Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings
Abstract
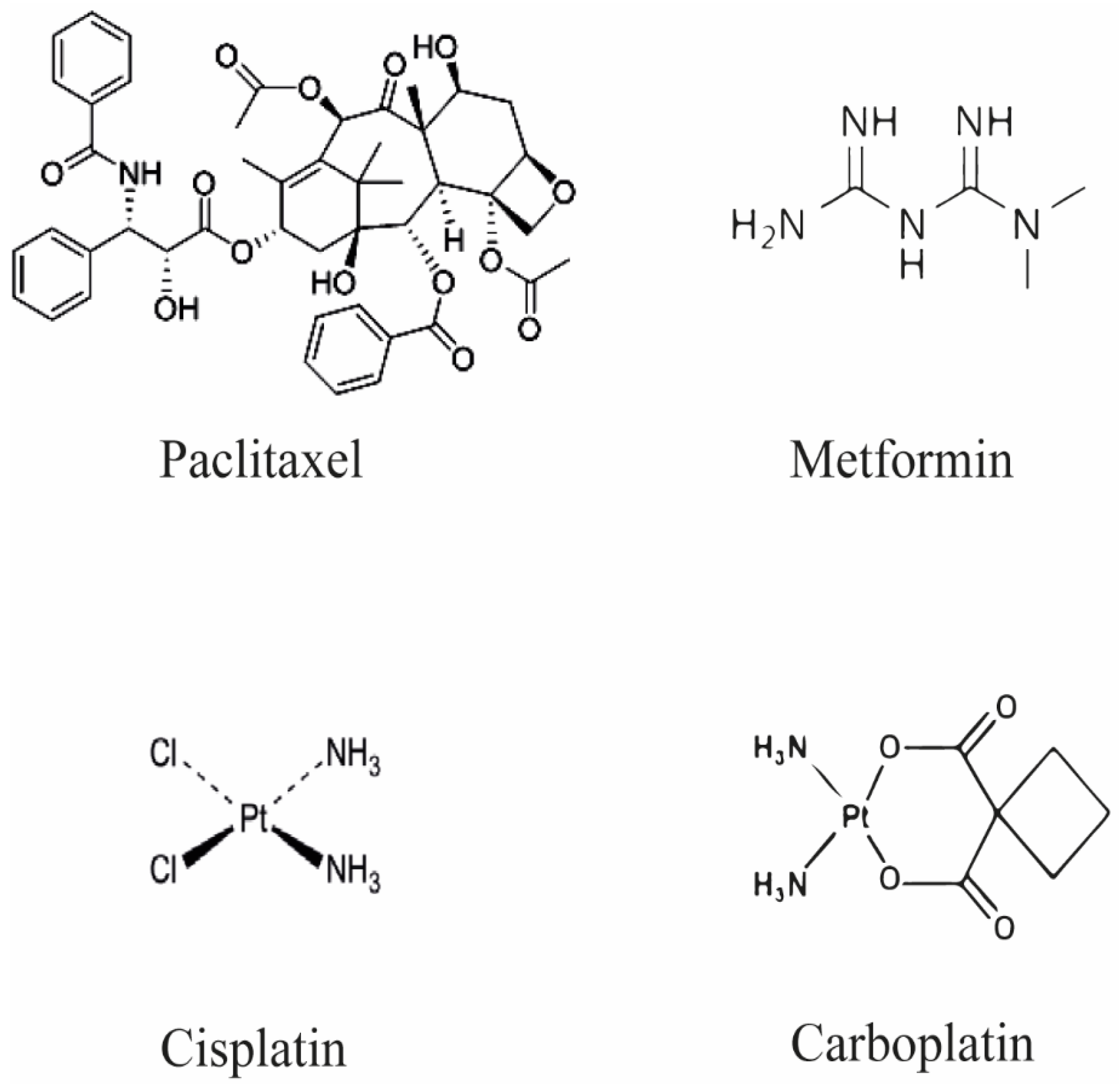
:1. Introduction
2. Metformin as Regulator of Cancer Cell Progression and Resistance
3. Role of Metformin in Regulating the Metabolic Pathway in Resistant Ovarian Cancer Cells
4. Role of Metformin in Cotreatment with SB203580 or Phenethyl Isothiocyanate (PEITC)
5. Conclusions and Further Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortiz, M.; Wabel, E.; Mitchell, K.; Horibata, S. Mechanisms of chemotherapy resistance in ovarian cancer. Cancer Drug Resist. 2022, 5, 304–316. [Google Scholar] [CrossRef]
- Rosen, D.G.; Yang, G.; Liu, G.; Mercado-Uribe, I.; Chang, B.; Xiao, X.S.; Zheng, J.; Xue, F.X.; Liu, J. Ovarian cancer: Pathology, biology, and disease models. Front. Biosci. 2009, 14, 2089–2102. [Google Scholar] [CrossRef] [Green Version]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef]
- Yang, L.; Xie, H.J.; Li, Y.Y.; Wang, X.; Liu, X.X.; Mai, J. Molecular mechanisms of platinumbased chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Samuel, S.M.; Varghese, E.; Koklesova, L.; Liskova, A.; Kubatka, P.; Busselberg, D. Counteracting Chemoresistance with Metformin in Breast Cancers: Targeting Cancer Stem Cells. Cancers 2020, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef]
- Campagna, R.; Bacchetti, T.; Salvolini, E.; Pozzi, V.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Ferretti, G.; Offidani, A.; et al. Paraoxonase-2 Silencing Enhances Sensitivity of A375 Melanoma Cells to Treatment with Cisplatin. Antioxidants 2020, 9, 1238. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 2022, 232, 114205. [Google Scholar] [CrossRef]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, A.; Su, F.; Grijalva, V.; Yalamanchi, M.; Yalamanchi, A.; Gao, F.; Trost, H.; Nwokedi, J.; Farias-Eisner, G.; Farias-Eisner, R.; et al. Paraoxonase 2 overexpression inhibits tumor development in a mouse model of ovarian cancer. Cell Death Dis. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmankaya, I.; Akar, S.; Ugras, S.; Guler, A.H.; Ezveci, H.; Aydogdu, M.; Celik, C. Nicotinamide N-methyltransferase overexpression may be associated with poor prognosis in ovarian cancer. J. Obstet. Gynaecol. 2021, 41, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef]
- Fumarola, S.; Cecati, M.; Sartini, D.; Ferretti, G.; Milanese, G.; Galosi, A.B.; Pozzi, V.; Campagna, R.; Morresi, C.; Emanuelli, M.; et al. Bladder Cancer Chemosensitivity is Affected by Paraoxonase-2 Expression. Antioxidants 2020, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Campagna, R.; Salvolini, E.; Pompei, V.; Pozzi, V.; Salvucci, A.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Offidani, A.; et al. Nicotinamide N-methyltransferase gene silencing enhances chemosensitivity of melanoma cell lines. Pigment Cell Melanoma Res. 2021, 34, 1039–1048. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- van Haren, M.J.; Zhang, Y.; Thijssen, V.; Buijs, N.; Gao, Y.; Mateuszuk, L.; Fedak, F.A.; Kij, A.; Campagna, R.; Sartini, D.; et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT). RSC Chem. Biol. 2021, 2, 1546–1555. [Google Scholar] [CrossRef]
- Gonzalez-Ballesteros, M.M.; Mejia, C.; Ruiz-Azuara, L. Metallodrugs: An approach against invasion and metastasis in cancer treatment. FEBS Open Bio. 2022, 12, 880–899. [Google Scholar] [CrossRef]
- Orr, G.A.; Verdier-Pinard, P.; McDaid, H.; Horwitz, S.B. Mechanisms of Taxol resistance related to microtubules. Oncogene 2003, 22, 7280–7295. [Google Scholar] [CrossRef]
- Kops, G.J.; Weaver, B.A.; Cleveland, D.W. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 2005, 5, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.; Kolluri, S.K.; Lin, F.; Liu, W.; Han, Y.H.; Cao, X.; Dawson, M.I.; Reed, J.C.; Zhang, X.K. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 2004, 116, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.K.; Sasaki, C.Y.; Hardwick, J.M.; Longo, D.L. Bcl-2-mediated drug resistance: Inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J. Exp. Med. 1999, 190, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Kumar, L.; Singh, N. Metformin and epithelial ovarian cancer therapeutics. Cell. Oncol. 2015, 38, 365–375. [Google Scholar] [CrossRef]
- Lou, E.; Fujisawa, S.; Morozov, A.; Barlas, A.; Romin, Y.; Dogan, Y.; Gholami, S.; Moreira, A.L.; Manova-Todorova, K.; Moore, M.A. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 2012, 7, e33093. [Google Scholar] [CrossRef] [Green Version]
- Pasquier, J.; Guerrouahen, B.S.; Al Thawadi, H.; Ghiabi, P.; Maleki, M.; Abu-Kaoud, N.; Jacob, A.; Mirshahi, M.; Galas, L.; Rafii, S.; et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 2013, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Thayanithy, V.; Dickson, E.L.; Steer, C.; Subramanian, S.; Lou, E. Tumor-stromal cross talk: Direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl. Res. 2014, 164, 359–365. [Google Scholar] [CrossRef]
- Desir, S.; Dickson, E.L.; Vogel, R.I.; Thayanithy, V.; Wong, P.; Teoh, D.; Geller, M.A.; Steer, C.J.; Subramanian, S.; Lou, E. Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget 2016, 7, 43150–43161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Zhao, N.; Li, D.; Zou, G.; Chen, Y. Metformin improves the sensitivity of ovarian cancer cells to chemotherapeutic agents. Oncol. Lett. 2019, 18, 2404–2411. [Google Scholar] [CrossRef] [Green Version]
- Aprile, M.; Costa, V.; Cimmino, A.; Calin, G.A. Emerging role of oncogenic long non-coding RNA as cancer biomarkers. Int. J. Cancer 2022. [Google Scholar] [CrossRef]
- Irfan, M.; Javed, Z.; Khan, K.; Khan, N.; Docea, A.O.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Apoptosis evasion via long non-coding RNAs in colorectal cancer. Cancer Cell Int. 2022, 22, 280. [Google Scholar] [CrossRef]
- Yang, J.; Qu, T.; Li, Y.; Ma, J.; Yu, H. Biological role of long non-coding RNA FTX in cancer progression. Biomed. Pharmacother. 2022, 153, 113446. [Google Scholar] [CrossRef]
- Hashemi, M.; Moosavi, M.S.; Abed, H.M.; Dehghani, M.; Aalipour, M.; Heydari, E.A.; Behroozaghdam, M.; Entezari, M.; Salimimoghadam, S.; Gunduz, E.S.; et al. Long non-coding RNA (lncRNA) H19 in human cancer: From proliferation and metastasis to therapy. Pharmacol. Res. 2022, 184, 106418. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Liang, C.; Su, X.; Ke, Y.; Mao, Y.; Fang, J.; Duan, S. Novel Insights into miR-944 in Cancer. Cancers 2022, 14, 4232. [Google Scholar] [CrossRef] [PubMed]
- Licini, C.; Avellini, C.; Picchiassi, E.; Mensa, E.; Fantone, S.; Ramini, D.; Tersigni, C.; Tossetta, G.; Castellucci, C.; Tarquini, F.; et al. Pre-eclampsia predictive ability of maternal miR-125b: A clinical and experimental study. Transl. Res. 2021, 228, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Avellini, C.; Licini, C.; Lazzarini, R.; Gesuita, R.; Guerra, E.; Tossetta, G.; Castellucci, C.; Giannubilo, S.R.; Procopio, A.; Alberti, S.; et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 2017, 8, 58642–58653. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, C.; Zhang, P.; Xie, H.; Hou, L.; Hui, Z.; Xu, Y.; Du, Q.; Zhou, X.; Su, B.; et al. Reduced miR-3127-5p expression promotes NSCLC proliferation/invasion and contributes to dasatinib sensitivity via the c-Abl/Ras/ERK pathway. Sci. Rep. 2014, 4, 6527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Wang, Y.; Wang, B.; Zhai, J. Metformin Affects Paclitaxel Sensitivity of Ovarian Cancer Cells Through Autophagy Mediated by Long Noncoding RNASNHG7/miR-3127-5p Axis. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef]
- Pozzi, V.; Salvolini, E.; Lucarini, G.; Salvucci, A.; Campagna, R.; Rubini, C.; Sartini, D.; Emanuelli, M. Cancer stem cell enrichment is associated with enhancement of nicotinamide N-methyltransferase expression. IUBMB Life 2020, 72, 1415–1425. [Google Scholar] [CrossRef]
- Zanoni, M.; Bravaccini, S.; Fabbri, F.; Arienti, C. Emerging Roles of Aldehyde Dehydrogenase Isoforms in Anti-cancer Therapy Resistance. Front. Med. 2022, 9, 795762. [Google Scholar] [CrossRef]
- Li, X.W.; Gao, H.Y.; Liu, J. The role of taurine in improving neural stem cells proliferation and differentiation. Nutr. Neurosci. 2017, 20, 409–415. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Xu, L.; Wu, T.; Cui, L.; Xu, D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids 2014, 46, 1673–1680. [Google Scholar] [CrossRef]
- Bishnu, A.; Sakpal, A.; Ghosh, N.; Choudhury, P.; Chaudhury, K.; Ray, P. Long term treatment of metformin impedes development of chemoresistance by regulating cancer stem cell differentiation through taurine generation in ovarian cancer cells. Int. J. Biochem. Cell Biol. 2019, 107, 116–127. [Google Scholar] [CrossRef]
- Graham, D.K.; DeRyckere, D.; Davies, K.D.; Earp, H.S. The TAM family: Phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 2014, 14, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.Y.; Lee, H.Y.; Lee, C. Metformin targets Axl and Tyro3 receptor tyrosine kinases to inhibit cell proliferation and overcome chemoresistance in ovarian cancer cells. Int. J. Oncol. 2015, 47, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Peng, Z.; Shi, M.; Ji, M.; Guo, H.; Shi, H. Metformin combined with p38 MAPK inhibitor improves cisplatin sensitivity in cisplatinresistant ovarian cancer. Mol. Med. Rep. 2014, 10, 2346–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.K.; Miskimins, W.K. Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures. J. Ovarian Res. 2012, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Ravindran, J.; Aggarwal, B.B. NF-κB and cancer: How intimate is this relationship. Mol. Cell. Biochem. 2010, 336, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Momeny, M.; Yousefi, H.; Eyvani, H.; Moghaddaskho, F.; Salehi, A.; Esmaeili, F.; Alishahi, Z.; Barghi, F.; Vaezijoze, S.; Shamsaiegahkani, S.; et al. Blockade of nuclear factor-κB (NF-κB) pathway inhibits growth and induces apoptosis in chemoresistant ovarian carcinoma cells. Int. J. Biochem. Cell Biol. 2018, 99, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.K.; Jia, Y.J.; Liu, S.H.; Ma, J. FSTL1 increases cisplatin sensitivity in epithelial ovarian cancer cells by inhibition of NF-κB pathway. Cancer Chemother. Pharmacol. 2021, 87, 405–414. [Google Scholar] [CrossRef]
- Dos Santos Guimaraes, I.; Ladislau-Magescky, T.; Tessarollo, N.G.; Dos Santos, D.Z.; Gimba, E.R.P.; Sternberg, C.; Silva, I.V.; Rangel, L.B.A. Chemosensitizing effects of metformin on cisplatin- and paclitaxel-resistant ovarian cancer cell lines. Pharmacol. Rep. 2018, 70, 409–417. [Google Scholar] [CrossRef]
- Deng, D.; Patel, R.; Chiang, C.Y.; Hou, P. Role of the Tumor Microenvironment in Regulating Pancreatic Cancer Therapy Resistance. Cells 2022, 11, 2952. [Google Scholar] [CrossRef]
- Xu, S.; Yang, Z.; Jin, P.; Yang, X.; Li, X.; Wei, X.; Wang, Y.; Long, S.; Zhang, T.; Chen, G.; et al. Metformin Suppresses Tumor Progression by Inactivating Stromal Fibroblasts in Ovarian Cancer. Mol. Cancer Ther. 2018, 17, 1291–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Na, H.K.; Surh, Y.J. Upregulation of VEGF by 15-deoxy-Delta12,14-prostaglandin J2 via heme oxygenase-1 and ERK1/2 signaling in MCF-7 cells. Ann. N. Y. Acad. Sci. 2006, 1090, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, C.; Peng, S.; Xu, H.; Wright, E.; Zhang, X.; Huo, X.; Cheng, E.; Pham, T.H.; Asanuma, K.; et al. Autocrine VEGF signaling promotes proliferation of neoplastic Barrett’s epithelial cells through a PLC-dependent pathway. Gastroenterology 2014, 146, 461–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Zhang, Y.; Peng, H.; Ke, Z.; Xu, L.; Su, T.; Tsung, A.; Tohme, S.; Huang, H.; Zhang, Q.; et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett. 2016, 373, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Chen, Q.; Rao, W.; Zhang, R.; Wang, Y.; Ge, H.; Wei, Q. OVA66 promotes tumour angiogenesis and progression through enhancing autocrine VEGF-VEGFR2 signalling. EBioMedicine 2019, 41, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Dang, J.H.; Jin, Z.J.; Liu, X.J.; Hu, D.; Wang, J.; Luo, Y.; Li, L.L. Metformin in combination with cisplatin inhibits cell viability and induces apoptosis of human ovarian cancer cells by inactivating ERK 1/2. Oncol. Lett. 2017, 14, 7557–7564. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.T.; Hankins, G.R.; Washington, M.K.; Orton, T.C.; Jirtle, R.L. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nat. Genet. 1995, 11, 447–449. [Google Scholar] [CrossRef]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Pollak, M. Targeting insulin and insulin-like growth factor signalling in oncology. Curr. Opin. Pharmacol. 2008, 8, 384–392. [Google Scholar] [CrossRef]
- Jakubikova, J.; Sedlak, J.; Mithen, R.; Bao, Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem. Pharmacol. 2005, 69, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Wada, M.; Kohno, K.; Nakamura, T.; Kawabe, T.; Kawakami, M.; Kagotani, K.; Okumura, K.; Akiyama, S.; Kuwano, M. A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 1996, 56, 4124–4129. [Google Scholar] [PubMed]
- Ma, J.J.; Chen, B.L.; Xin, X.Y. Inhibition of multi-drug resistance of ovarian carcinoma by small interfering RNA targeting to MRP2 gene. Arch. Gynecol. Obstet. 2009, 279, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shi, H.R.; Ren, F.; Wang, J.L.; Wu, Q.H.; Li, X.; Zhang, R.T. Inhibition of the IGF signaling pathway reverses cisplatin resistance in ovarian cancer cells. BMC Cancer 2017, 17, 851. [Google Scholar] [CrossRef]
- Ricci, F.; Brunelli, L.; Affatato, R.; Chila, R.; Verza, M.; Indraccolo, S.; Falcetta, F.; Fratelli, M.; Fruscio, R.; Pastorelli, R.; et al. Overcoming platinum-acquired resistance in ovarian cancer patient-derived xenografts. Ther. Adv. Med. Oncol. 2019, 11, 1758835919839543. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Rasola, A.; Neckers, L.; Picard, D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells. Trends Cell Biol. 2014, 24, 455–463. [Google Scholar] [CrossRef]
- Matassa, D.S.; Amoroso, M.R.; Lu, H.; Avolio, R.; Arzeni, D.; Procaccini, C.; Faicchia, D.; Maddalena, F.; Simeon, V.; Agliarulo, I.; et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016, 23, 1542–1554. [Google Scholar] [CrossRef] [Green Version]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L.; et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013, 24, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006, 25, 4777–4786. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.A.; Kim, H.; Kim, M.K.; Kim, H.S.; Chung, H.H.; Kim, Y.B.; Song, Y.S. Association of overexpression of hexokinase II with chemoresistance in epithelial ovarian cancer. Clin. Exp. Med. 2014, 14, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Alessi, D.R. The PI3K-PDK1 connection: More than just a road to PKB. Biochem. J. 2000, 346 Pt 3, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Moll, U.M.; Wolff, S.; Speidel, D.; Deppert, W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 2005, 17, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Salani, B.; Marini, C.; Rio, A.D.; Ravera, S.; Massollo, M.; Orengo, A.M.; Amaro, A.; Passalacqua, M.; Maffioli, S.; Pfeffer, U.; et al. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci. Rep. 2013, 3, 2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.Y.; Patten, D.A.; Lee, S.G.; Parks, R.J.; Chan, D.W.; Harper, M.E.; Tsang, B.K. p53 Promotes chemoresponsiveness by regulating hexokinase II gene transcription and metabolic reprogramming in epithelial ovarian cancer. Mol. Carcinog. 2019, 58, 2161–2174. [Google Scholar] [CrossRef]
- Chan, D.A.; Sutphin, P.D.; Nguyen, P.; Turcotte, S.; Lai, E.W.; Banh, A.; Reynolds, G.E.; Chi, J.T.; Wu, J.; Solow-Cordero, D.E.; et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci. Transl. Med. 2011, 3, 94ra70. [Google Scholar] [CrossRef] [Green Version]
- Fiume, L.; Manerba, M.; Vettraino, M.; Di Stefano, G. Impairment of aerobic glycolysis by inhibitors of lactic dehydrogenase hinders the growth of human hepatocellular carcinoma cell lines. Pharmacology 2010, 86, 157–162. [Google Scholar] [CrossRef]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Queckborner, S.; Turnbull, A.; Michie, C.O.; Williams, A.R.W.; Rye, T.; Gourley, C.; Langdon, S.P. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment. BMC Cancer 2018, 18, 636. [Google Scholar] [CrossRef] [Green Version]
- Xing, D.; Orsulic, S. Modeling resistance to pathway-targeted therapy in ovarian cancer. Cell Cycle 2005, 4, 1004–1006. [Google Scholar] [CrossRef]
- Anjum, J.; Mitra, S.; Das, R.; Alam, R.; Mojumder, A.; Emran, T.B.; Islam, F.; Rauf, A.; Hossain, M.J.; Aljohani, A.S.M.; et al. A renewed concept on the MAPK signaling pathway in cancers: Polyphenols as a choice of therapeutics. Pharmacol. Res. 2022, 184, 106398. [Google Scholar] [CrossRef]
- Kumar, S.; Principe, D.R.; Singh, S.K.; Viswakarma, N.; Sondarva, G.; Rana, B.; Rana, A. Mitogen-Activated Protein Kinase Inhibitors and T-Cell-Dependent Immunotherapy in Cancer. Pharmaceuticals 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tossetta, G.; Fantone, S.; Licini, C.; Marzioni, D.; Mattioli-Belmonte, M. The multifaced role of HtrA1 in the development of joint and skeletal disorders. Bone 2022, 157, 116350. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marzioni, D. The Multifaced Actions of Curcumin in Pregnancy Outcome. Antioxidants 2021, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Perugini, J.; Di Mercurio, E.; Tossetta, G.; Severi, I.; Monaco, F.; Reguzzoni, M.; Tomasetti, M.; Dani, C.; Cinti, S.; Giordano, A. Biological Effects of Ciliary Neurotrophic Factor on hMADS Adipocytes. Front. Endocrinol. 2019, 10, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef]
- Gavrilas, L.I.; Cruceriu, D.; Mocan, A.; Loghin, F.; Miere, D.; Balacescu, O. Plant-Derived Bioactive Compounds in Colorectal Cancer: Insights from Combined Regimens with Conventional Chemotherapy to Overcome Drug-Resistance. Biomedicines 2022, 10, 1948. [Google Scholar] [CrossRef]
- Ioannides, C.; Konsue, N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab. Rev. 2015, 47, 356–373. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta 2014, 1846, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Chen, C.; Zhou, J.; Chen, X.; Cai, K.; Shen, M.; Chen, X.; Jiang, L.; Wang, G. Inhibition of Autophagy Promotes the Anti-Tumor Effect of Metformin in Oral Squamous Cell Carcinoma. Cancers 2022, 14, 4185. [Google Scholar] [CrossRef]
- Mei, J.; Tian, H.; Huang, H.S.; Wu, N.; Liou, Y.L.; Chu, T.Y.; Wang, J.; Zhang, W. CCNE1 is a potential target of Metformin for tumor suppression of ovarian high-grade serous carcinoma. Cell Cycle 2022, 1–15. [Google Scholar] [CrossRef]
- Ni, W.; Wu, J.; Feng, Y.; Hu, Y.; Liu, H.; Chen, J.; Chen, F.; Tian, H. Metformin reprograms tumor microenvironment and boosts chemoimmunotherapy in colorectal cancer. Biomater. Sci. 2022, 10, 5596–5607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, J.; Zhang, Y.; Xun, P.; Chen, Z. Metformin and thyroid carcinoma incidence and prognosis: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0271038. [Google Scholar] [CrossRef] [PubMed]
- Siddharth, S.; Kuppusamy, P.; Wu, Q.; Nagalingam, A.; Saxena, N.K.; Sharma, D. Metformin Enhances the Anti-Cancer Efficacy of Sorafenib via Suppressing MAPK/ERK/Stat3 Axis in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 8083. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer Res. Treat. 2014, 145, 785–790. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin reduces ovarian cancer risk in Taiwanese women with type 2 diabetes mellitus. Diabetes/Metab. Res. Rev. 2015, 31, 619–626. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol. Oncol. 2015, 138, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin use is associated with a lower risk of uterine leiomyoma in female type 2 diabetes patients. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819895159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial respiratory complex I: Structure, function and implication in human diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef]

| Model | Target | Results | Ref. |
|---|---|---|---|
| A2780, C200, SKOV3, and IOSE cell lines | Tunneling nanotubes (TNTs) | Metformin decreased TNT formation, suppressing mTOR signaling by AMPK activation. | [31] |
| SKOV3/CDDP and SKOV3 cells | Autophagy | Metformin increased LC3 expression, inducing autophagy. Combining metformin with DDP or MTX decreased the IC50 of CDDP and MTX in the drug-resistant cancer cell lines SKOV3/CDDP. | [35] |
| Paclitaxel-resistant A2780/PR and SKOV3/PR cells | SNHG7/miR-3127-5p axis | Metformin inhibited cell viability, migration, invasion, and autophagy and promoted apoptosis by reducing SNHG7 expression. Metformin treatment reversed SNHG7-mediated paclitaxel sensitivity and autophagy by increasing miR-3127-5p expression. Metformin decreased tumor growth and autophagy in xenografts of A2780/PR by SNHG7 overexpression. | [45] |
| A2780 and OAW42 cells resistant and sensitive to cisplatin and paclitaxel | Cancer stem cells (CSCs) | Metformin cotreatment significantly reduced cell proliferation and migration and increased chemosensitivity by reducing the CSC population by increasing taurine levels. | [50] |
| A2780, SKOV3, cisplatin resistant A2780/CDDP and taxol-resistant SKOV3/TR cells | AXL and TYRO3 | Metformin decreased sensitive and cisplatin/taxol-resistant ovarian cancer cell viability. Metformin decreased both AXL and TYRO3 mRNA and protein levels. Metformin treatment reduced ERK and STAT3 phosphorylation in both sensitive and resistant cell lines. | [53] |
| Paclitaxel-sensitive (A2780) and -resistant (A2780/PR) Cisplatin-sensitive (A2780) and -resistant (A2780/CDDP) cells | NF-κB | The combination of metformin with cisplatin or paclitaxel improved the efficiency of treatment, reducing the cell proliferation rate in both sensitive and resistant cells. Metformin reduced the NF-κB signaling pathway and cytokine production. | [59] |
| Ovarian cancer samples, MRC5 and SKOV3 cells | NF-κB | Tumor stroma of samples from patients with routine metformin administration exhibited lower IL-6 expression. Metformin cotreatment reduced IL-6 secretion in cisplatin-stimulated MRC5 cells, reducing tumor growth in 3D cocultures with SKOV3 and in murine xenograft models. Metformin inhibited NFκB signaling. | [61] |
| HO-8910 cells | ERK1/2 activation | Metformin combined with cisplatin inhibited cell viability and induced apoptosis. Metformin reduced pERK1/2, VEGF, VEGFR2, and Bcl-2 expression when used as a cotreatment with cisplatin, whereas the expression of Bax and caspase-3 was upregulated. | [67] |
| Cisplatin-resistant CP70 cells | IGF/IGF1R/AKT/MRP2 axis | Metformin reduced the IC50 value of cisplatin in a concentration-dependent manner. Metformin increased apoptosis and the cell number in the G0/G1 phase of the cell cycle. Metformin reduced MRP2, IGF1, IGF1R, pIGF1, pIGF1R, AKT, and pAkt expression. | [74] |
| Model | Target | Results | Ref. |
|---|---|---|---|
| Ovarian cancer patient-derived xenografts (PDXs) resistant and sensitive to cisplatin | Mitochondrial activity? | Metformin reversed platinum resistance in cisplatin-resistant PDXs. | [75] |
| Platinum-sensitive (PEA1) and platinum-resistant (PEA2) cells | Mitochondrial activity | Cisplatin resistance was reversible upon adding metformin to the cisplatin treatment, increasing cell death in TRAP1-silenced cells. | [78] |
| Hey (p53-wt) and OV-90 (p53-mutant) cell lines | HKII and pPDK1 | Metformin enhanced the apoptotic rate in cisplatin-resistant Hey cells and decreased HKII and pPDK1 expression in Hey cells but not in p53-mutant OV-90 cells. Metformin decreased the glucose consumption level in Hey cells treated with CDDP, contributing to increased apoptosis. | [85] |
| Platinum-sensitive (PEA1) and platinum-resistant (PEA2) cells | Mitochondrial activity? | Metformin synergically increased the effects of STF31 and oxamic acid when used as a cotreatment, resulting in a significant increase in cell death in both chemosensitive and chemoresistant ovarian cancer cell lines. | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tossetta, G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. Int. J. Mol. Sci. 2022, 23, 12893. https://doi.org/10.3390/ijms232112893
Tossetta G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. International Journal of Molecular Sciences. 2022; 23(21):12893. https://doi.org/10.3390/ijms232112893
Chicago/Turabian StyleTossetta, Giovanni. 2022. "Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings" International Journal of Molecular Sciences 23, no. 21: 12893. https://doi.org/10.3390/ijms232112893





