Metallothionein Expression as a Physiological Response against Metal Toxicity in the Striped Rockcod Trematomus hansoni
Abstract
1. Introduction
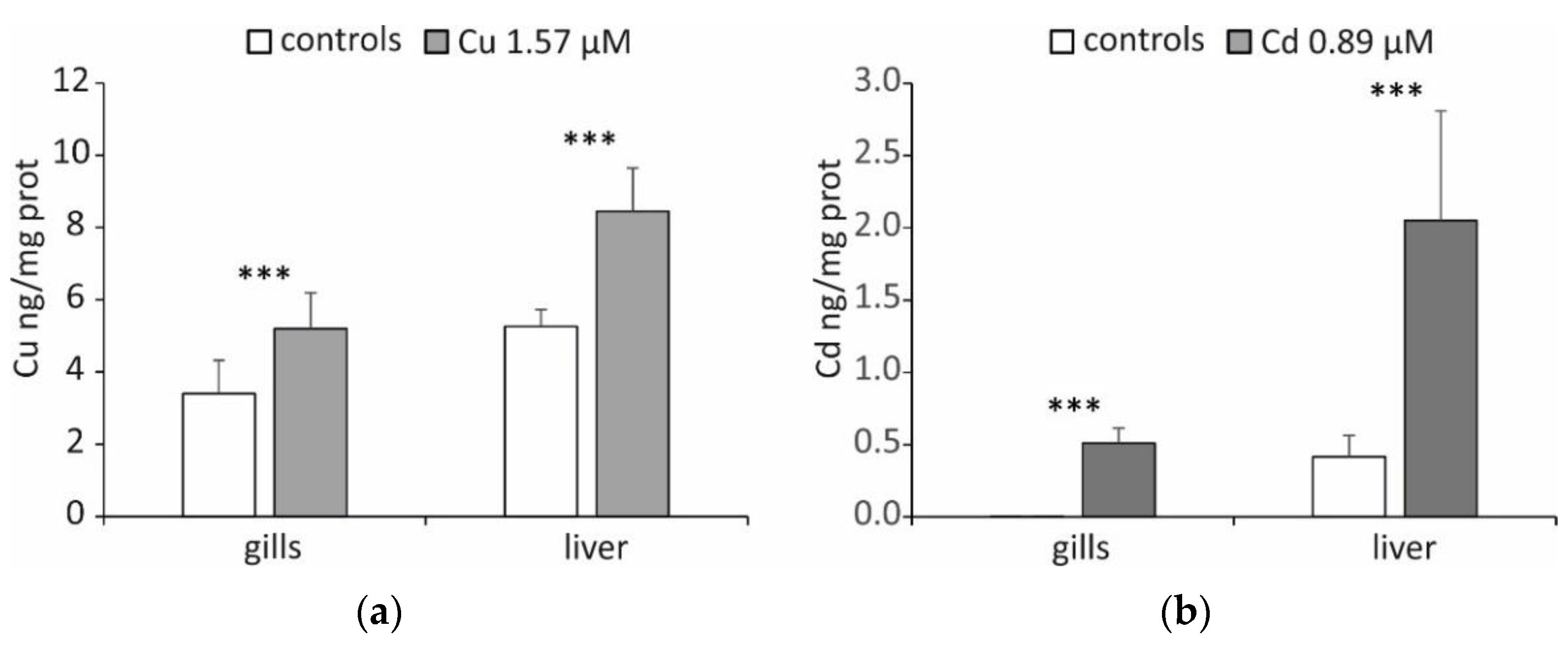
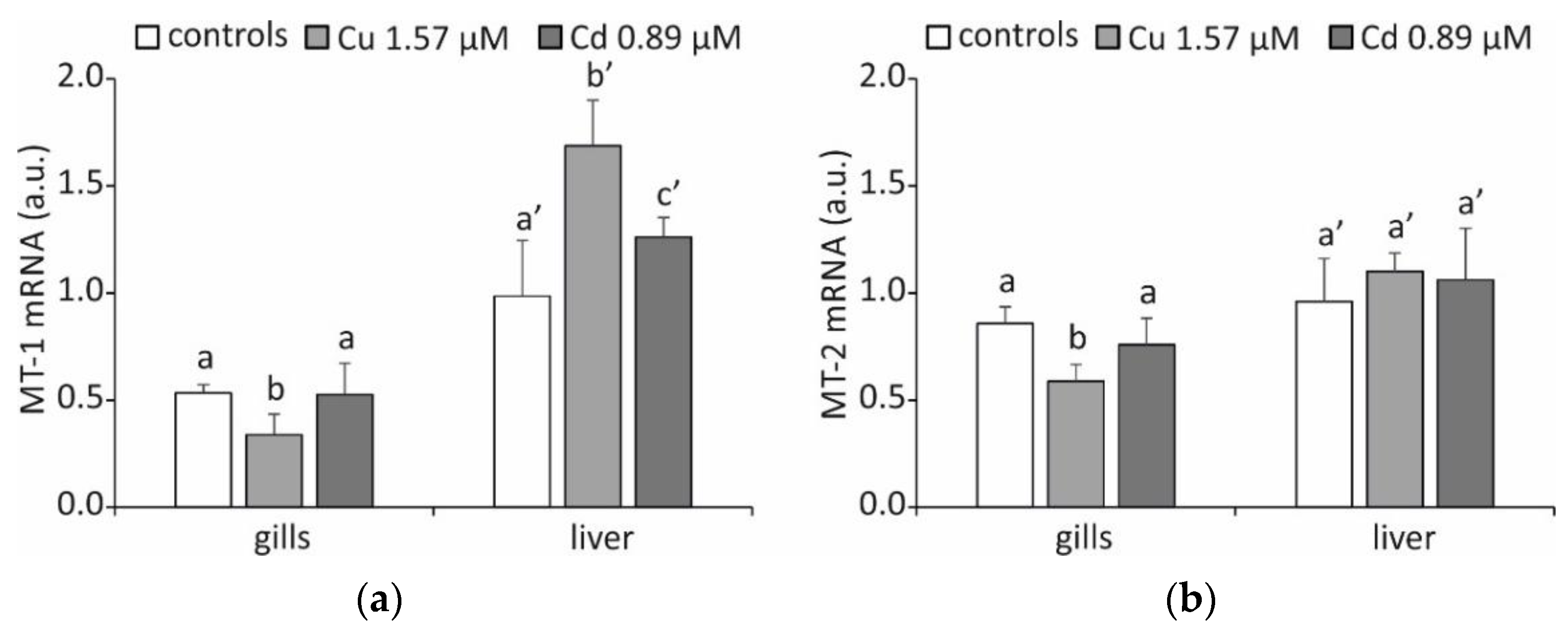
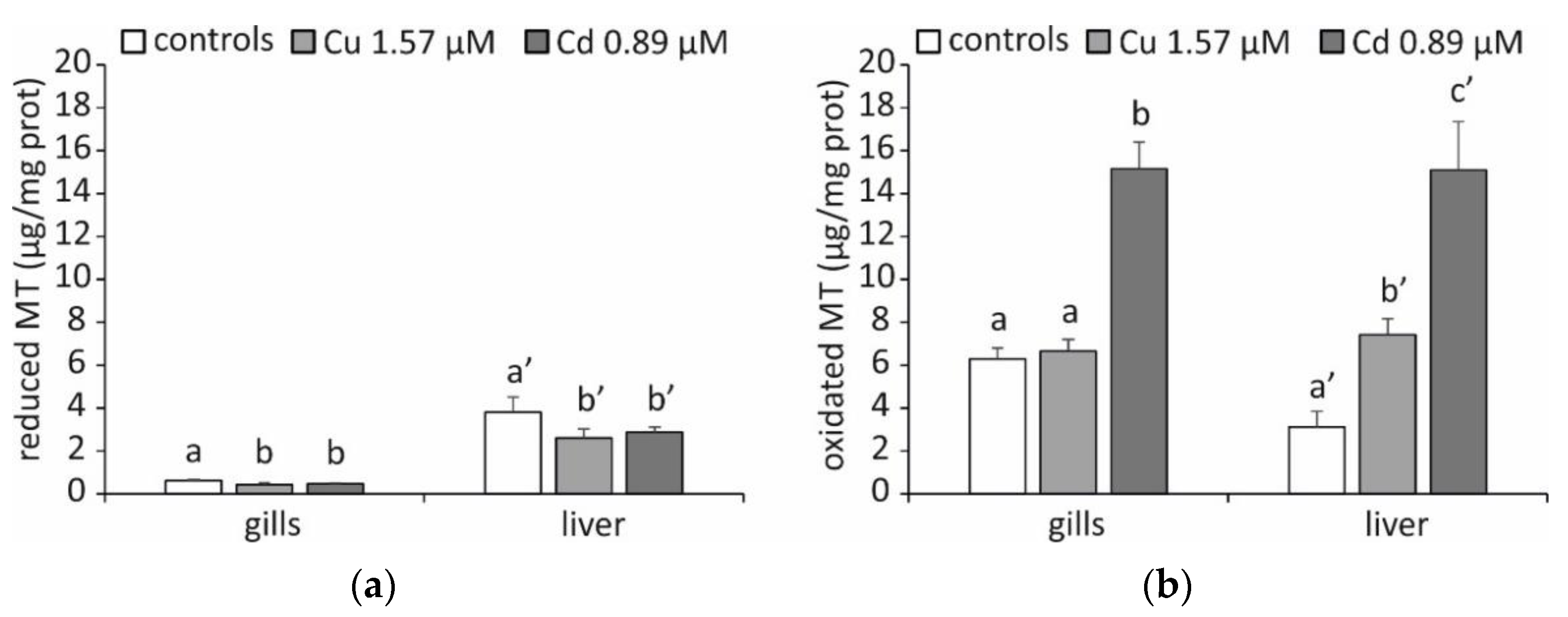
2. Results and Discussion
3. Materials and Methods
3.1. Ethical Procedures
3.2. Experimental Animals
3.3. Primers Design, Total RNA Extraction, mt-1, and mt-2 cDNA Synthesis
3.4. qRT-PCR Analysis
3.5. Estimation of Metal and Metallothionein Concentrations
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marrone, A.; La Russa, D.; Brunelli, E.; Santovito, G.; La Russa, M.F.; Barca, D.; Pellegrino, D. Antarctic Fish as a Global Pollution Sensor: Metals Biomonitoring in a Twelve-Year Period. Front. Mol. Biosci. 2021, 8, 794946. [Google Scholar] [CrossRef] [PubMed]
- Sidell, B.D. Life at body temperatures below 0 degrees C: The physiology and biochemistry of Antarctic fishes. Gravit. Space Biol. Bull. 2000, 13, 25–34. [Google Scholar] [PubMed]
- Peck, L.S. Prospects for surviving climate change in Antarctic aquatic species. Front. Zool. 2005, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Lauro, F.M.; Grzymski, J.J.; Read, R.; Bakiu, R.; Santovito, G.; Luporini, P.; Vallesi, A. The antioxidant defense system of the marine polar ciliate Euplotes nobilii: Characterization of the msrB gene family. Biology 2017, 6, 4. [Google Scholar] [CrossRef]
- Chatzidimitriou, E.; Bisaccia, P.; Corrà, F.; Bonato, M.; Irato, P.; Manuto, L.; Toppo, S.; Bakiu, R.; Santovito, G. Copper/zinc superoxide dismutase from the crocodile icefish Chionodraco hamatus: Antioxidant defense at constant sub-zero temperature. Antioxidants 2020, 9, 325. [Google Scholar] [CrossRef]
- Westerlund, S.; Ohoman, P. Cadmium, copper, cobalt, nickel, lead, and zinc in the water column of the Weddel Sea, Antarctica. Geochem. Cosmochim. Acta 1991, 55, 2127–2146. [Google Scholar] [CrossRef]
- Bargagli, R.; Nelli, L.; Ancora, S.; Focardi, S. Elevated cadmium accumulation in marine organisms from Terra Nova Bay (Antarctica). Polar Biol. 1996, 16, 513–520. [Google Scholar] [CrossRef]
- Wicklund, A.; Runn, P.; Norrgren, L. Cadmium and zinc interactions in fish: Effects of zinc on the uptake, organ distribution, and elimination of 109Cd in the zebrafish, Brachydanio rerio. Arch. Environ. Contam. Toxicol. 1988, 17, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.S. Copper, cadmium and zinc concentrations in oceanic amphipod and euphausiid crustaceans, as a source of heavy metals to pelagic seabirds. Mar. Biol. 1989, 103, 513–518. [Google Scholar] [CrossRef]
- Scheller, J.S.; Irvine, G.W.; Stillman, M.J. Unravelling the mechanistic details of metal binding to mammalian metallothioneins from stoichiometric, kinetic, and binding affinity data. Dalton Trans. 2018, 47, 3613–3637. [Google Scholar] [CrossRef]
- Motta, C.M.; Simoniello, P.; Di Lorenzo, M.; Migliaccio, V.; Panzuto, R.; Califano, E.; Santovito, G. Endocrine disrupting effects of copper and cadmium in the oocytes of the Antarctic Emerald rockcod Trematomus bernacchii. Chemosphere 2021, 268, 129282. [Google Scholar] [CrossRef]
- Grosell, M. Copper. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: London, UK, 2012; pp. 53–133. [Google Scholar]
- Wilhelm Filho, D. Reactive oxygen species, antioxidants and fish mitochondria. Front. Biosci. 2007, 12, 1229–1237. [Google Scholar] [CrossRef]
- Wiens, L.; Banh, S.; Sotiri, E.; Jastroch, M.; Block, B.A.; Brand, M.D.; Treberg, J.R. Comparison of Mitochondrial Reactive Oxygen Species Production of Ectothermic and Endothermic Fish Muscle. Front. Physiol. 2017, 8, 704. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef]
- Shahjahan; Taslima, K.; Rahman, M.S.; Emran, A.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Boldrin, F.; Santovito, G.; Formigari, A.; Bisharyan, Y.; Cassidy-Hanley, D.; Clark, T.G.; Piccinni, E. MTT2, a copper-inducible metallothionein gene from Tetrahymena thermophila. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 232–240. [Google Scholar] [CrossRef]
- Formigari, A.; Boldrin, F.; Santovito, G.; Cassidy-Hanley, D.; Clark, T.G.; Piccinni, E. Functional characterization of the 5′-upstream region of MTT5 metallothionein gene from Tetrahymena thermophila. Protist 2010, 161, 71–77. [Google Scholar] [CrossRef]
- Basu, A.; Lazo, J.S. A hypothesis regarding the protective role of metallothioneins against the toxicity of DNA interactive anticancer drugs. Toxicol. Lett. 1990, 50, 123–135. [Google Scholar] [CrossRef]
- Kägi, J.H.R. Overview of metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar] [CrossRef]
- Mieiro, C.L.; Bervoets, L.; Joosen, S.; Blust, R.; Duarte, A.C.; Pereira, M.E.; Pacheco, M. Metallothioneins failed to reflect mercury external levels of exposure and bioaccumulation in marine fish—Considerations on tissue and species specific responses. Chemosphere 2011, 85, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Fields, L.G.; DeVries, A.L. Variation in blood serum antifreeze activity of Antarctic Trematomus fishes across habitat temperature and depth. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 185, 43–50. [Google Scholar] [CrossRef]
- Benedetti, M.; Nigro, M.; Regoli, F. Characterisation of antioxidant defences in three Antarctic notothenioid species from Terra Nova Bay (Ross Sea). Chem. Ecol. 2010, 26, 305–314. [Google Scholar] [CrossRef]
- Sattin, G.; Santovito, G.; Cassini, A. Physiological antioxidant responses against high environmental oxygen concentration: Glutathione peroxidase from the Antarctic teleost Trematomus eulepidotus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 151, S27. [Google Scholar] [CrossRef]
- Bourdineaud, J.P.; Baudrimont, M.; Gonzalez, P.; Moreau, J.L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Desouky, M.M. Tissue distribution and subcellular localization of trace metals in the pond snail Lymnaea stagnalis with special reference to the role of lysosomal granules in metal sequestration. Aquat. Toxicol. 2006, 77, 143–152. [Google Scholar] [CrossRef]
- Leonard, E.M.; Banerjee, U.; D'Silva, J.J.; Wood, C.M. Chronic nickel bioaccumulation and sub-cellular fractionation in two freshwater teleosts, the round goby and the rainbow trout, exposed simultaneously to waterborne and dietborne nickel. Aquat. Toxicol. 2014, 154, 141–153. [Google Scholar] [CrossRef]
- Dalla Riva, S.; Abelmoschi, M.L.; Magi, E.; Soggia, F. The utilization of the Antarctic environmental specimen bank (BCAA) in monitoring Cd and Hg in an Antarctic coastal area in Terra Nova Bay (Ross Sea—Northern Victoria Land). Chemosphere 2004, 56, 59–69. [Google Scholar] [CrossRef]
- Santovito, G.; Piccinni, E.; Boldrin, F.; Irato, P. Comparative study on metal homeostasis and detoxification in two Antarctic teleosts. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 155, 580–586. [Google Scholar] [CrossRef]
- Benedetti, M.; Martuccio, G.; Fattorini, D.; Canapa, A.; Barucca, M.; Nigro, M.; Regoli, F. Oxidative and modulatory effects of trace metals on metabolism of polycyclic aromatic hydrocarbons in the Antarctic fish Trematomus bernacchii. Aquat. Toxicol. 2007, 85, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Carginale, V.; Scudiero, R.; Capasso, C.; Capasso, A.; Kille, P.; di Prisco, G.; Parisi, E. Cadmium-induced differential accumulation of metallothionein isoforms in the Antarctic icefish, which exhibits no basal metallothionein protein but high endogenous mRNA levels. Biochem. J. 1998, 332, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Carnera, M.; Schumann, S.; Piva, E.; Bakiu, R.; Irato, P.; Santovito, G. Gene expression of metallothioneins in Trematomus eulepidotus as a physiological response to an experimental variation of the environmental concentration of metal ions. submitted.
- Scudiero, R.; Carginale, V.; Capasso, C.; Riggio, M.; Filosa, S.; Parisi, E. Structural and functional analysis of metal regulatory elements in the promoter region of genes encoding metallothionein isoforms in the Antarctic fish Chionodraco hamatus (icefish). Gene 2001, 274, 199–208. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Cherian, M.G. Quantification of metallothionein by silver saturation. Methods Enzymol. 1991, 205, 78–83. [Google Scholar] [CrossRef]
- Santovito, G.; Piccinni, E.; Irato, P. An Improved method for rapid determination of the reduced and oxidized states of metallothioneins in biological samples. In Marine Pollution: New Research; Hofer, T.N., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2008; pp. 101–124. [Google Scholar]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef]
- Franchi, N.; Ferro, D.; Ballarin, L.; Santovito, G. Transcription of genes involved in glutathione biosynthesis in the solitary tunicate Ciona intestinalis exposed to metals. Aquat. Toxicol. 2012, 114–115, 14–22. [Google Scholar] [CrossRef]
- Basaga, H.S. Biochemical aspects of free radicals. Biochem. Cell Biol. 1990, 68, 989–998. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Zangger, K.; Shen, G.; Oz, G.; Otvos, J.D.; Armitage, I.M. Oxidative dimerization in metallothionein is a result of intermolecular disulphide bonds between cysteines in the alpha-domain. Biochem. J. 2001, 359, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Bremner, I. Oxygen free radicals and metallothionein. Free Radic. Biol. Med. 1993, 14, 325–337. [Google Scholar] [CrossRef]
- Santovito, G.; Trentin, E.; Gobbi, I.; Bisaccia, P.; Tallandini, L.; Irato, P. Non-enzymatic antioxidant responses of Mytilus galloprovincialis: Insights into the physiological role against metal-induced oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 240, 108909. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.H.; Tam, S.C.; Beattie, J.H.; Hesketh, J.E. Evidence for differences in the post-transcriptional regulation of rat metallothionein isoforms. Biochem. J. 1996, 315, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Lavut, A.; Raveh, D. Sequestration of highly expressed mRNAs in cytoplasmic granules, P-bodies, and stress granules enhances cell viability. PLoS Genet. 2012, 8, e1002527. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Bakiu, R.; De Pittà, C.; Boldrin, F.; Cattalini, F.; Pucciarelli, S.; Miceli, C.; Santovito, G. Cu,Zn superoxide dismutases from Tetrahymena thermophila: Molecular evolution and gene expression of the first line of antioxidant defenses. Protist 2015, 166, 131–145. [Google Scholar] [CrossRef]
- Sattin, G.; Bakiu, R.; Tolomeo, A.M.; Carraro, A.; Coppola, D.; Ferro, D.; Patarnello, T.; Santovito, G. Characterization and expression of a new cytoplasmic glutathione peroxidase 1 gene in the Antarctic fish Trematomus bernacchii. Hydrobiologia 2015, 761, 363–372. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Carraro, A.; Bakiu, R.; Toppo, S.; Place, S.P.; Ferro, D.; Santovito, G. Peroxiredoxin 6 from the Antarctic emerald rockcod: Molecular characterization of its response to warming. J. Comp. Physiol. B 2016, 186, 59–71. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Carraro, A.; Bakiu, R.; Toppo, S.; Garofalo, F.; Pellegrino, D.; Gerdol, M.; Ferro, D.; Place, S.P.; Santovito, G. Molecular characterization of novel mitochondrial peroxiredoxins from the Antarctic emerald rockcod and their gene expression in response to environmental warming. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 255, 108580. [Google Scholar] [CrossRef]
- Drago, L.; Ferro, D.; Bakiu, R.; Ballarin, L.; Santovito, G. Typical 2-Cys Peroxiredoxins as a Defense Mechanism against Metal-Induced Oxidative Stress in the Solitary Ascidian Ciona robusta. Antioxidants 2022, 11, 93. [Google Scholar] [CrossRef]
- Waris, S.; Wilce, M.C.; Wilce, J.A. RNA recognition and stress granule formation by TIA proteins. Int. J. Mol. Sci. 2014, 15, 23377–23388. [Google Scholar] [CrossRef]
- Nicorelli, E.; Gerdol, M.; Buonocore, F.; Pallavicini, A.; Scapigliati, G.; Guidolin, L.; Irato, P.; Corrà, F.; Santovito, G. First evidence of T cell restricted intracellular antigen (TIA) protein gene expression in Antarctic fish. ISJ Invertebr. Surviv. J. 2018, 15, 127. [Google Scholar]
- Drago, L.; Peronato, A.; Franchi, N.; Ballarin, L.; Bakiu, R.; Santovito, G. Stress granules in Ciona robusta: First evidences of TIA-1-related nucleolysin and tristetraprolin gene expression under metal exposure. Comp. Biochem. Physiol. C 2021, 243, 108977. [Google Scholar] [CrossRef]
- Piva, E.; Schumann, S.; Dotteschini, S.; Brocca, G.; Radaelli, G.; Marion, A.; Irato, P.; Bertotto, D.; Santovito, G. Antioxidant Responses Induced by PFAS Exposure in Freshwater Fish in the Veneto Region. Antioxidants 2022, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Santovito, G.; Marino, S.; Sattin, G.; Cappellini, R.; Bubacco, L.; Beltramini, M. Cloning and characterization of cytoplasmic carbonic anhydrase from gills of four Antarctic fish: Insights into the evolution of fish carbonic anhydrase and cold adaptation. Polar Biol. 2012, 35, 1587–1600. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakiu, R.; Pacchini, S.; Piva, E.; Schumann, S.; Tolomeo, A.M.; Ferro, D.; Irato, P.; Santovito, G. Metallothionein Expression as a Physiological Response against Metal Toxicity in the Striped Rockcod Trematomus hansoni. Int. J. Mol. Sci. 2022, 23, 12799. https://doi.org/10.3390/ijms232112799
Bakiu R, Pacchini S, Piva E, Schumann S, Tolomeo AM, Ferro D, Irato P, Santovito G. Metallothionein Expression as a Physiological Response against Metal Toxicity in the Striped Rockcod Trematomus hansoni. International Journal of Molecular Sciences. 2022; 23(21):12799. https://doi.org/10.3390/ijms232112799
Chicago/Turabian StyleBakiu, Rigers, Sara Pacchini, Elisabetta Piva, Sophia Schumann, Anna Maria Tolomeo, Diana Ferro, Paola Irato, and Gianfranco Santovito. 2022. "Metallothionein Expression as a Physiological Response against Metal Toxicity in the Striped Rockcod Trematomus hansoni" International Journal of Molecular Sciences 23, no. 21: 12799. https://doi.org/10.3390/ijms232112799
APA StyleBakiu, R., Pacchini, S., Piva, E., Schumann, S., Tolomeo, A. M., Ferro, D., Irato, P., & Santovito, G. (2022). Metallothionein Expression as a Physiological Response against Metal Toxicity in the Striped Rockcod Trematomus hansoni. International Journal of Molecular Sciences, 23(21), 12799. https://doi.org/10.3390/ijms232112799








