. [Google Scholar] [CrossRef]
- Denison, F.C.; Calder, A.A.; Kelly, R.W. The action of prostaglandin E2 on the human cervix: Stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am. J. Obstet. Gynecol. 1999, 180, 614–620. [Google Scholar] [CrossRef]
- Watari, M.; Watari, H.; DiSanto, M.E.; Chacko, S.; Shi, G.P.; Strauss, J.F., III. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am. J. Pathol. 1999, 154, 1755–1762. [Google Scholar] [CrossRef] [Green Version]
- Osmers, R.G.W.; Blaser, J.; Kuhn, W.; Tschesche, H. Interleukin-8 synthesis and the onset of labor. Obstet. Gynecol. 1995, 86, 223–229. [Google Scholar] [CrossRef]
- Winkler, M.; Fischer, D.-C.C.; Ruck, P.; Marx, T.; Kaiserling, E.; Oberpichler, A.; Tschesche, H.; Rath, W.; Kaiserlîng, E.; Oberpichler, A.; et al. Parturition at term: Parallel increases in interleukin-8 and proteinase concentrations and neutrophil count in the lower uterine segment. Hum. Reprod. 1999, 14, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Khatun, S.; Kanayama, N.; Belayet, H.M.; Yonezawa, M.; Kobayashi, T.; Terao, T. Interleukin-8 potentiates the effect of interleukin-1-induced uterine contractions. Hum. Reprod. 1999, 14, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yan, C.; Gieling, R.G.; Kida, Y.; Garner, W.; Li, W.; Han, Y.-P.P. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated Kinase-1. BMC Immunol. 2009, 10, 15. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cindrova-Davies, T.; Spasic-Boskovic, O.; Jauniaux, E.; Charnock-Jones, D.S.; Burton, G.J. Nuclear Factor-κB, p38, and Stress-Activated Protein Kinase Mitogen-Activated Protein Kinase Signaling Pathways Regulate Proinflammatory Cytokines and Apoptosis in Human Placental Explants in Response to Oxidative Stress: Effects of Antioxidant Vitamins. Am. J. Pathol. 2007, 170, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-T.; Wei, W.; Ma, B.; Xu, Y.; Liu, W.-J.; Wang, Y.; Lv, K.-Y.; Tang, H.-T.; Wei, D.; Xia, Z.-F. Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J. Immunol. 2007, 179, 7808–7819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Richardson, L.; Sheller-Miller, S.; Zhong, N.; Menon, R. Oxidative stress induces p38MAPK-dependent senescence in the feto-maternal interface cells. Placenta 2018, 67, 15–23. [Google Scholar] [CrossRef]
- Svineng, G.; Ravuri, C.; Rikardsen, O.; Huseby, N.E.; Winberg, J.O. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect. Tissue Res. 2008, 49, 197–202. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; Florido, J.; Kajarabille, N.; Prados, S.; De Paco, C.; Ocon, O.; Pulido-Moran, M.; Ochoa, J.J. A New Approach to Oxidative Stress and Inflammatory Signaling during Labour in Healthy Mothers and Neonates. Oxid. Med. Cell. Longev. 2015, 2015, 178536. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory Cytokines Increase Reactive Oxygen Species through Mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp. Eye Res. 2007, 85, 462. [Google Scholar] [CrossRef] [Green Version]
- Stjernholm-Vladic, Y.; Stygar, D.; Mansson, C.; Masironi, B.; Akerberg, S.; Wang, H.; Ekman-Ordeberg, G.; Sahlin, L. Factors involved in the inflammatory events of cervical ripening in humans. Reprod. Biol. Endocrinol. RBE 2004, 2, 74. [Google Scholar] [CrossRef] [Green Version]
- Kalkhoven, E.; Wissink, S.; Van Der Saag, P.T.; Van Der Burg, B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J. Biol. Chem. 1996, 271, 6217–6224. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoonbroodt, S.; Ferreira, V.; Best-Belpomme, M.; Boelaert, J.R.; Legrand-Poels, S.; Korner, M.; Piette, J. Crucial Role of the Amino-Terminal Tyrosine Residue 42 and the Carboxyl-Terminal PEST Domain of IκBα in NF-κB Activation by an Oxidative Stress. J. Immunol. 2000, 164, 4292–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allport, V.C.; Pieber, D.; Slater, D.M.; Newton, R.; White, J.O.; Bennett, P.R. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the “functional progesterone withdrawal”. Mol. Hum. Reprod. 2001, 7, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Madrid, L.V.; Wang, C.-Y.; Guttridge, D.C.; Schottelius, A.J.G.; Baldwin, A.S.; Mayo, M.W. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol. Cell. Biol. 2000, 20, 1626–1638. [Google Scholar] [CrossRef] [Green Version]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, H.J.; Kim, C.K.; Kim, J.Y.; Ha, K.S.; Lee, H.; Chung, H.T.; Kwon, H.J.; Kwon, Y.G.; Kim, Y.M. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: Role of H(2)O(2) in NF-kappaB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef]
- Li, Q.; Engelhardt, J.F. Interleukin-1beta induction of NFkappaB is partially regulated by H2O2-mediated activation of NFkappaB-inducing kinase. J. Biol. Chem. 2006, 281, 1495–1505. [Google Scholar] [CrossRef] [Green Version]
- Tschugguel, W.; Schneeberger, C.; Lass, H.; Stonek, F.; Zaghlula, M.B.; Czerwenka, K.; Schatten, C.; Kaider, A.; Husslein, P.; Huber, J.C. Human Cervical Ripening Is Associated with an Increase in Cervical Inducible Nitric Oxide Synthase Expression. Biol. Reprod. 1999, 60, 1367–1372. [Google Scholar] [CrossRef] [Green Version]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Chwalisz, K.; Shao-Qing, S.; Garfield, R.E.; Beier, H.M. Cervical ripening in guinea-pigs after a local application of nitric oxide. Hum. Reprod. Oxf. Engl. 1997, 12, 2093–2101. [Google Scholar] [CrossRef]
- Tiboni, G.M.; Giampietro, F. Inhibition of nitric oxide synthesis causes preterm delivery in the mouse. Hum. Reprod. 2000, 15, 1838–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, T.; Nakanishi, T.; Kimura, Y.; Hattori, T.; Sasaki, K.; Norimatsu, H.; Takahashi, K.; Takigawa, M. Nitric oxide mediates interleukin-1-induced matrix degradation and basic fibroblast growth factor release in cultured rabbit articular chondrocytes: A possible mechanism of pathological neovascularization in arthritis. Endocrinology 1996, 137, 3729–3737. [Google Scholar] [CrossRef] [Green Version]
- Väisänen-Tommiska, M.R.H.H. Nitric oxide in the human uterine cervix: Endogenous ripening factor. Ann. Med. 2008, 40, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, C.C.; Madara, P.J.; Van Dervort, A.L.; Tropea, M.M.; Wesley, R.A.; Danner, R.L.; Van Dervort, A.L.; Tropea, M.M.; Wesley, R.A.; Danner, R.L. Effects of nitric oxide on chemotaxis and endotoxin-induced interleukin- 8 production in human neutrophils. J. Infect. Dis. 1998, 177, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekerhovd, E.; Weijdegård, B.; Brännström, M.; Mattsby-Baltzer, I.; Norström, A. Nitric oxide induced cervical ripening in the human: Involvement of cyclic guanosine monophosphate, prostaglandin F(2 alpha), and prostaglandin E(2). Am. J. Obstet. Gynecol. 2002, 186, 745–750. [Google Scholar] [CrossRef]
- Ledingham, M.A.; Denison, F.C.; Kelly, R.W.; Young, A.; Norman, J.E. Nitric oxide donors stimulate prostaglandin F(2alpha) and inhibit thromboxane B(2) production in the human cervix during the first trimester of pregnancy. Mol. Hum. Reprod. 1999, 5, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Salvemini, D.; Masferrer, J.L. Interactions of nitric oxide with cyclooxygenase: In vitro, ex vivo, and in vivo studies. Methods Enzymol. 1996, 269, 12–25. [Google Scholar] [CrossRef]
- Väisänen-Tommiska, M.; Mikkola, T.S.; Ylikorkala, O. Misoprostol induces cervical nitric oxide release in pregnant, but not in nonpregnant, women. Am. J. Obstet. Gynecol. 2005, 193, 790–796. [Google Scholar] [CrossRef]
- Kublickiene, K.R.; Cockell, A.P.; Nisell, H.; Poston, L. Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. Am. J. Obstet. Gynecol. 1997, 177, 1263–1269. [Google Scholar] [CrossRef]
- Learmont, J.G.; Poston, L. Nitric oxide is involved in flow-induced dilation of isolated human small fetoplacental arteries. Am. J. Obstet. Gynecol. 1996, 174, 583–588. [Google Scholar] [CrossRef]
- Garfield, R.E.; Saade, G.; Buhimschi, C.; Buhimschi, I.; Shi, L.; Shi, S.Q.; Chwalisz, K. Control and assessment of the uterus and cervix during pregnancy and labour. Hum. Reprod. Update 1998, 4, 673–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sladek, S.M.; Regenstein, A.C.; Lykins, D.; Roberts, J.M. Nitric oxide synthase activity in pregnant rabbit uterus decreases on the last day of pregnancy. Am. J. Obstet. Gynecol. 1993, 169, 1285–1291. [Google Scholar] [CrossRef]
- Chwalisz, K.; Garfield, R.E. Role of nitric oxide in the uterus and cervix: Implications for the management of labor. J. Perinat. Med. 1998, 26, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.M.; Pae, H.O.; Jang, S.I.; Kim, Y.M.; Chung, H.T. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. J. Biochem. Mol. Biol. 2002, 35, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.K.M.; Zamora, R.; Petrosko, P.; Billiar, T.R. The regulatory role of nitric oxide in apoptosis. Int. Immunopharmacol. 2001, 1, 1421–1441. [Google Scholar] [CrossRef]
- Clancy, R.M.; Gomez, P.F.; Abramson, S.B. Nitric oxide sustains nuclear factor kappaB activation in cytokine-stimulated chondrocytes. Osteoarthr. Cartil. 2004, 12, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Lattey, K.R.; Kelly, A.J. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database Syst. Rev. 2016, 12, CD006901. [Google Scholar] [CrossRef]
- Mehmood, M.; Rizwan, M.; Gregus Ml, M.; Abbas, S. Machine Learning Assisted Cervical Cancer Detection. Front. Public Health 2021, 9, 788376. [Google Scholar] [CrossRef]
- Sato, M.; Morimoto, K.; Kajihara, S.; Tateishi, R.; Shiina, S.; Koike, K.; Yatomi, Y. Machine-learning Approach for the Development of a Novel Predictive Model for the Diagnosis of Hepatocellular Carcinoma. Sci. Rep. 2019, 9, 7704. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socha, M.W.; Flis, W.; Wartęga, M.; Stankiewicz, M. Impact of Oxidative Stress on Molecular Mechanisms of Cervical Ripening in Pregnant Women. Int. J. Mol. Sci. 2022, 23, 12780. https://doi.org/10.3390/ijms232112780
Socha MW, Flis W, Wartęga M, Stankiewicz M. Impact of Oxidative Stress on Molecular Mechanisms of Cervical Ripening in Pregnant Women. International Journal of Molecular Sciences. 2022; 23(21):12780. https://doi.org/10.3390/ijms232112780
Chicago/Turabian StyleSocha, Maciej W., Wojciech Flis, Mateusz Wartęga, and Martyna Stankiewicz. 2022. "Impact of Oxidative Stress on Molecular Mechanisms of Cervical Ripening in Pregnant Women" International Journal of Molecular Sciences 23, no. 21: 12780. https://doi.org/10.3390/ijms232112780






