Decellularized Extracellular Matrix-Based Bioinks for Tendon Regeneration in Three-Dimensional Bioprinting
Abstract
1. Introduction
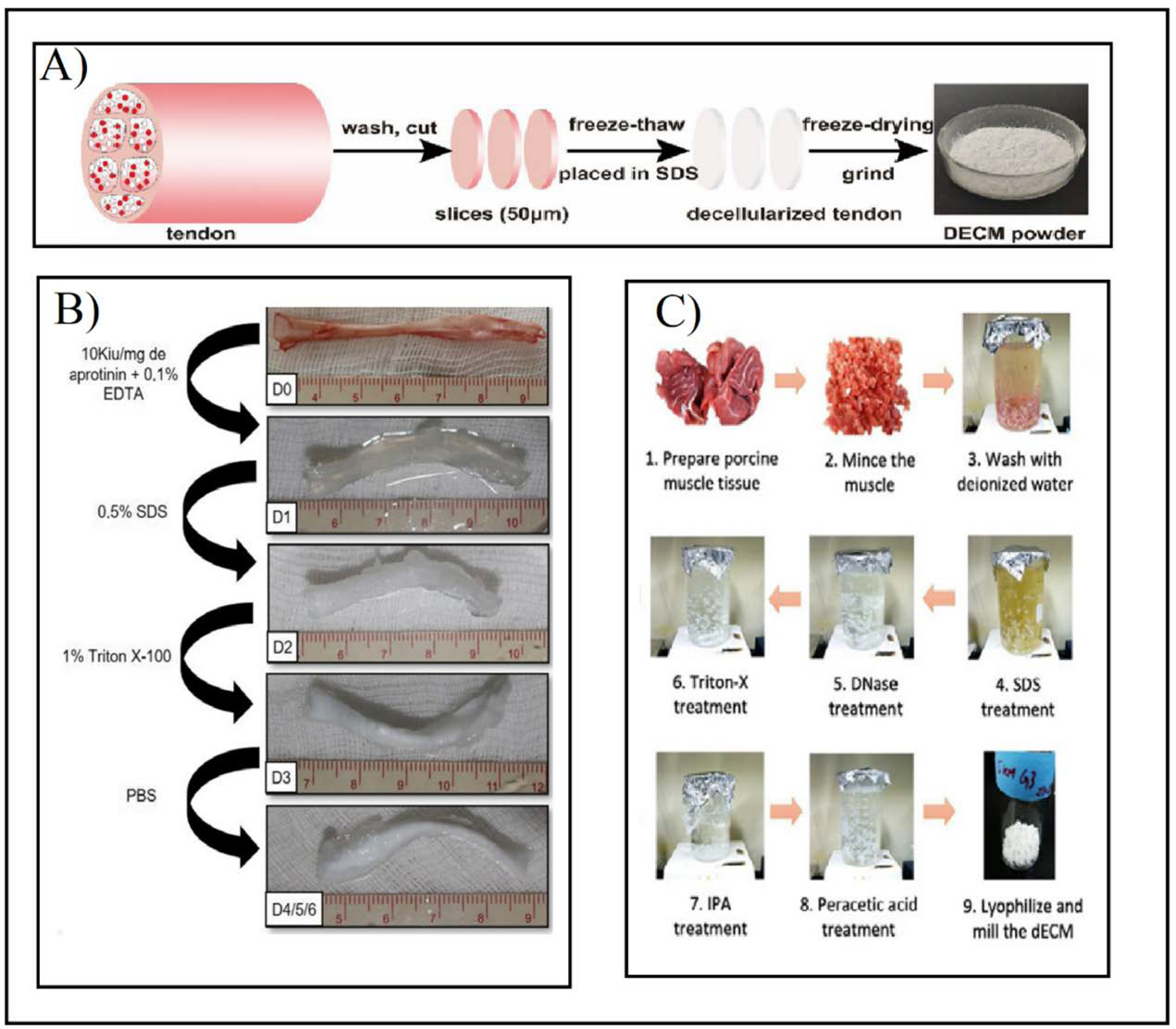
2. Decellularization Process
2.1. Chemical Decellularization
2.2. Biological Decellularization
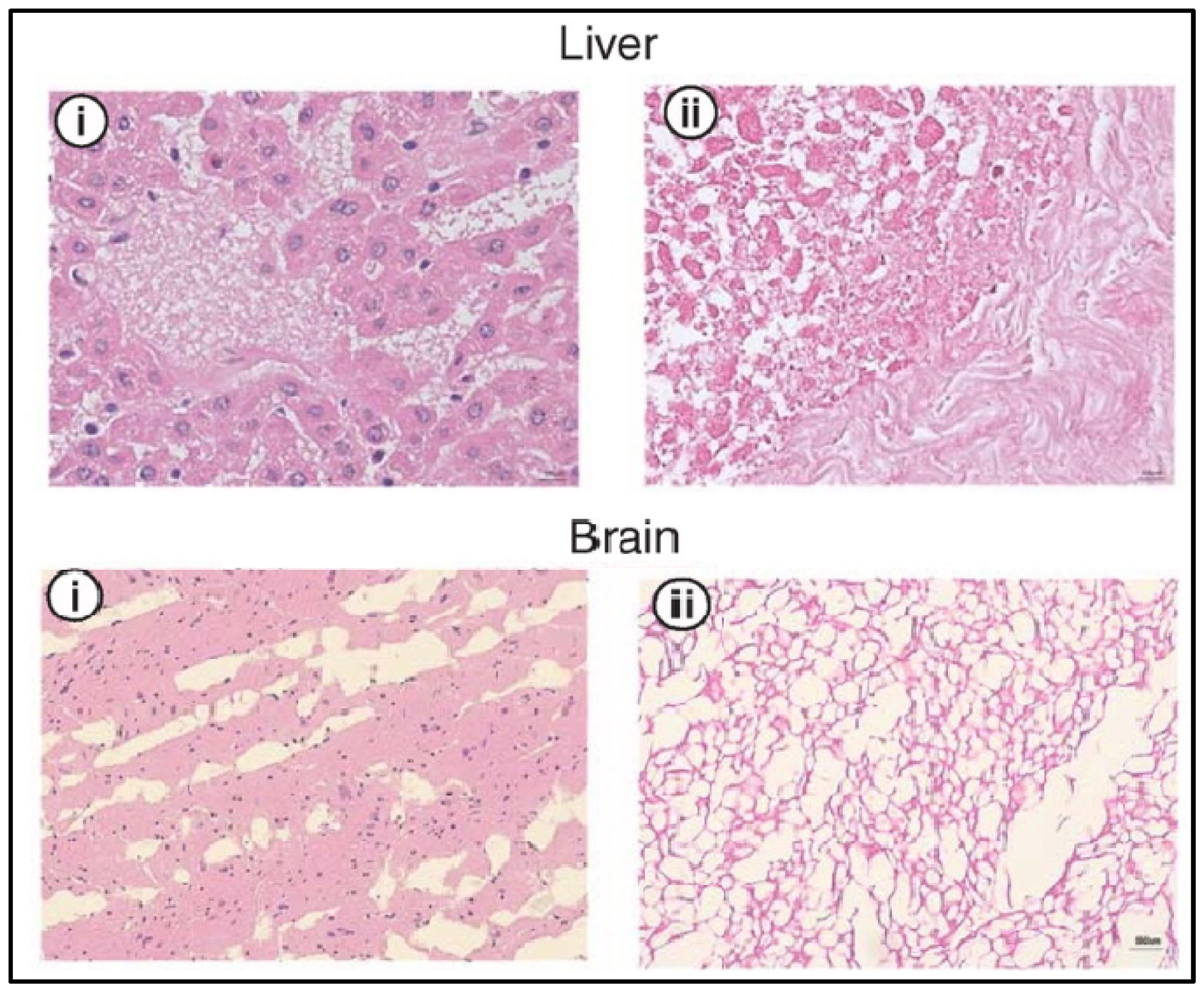
2.3. Physical Decellularization
3. Analysis of the dECM
4. dECM Sterilization and Preservation
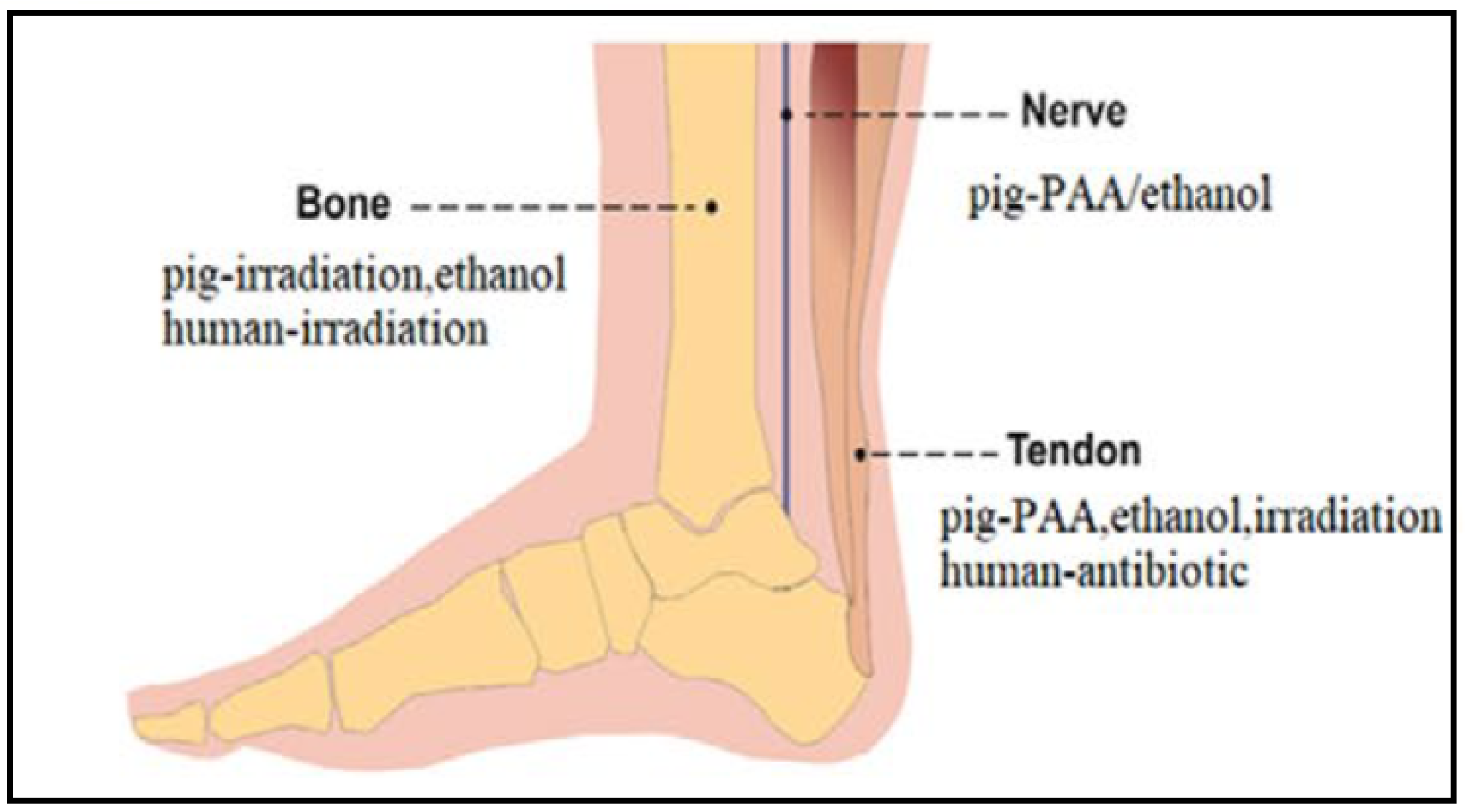
5. Decellularization Protocols for Tendons
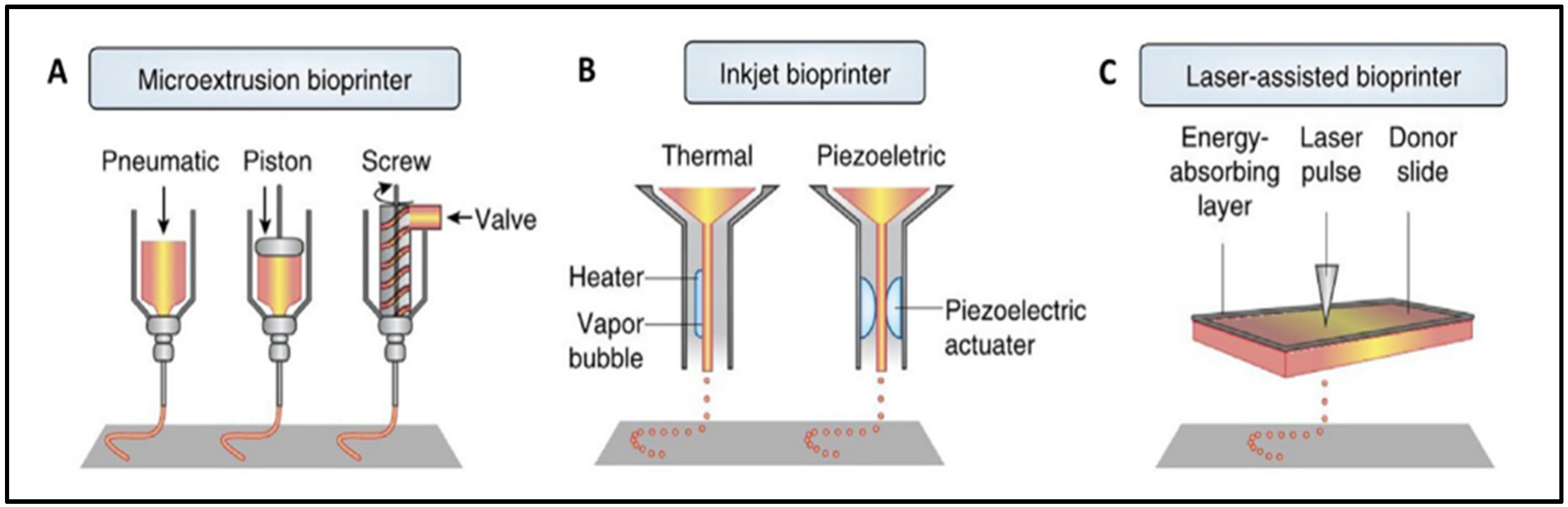
6. Tendon-Derived dECM Bioinks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbull, G.; Clarke, J.; Picard, F.; Zhang, W.; Riches, P.; Li, B.; Shu, W. 3D biofabrication for soft tissue and cartilage engineering. Med. Eng. Phys. 2020, 82, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Shiffman, M. Regenerative Medicine and Plastic Surgery; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Ruiz-Alonso, S.; Lafuente-Merchan, M.; Ciriza, J.; Saenz-Del-Burgo, L.; Pedraz, J.L. Tendon tissue engineering: Cells, growth factors, scaffolds and production techniques. J. Control. Release 2021, 333, 448–486. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef] [PubMed]
- Rinoldi, C.; Costantini, M.; Kijeńska-Gawrońska, E.; Testa, S.; Fornetti, E.; Heljak, M.; Ćwiklińska, M.; Buda, R.; Baldi, J.; Cannata, S.; et al. Aligned Cell-Laden Yarns: Tendon Tissue Engineering: Effects of Mechanical and Biochemical Stimulation on Stem Cell Alignment on Cell-Laden Hydrogel Yarns. Adv. Healthc. Mater. 2019, 8, 1970025. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Li, M.; Xiao, H.; Shi, Q.; Zhang, T.; Li, X.; Zhao, C.; Hu, J.; Lu, H. Functional decellularized fibrocartilaginous matrix graft for rotator cuff enthesis regeneration: A novel technique to avoid in-vitro loading of cells. Biomaterials 2020, 250, 119996. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, K.; Bai, J.; Tian, D.; Liu, G. Experimental study of tendon sheath repair via decellularized amnion to prevent tendon adhesion. PLoS ONE 2018, 13, e0205811. [Google Scholar] [CrossRef]
- Potyondy, T.; Uquillas, J.A.; Tebon, P.J.; Byambaa, B.; Hasan, A.; Tavafoghi, M.; Mary, H.; Aninwene, G.E.; Pountos, I.; Khademhosseini, A.; et al. Recent advances in 3D bioprinting of musculoskeletal tissues. Biofabrication 2021, 13, 022001. [Google Scholar] [CrossRef]
- Cui, J.; Ning, L.-J.; Wu, F.-P.; Hu, R.-N.; Li, X.; He, S.-K.; Zhang, Y.-J.; Luo, J.-J.; Luo, J.-C.; Qin, T.-W. Biomechanically and biochemically functional scaffold for recruitment of endogenous stem cells to promote tendon regeneration. npj Regen. Med. 2022, 7, 26. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Hafeez, M.N.; D’Avanzo, N.; Russo, V.; Di Marzio, L.; Cilurzo, F.; Paolino, D.; Fresta, M.; Barboni, B.; Santos, H.A.; Celia, C. Tendon Tissue Repair in Prospective of Drug Delivery, Regenerative Medicines, and Innovative Bioscaffolds. Stem Cells Int. 2021, 2021, 1488829. [Google Scholar] [CrossRef]
- Hanai, H.; Jacob, G.; Nakagawa, S.; Tuan, R.S.; Nakamura, N.; Shimomura, K. Potential of Soluble Decellularized Extracellular Matrix for Musculoskeletal Tissue Engineering—Comparison of Various Mesenchymal Tissues. Front. Cell Dev. Biol. 2020, 8, 581972. [Google Scholar] [CrossRef]
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S.; et al. Naturally-Derived Biomaterials for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1077, 421–449. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Fu, C.-P.; Li, X.-Y.; Lu, X.-C.; Hu, L.-G.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Three-Dimensional Bioprinting of Decellularized Extracellular Matrix-Based Bioinks for Tissue Engineering. Molecules 2022, 27, 3442. [Google Scholar] [CrossRef]
- Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: Prospects for the future. Methods 2020, 171, 108–118. [Google Scholar] [CrossRef]
- Chae, S.; Sun, Y.; Choi, Y.-J.; Ha, D.-H.; Jeon, I.; Cho, D.-W. 3D cell-printing of tendon-bone interface using tissue-derived extracellular matrix bioinks for chronic rotator cuff repair. Biofabrication 2021, 13, 035005. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Fendereski, M.; Hwang, N.S.; Hwang, Y. Recent Advancements in Decellularized Matrix-Based Biomaterials for Musculoskeletal Tissue Regeneration. Adv. Exp. Med. Biol. 2018, 1077, 149–162. [Google Scholar] [CrossRef]
- Kuzucu, M.; Vera, G.; Beaumont, M.; Fischer, S.; Wei, P.; Shastri, V.P.; Forget, A. Extrusion-Based 3D Bioprinting of Gradients of Stiffness, Cell Density, and Immobilized Peptide Using Thermogelling Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 2192–2197. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Di Nardo, P.; Manzari, V.; Carotenuto, F.; Teodori, L. 3D Printing Decellularized Extracellular Matrix to Design Biomimetic Scaffolds for Skeletal Muscle Tissue Engineering. BioMed Res. Int. 2020, 2020, 2689701. [Google Scholar] [CrossRef]
- Pennarossa, G.; Arcuri, S.; De Iorio, T.; Gandolfi, F.; Brevini, T.A.L. Current Advances in 3D Tissue and Organ Reconstruction. Int. J. Mol. Sci. 2021, 22, 830. [Google Scholar] [CrossRef]
- Bianchi, E.; Ruggeri, M.; Rossi, S.; Vigani, B.; Miele, D.; Bonferoni, M.; Sandri, G.; Ferrari, F. Innovative Strategies in Tendon Tissue Engineering. Pharmaceutics 2021, 13, 89. [Google Scholar] [CrossRef]
- Pedroza-González, S.C.; Rodriguez-Salvador, M.; Benítez, B.E.P.; Alvarez, M.M.; Santiago, G.T.-D. Bioinks for 3D Bioprinting: A Scientometric Analysis of Two Decades of Progress. Int. J. Bioprinting 2021, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Silva, I.V.; Duarte, M.M.; Oliveira, A.L. Current Trends on Protein Driven Bioinks for 3D Printing. Pharmaceutics 2021, 13, 1444. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.M.; Raitman, I.; Schwarzbauer, J.E. Cell-derived decellularized extracellular matrices. Methods Extracell. Matrix Biol. 2017, 143, 97–114. [Google Scholar] [CrossRef]
- Tao, C.; Nie, X.; Zhu, W.; Iqbal, J.; Xu, C.; Wang, D.-A. Autologous cell membrane coatings on tissue engineering xenografts for suppression and alleviation of acute host immune responses. Biomaterials 2020, 258, 120310. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi Sadrabadi, A.; Baei, P.; Hosseini, S.; Baghaban Eslaminejad, M. Decellularized Extracellular Matrix as a Potent Natural Biomaterial for Regenerative Medicine. Adv. Exp. Med. Biol. 2020, 13, 27–43. [Google Scholar]
- Yun, H.-W.; Song, B.R.; Shin, D.I.; Yin, X.Y.; Truong, M.-D.; Noh, S.; Jin, Y.J.; Kwon, H.J.; Min, B.-H.; Park, D.Y. Fabrication of decellularized meniscus extracellular matrix according to inner cartilaginous, middle transitional, and outer fibrous zones result in zone-specific protein expression useful for precise replication of meniscus zones. Mater. Sci. Eng. C 2021, 128, 112312. [Google Scholar] [CrossRef]
- Sotnichenko, A.; Gilevich, I.; Melkonyan, K.; Yutskevich, Y.; Rusinova, T.; Karakulev, A.; Bogdanov, S.B.; Aladina, V.A.; Belich, Y.A.; Gumenyuk, S.E.; et al. Comparative Morphological Characteristics of the Results of Implantation of Decellularized and Recellularized Porcine Skin Scaffolds. Bull. Exp. Biol. Med. 2021, 170, 378–383. [Google Scholar] [CrossRef]
- Jin, R.; Cui, Y.; Chen, H.; Zhang, Z.; Weng, T.; Xia, S.; Yu, M.; Zhang, W.; Shao, J.; Yang, M.; et al. Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater. 2021, 131, 248–261. [Google Scholar] [CrossRef]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Lively, M.O.; Willey, J.S.; Smith, T.L.; Van Dyke, M.E.; Whitlock, P.W. A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure. J. Funct. Biomater. 2018, 9, 45. [Google Scholar] [CrossRef]
- Jacintho Delgado, A.; Oliveira Carreira, A.; Costa de Carvalho, H.; da Palma, R.; de Castro Sasahara, T.; Figueiredo de Carvalho, C.; León, M.; Barreto, R.d.N.; Miglino, M.A. Development of a new decellularization protocol for the whole porcine heart. J. Clin. Transl. Res. 2021, 7, 563–574. [Google Scholar]
- Ayariga, J.A.; Huang, H.; Dean, D. Decellularized Avian Cartilage, a Promising Alternative for Human Cartilage Tissue Regeneration. Materials 2022, 15, 1974. [Google Scholar] [CrossRef]
- Gvaramia, D.; Kern, J.; Jakob, Y.; Tritschler, H.; Brenner, R.E.; Breiter, R.; Kzhyshkowska, J.; Rotter, N. Modulation of the inflammatory response to decellularized collagen matrix for cartilage regeneration. J. Biomed. Mater. Res. Part A 2021, 110, 1021–1035. [Google Scholar] [CrossRef]
- Daugs, A.; Lehmann, N.; Eroglu, D.; Meinke, M.; Markhoff, A.; Bloch, O. In Vitro Detection System to Evaluate the Immunogenic Potential of Xenografts. Tissue Eng. Part C Methods 2018, 24, 280–288. [Google Scholar] [CrossRef]
- Zheng, Z.-W.; Chen, Y.-H.; Wu, D.-Y.; Wang, J.; Lv, M.-M.; Wang, X.-S.; Sun, J.; Zhang, Z.-Y. Development of an Accurate and Proactive Immunomodulatory Strategy to Improve Bone Substitute Material-Mediated Osteogenesis and Angiogenesis. Theranostics 2018, 8, 5482–5500. [Google Scholar] [CrossRef]
- Das, P.; Singh, Y.P.; Mandal, B.B.; Nandi, S.K. Tissue-derived decellularized extracellular matrices toward cartilage repair and regeneration. Cell-Deriv. Matrices—Part B 2020, 157, 185–221. [Google Scholar] [CrossRef]
- Hsieh, D.-J.; Srinivasan, P.; Yen, K.-C.; Yeh, Y.-C.; Chen, Y.-J.; Wang, H.-C.; Tarng, Y.-W. Protocols for the preparation and characterization of decellularized tissue and organ scaffolds for tissue engineering. BioTechniques 2021, 70, 107–115. [Google Scholar] [CrossRef]
- de Lima Santos, A.; da Silva, C.; de Sá Barreto, L.; Leite, K.; Tamaoki, M.; Ferreira, L.; de Almeida, F.G.; Faloppa, F. A new decellularized tendon scaffold for rotator cuff tears—Evaluation in rabbits. BMC Musculoskelet. Disord. 2020, 21, 689. [Google Scholar] [CrossRef]
- Liu, G.-M.; Pan, J.; Zhang, Y.; Ning, L.-J.; Luo, J.-C.; Huang, F.-G.; Qin, T.-W. Bridging Repair of Large Rotator Cuff Tears Using a Multilayer Decellularized Tendon Slices Graft in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 2569–2578. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Alimoradi, N. Contribution of physical methods in decellularization of animal tissues. J. Med. Signals Sensors 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Zhang, E.; Zhang, R.; Cai, D.; Zhu, S.; Ran, J.; Bunpetch, V.; Cai, Y.; Heng, B.C.; et al. Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 2017, 56, 129–140. [Google Scholar] [CrossRef]
- Tao, M.; Liang, F.; He, J.; Ye, W.; Javed, R.; Wang, W.; Yu, T.; Fan, J.; Tian, X.; Wang, X.; et al. Decellularized tendon matrix membranes prevent post-surgical tendon adhesion and promote functional repair. Acta Biomater. 2021, 134, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Ito, A.; Kimura, T.; Kishida, A. Extracellular Matrix Induces Periodontal Ligament Reconstruction In Vivo. Int. J. Mol. Sci. 2019, 20, 3277. [Google Scholar] [CrossRef] [PubMed]
- Forouzesh, F.; Rabbani, M.; Bonakdar, S. A Comparison between Ultrasonic Bath and Direct Sonicator on Osteochondral Tissue Decellularization. J. Med. Signals Sens. 2019, 9, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Yee, M.; Sheng, Z.L.J.; Amirul, A.; Naing, M.W. Decellularization systems and devices: State-of-the-art. Acta Biomater. 2020, 115, 51–59. [Google Scholar] [CrossRef]
- Park, W.; Gao, G.; Cho, D.-W. Tissue-Specific Decellularized Extracellular Matrix Bioinks for Musculoskeletal Tissue Regeneration and Modeling Using 3D Bioprinting Technology. Int. J. Mol. Sci. 2021, 22, 7837. [Google Scholar] [CrossRef] [PubMed]
- Behmer Hansen, R.; Wang, X.; Kaw, G.; Pierre, V.; Senyo, S. Accounting for Material Changes in Decellularized Tissue with Underutilized Methodologies. BioMed Res. Int. 2021, 2021, 6696295. [Google Scholar] [CrossRef]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef]
- Nam, S.Y.; Park, S.-H. ECM Based Bioink for Tissue Mimetic 3D Bioprinting. Adv. Exp. Med. Biol. 2018, 1064, 335–353. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, B.; Huang, W.; Tang, Y.; Chen, X. Preparation and biological characteristics of a bovine acellular tendon fiber material. J. Biomed. Mater. Res. Part A 2021, 109, 1931–1941. [Google Scholar] [CrossRef]
- Stone, R.; Frahs, S.; Hardy, M.; Fujimoto, A.; Pu, X.; Keller-Peck, C.; Oxford, J. Decellularized Porcine Cartilage Scaffold; Validation of Decellularization and Evaluation of Biomarkers of Chondrogenesis. Int. J. Mol. Sci. 2021, 22, 6241. [Google Scholar] [CrossRef]
- Wu, G.; Sun, B.; Zhao, C.; Wang, Z.; Teng, S.; Yang, M.; Cui, Z.; Zhu, G.; Yu, Y. Three-Dimensional Tendon Scaffold Loaded with TGF-β1 Gene Silencing Plasmid Prevents Tendon Adhesion and Promotes Tendon Repair. ACS Biomater. Sci. Eng. 2021, 7, 5739–5748. [Google Scholar] [CrossRef]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef]
- Yang, J.; Dang, H.; Xu, Y. Recent advancement of decellularization extracellular matrix for tissue engineering and biomedical application. Artif. Organs 2021, 46, 549–567. [Google Scholar] [CrossRef]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef]
- Chu, W.; Hu, G.; Peng, L.; Zhang, W.; Ma, Z. The use of a novel deer antler decellularized cartilage-derived matrix scaffold for repair of osteochondral defects. J. Biol. Eng. 2021, 15, 23. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhang, H.; Tang, Z.; Cui, X.; Li, X.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Solubilized Cartilage ECM Facilitates the Recruitment and Chondrogenesis of Endogenous BMSCs in Collagen Scaffolds for Enhancing Microfracture Treatment. ACS Appl. Mater. Interfaces 2021, 13, 24553–24564. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, F.; Lu, Y.; Pan, S.; Yang, W.; Zhang, G.; Ma, J.; Shi, H. Rapid preparation of decellularized trachea as a 3D scaffold for organ engineering. Int. J. Artif. Organs 2020, 44, 55–64. [Google Scholar] [CrossRef]
- Milian, L.; Sancho-Tello, M.; Roig-Soriano, J.; Foschini, G.; Martínez-Hernández, N.J.; Más-Estellés, J.; Ruiz-Sauri, A.; Zurriaga, J.; Carda, C.; Mata, M. Optimization of a decellularization protocol of porcine tracheas. Long-term effects of cryopreservation. A histological study. Int. J. Artif. Organs 2021, 44, 998–1012. [Google Scholar] [CrossRef]
- Khajavi, M.; Hajimoradloo, A.; Zandi, M.; Pezeshki-Modaress, M.; Bonakdar, S.; Zamani, A. Fish cartilage: A promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2021, 109, 1737–1750. [Google Scholar] [CrossRef]
- Mousavi, M.S.; Amoabediny, G.; Mahfouzi, S.H.; Tali, S.H.S. Enhanced articular cartilage decellularization using a novel perfusion-based bioreactor method. J. Mech. Behav. Biomed. Mater. 2021, 119, 104511. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, H.; Wu, J.; Wang, A.; Chen, X.; Hu, S.; Zhang, Y.; Bai, D.; Jin, Z. COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of periodontal ligament stem cells. Theranostics 2019, 9, 4265–4286. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Jeon, M.; Choi, H.-J.; Lee, H.-S.; Kim, I.-H.; Kang, C.-M.; Song, J.S. Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 2019, 14, e0221236. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Gruber, R.; Kim, C. Periodontal healing by periodontal ligament fiber with or without cells: A preclinical study of the decellularized periodontal ligament in a tooth replantation model. J. Periodontol. 2019, 91, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Volpato, F.Z.; Hutmacher, D.W.; Ivanovski, S. Assessment of static and perfusion methods for decellularization of PCL membrane-supported periodontal ligament cell sheet constructs. Arch. Oral Biol. 2018, 88, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, T.; Wang, L.; Jiang, J.; Xie, G.; Huangfu, X.; Dong, S.; Zhao, J. Tissue-Engineered Decellularized Allografts for Anterior Cruciate Ligament Reconstruction. ACS Biomater. Sci. Eng. 2020, 6, 5700–5710. [Google Scholar] [CrossRef]
- Sherifi, I.; Bachy, M.; Laumonier, T.; Petite, H.; Hannouche, D. Use of supercritical carbon dioxide technology for fabricating a tissue engineering scaffold for anterior cruciate ligament repair. Sci. Rep. 2020, 10, 14030. [Google Scholar] [CrossRef]
- Lohan, A.; Kohl, B.; Meier, C.; Schulze-Tanzil, G. Tenogenesis of Decellularized Porcine Achilles Tendon Matrix Reseeded with Human Tenocytes in the Nude Mice Xenograft Model. Int. J. Mol. Sci. 2018, 19, 2059. [Google Scholar] [CrossRef]
- Jones, G.; Herbert, A.; Berry, H.; Edwards, J.H.; Fisher, J.; Ingham, E. Decellularization and Characterization of Porcine Superflexor Tendon: A Potential Anterior Cruciate Ligament Replacement. Tissue Eng. Part A 2017, 23, 124–134. [Google Scholar] [CrossRef]
- Edwards, J.H.; Herbert, A.; Jones, G.L.; Manfield, I.W.; Fisher, J.; Ingham, E. The effects of irradiation on the biological and biomechanical properties of an acellular porcine superflexor tendon graft for cruciate ligament repair. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 2477–2486. [Google Scholar] [CrossRef]
- Roth, S.P.; Schubert, S.; Scheibe, P.; Groß, C.; Brehm, W.; Burk, J. Growth Factor-Mediated Tenogenic Induction of Multipotent Mesenchymal Stromal Cells Is Altered by the Microenvironment of Tendon Matrix. Cell Transplant. 2018, 27, 1434–1450. [Google Scholar] [CrossRef]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Health Mater. 2020, 9, e2000734. [Google Scholar] [CrossRef]
- Toprakhisar, B.; Nadernezhad, A.; Bakirci, E.; Khani, N.; Skvortsov, G.A.; Koc, B. Development of Bioink from Decellularized Tendon Extracellular Matrix for 3D Bioprinting. Macromol. Biosci. 2018, 18, e1800024. [Google Scholar] [CrossRef]
- De Santis, M.; Alsafadi, H.; Tas, S.; Bölükbas, D.; Prithiviraj, S.; Da Silva, I.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2020, 33, 2005476. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Luo, C.; Zhai, C.; Li, Z.; Zhang, Y.; Yuan, T.; Dong, S.; Zhang, J.; Fan, W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C 2021, 118, 111388. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zuo, Q.; Wang, Q.; Li, Z.; Yan, K.; Shen, K.; Xie, R.; Fan, W. 3D Bioprinting of Biomimetic Bilayered Scaffold Consisting of Decellularized Extracellular Matrix and Silk Fibroin for Osteochondral Repair. Int. J. Bioprinting 2021, 7, 401. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Zhou, X.; Zhang, J.; Zhou, H.; Hao, H.; Ding, L.; Li, H.; Gu, Y.; Ma, J.; et al. An Overview of Extracellular Matrix-Based Bioinks for 3D Bioprinting. Front. Bioeng. Biotechnol. 2022, 10, 905438. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, J.; Zhang, J.; Yu, H.; Dai, W.; Yan, W.; Sun, M.; Ding, G.; Li, Q.; Meng, Q.; et al. Comparison of three different acidic solutions in tendon decellularized extracellular matrix bio-ink fabrication for 3D cell printing. Acta Biomater. 2021, 131, 262–275. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, J.; Sun, M.; Yu, H.; Wu, N.; Li, Z.; Zhang, J.; Li, Q.; Yang, P.; Liu, Q.; et al. Digestion degree is a key factor to regulate the printability of pure tendon decellularized extracellular matrix bio-ink in extrusion-based 3D cell printing. Biofabrication 2020, 12, 045011. [Google Scholar] [CrossRef]
- Chae, S.; Yong, U.; Park, W.; Choi, Y.-M.; Jeon, I.-H.; Kang, H.; Jang, J.; Choi, H.S.; Cho, D.-W. 3D cell-printing of gradient multi-tissue interfaces for rotator cuff regeneration. Bioact. Mater. 2023, 19, 611–625. [Google Scholar] [CrossRef]
- Chae, S.; Choi, Y.-J.; Cho, D.-W. Mechanically and biologically promoted cell-laden constructs generated using tissue-specific bioinks for tendon/ligament tissue engineering applications. Biofabrication 2022, 14, 025013. [Google Scholar] [CrossRef]





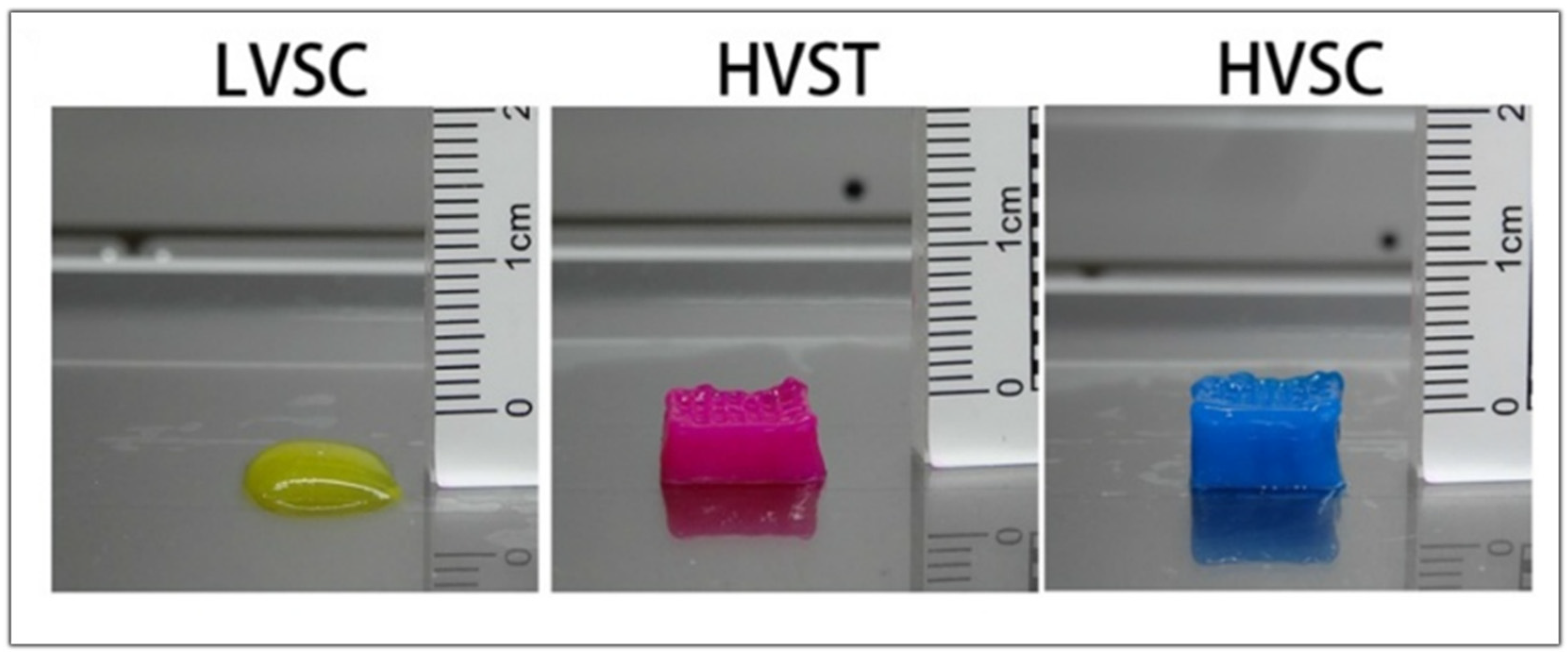

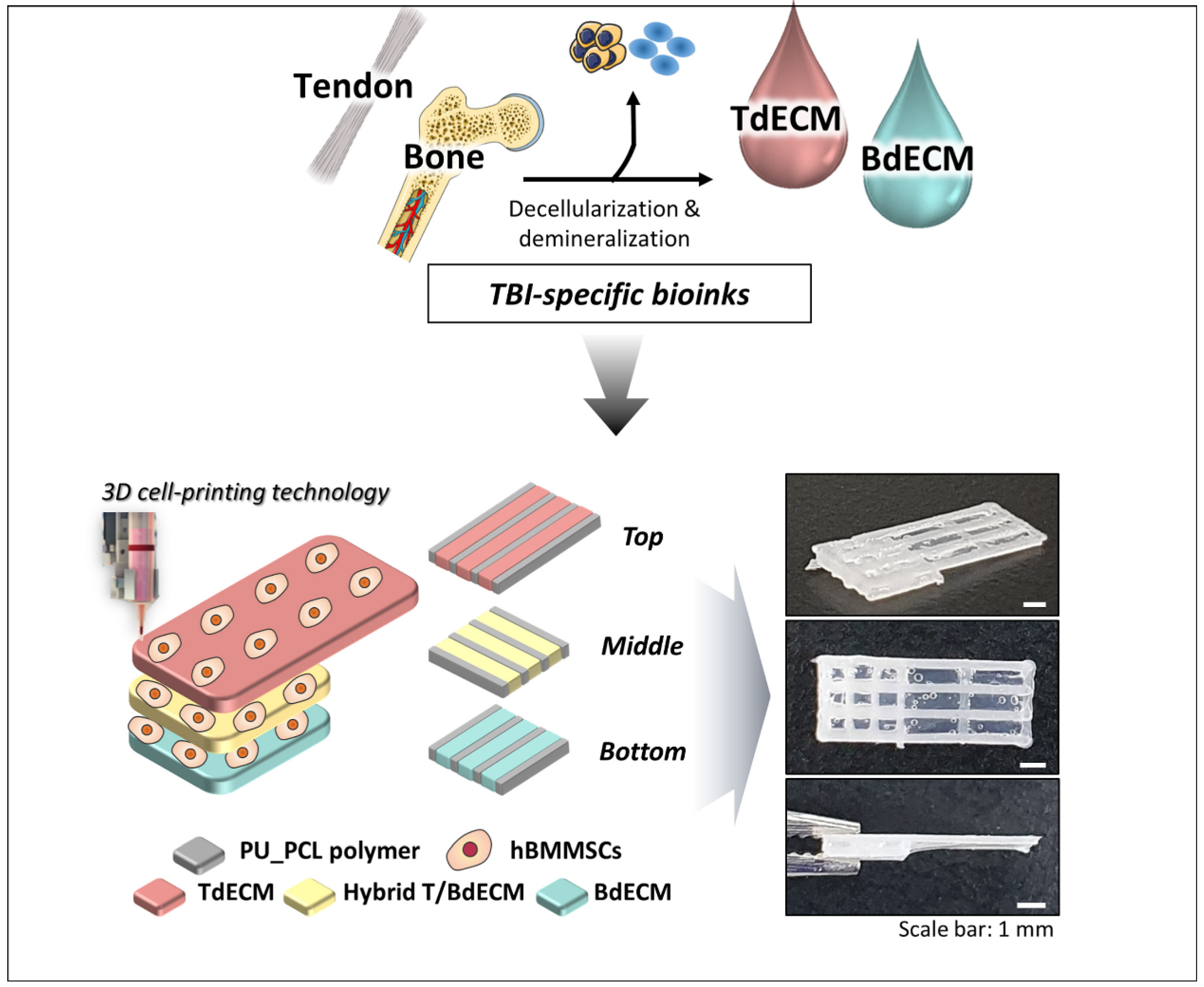

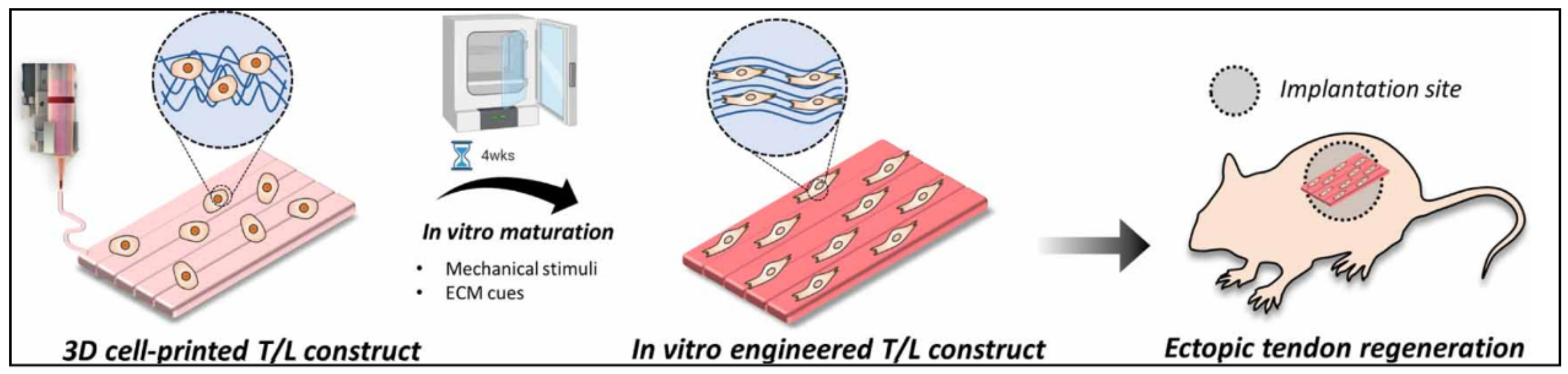
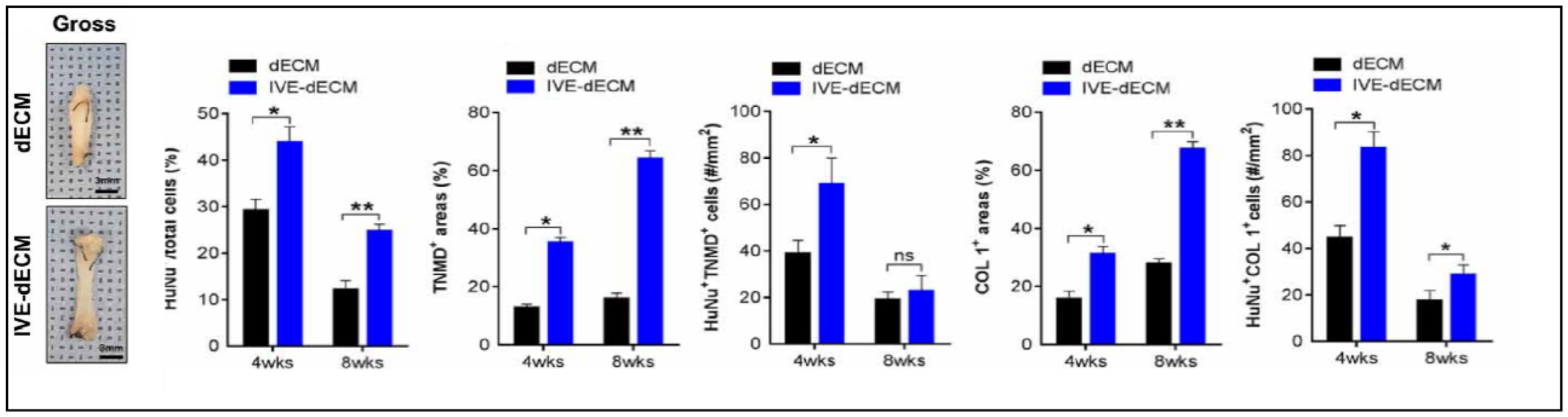
| Specific Tissue Genes | |
|---|---|
| Chondrogenic differentiation markers | COL2A1, ACAN, SOX-9 |
| Tenogenic differentiation markers | SCX, TNMD, TNC |
| Cartilage marker | GAPDH, HPRT1 |
| Tendon marker | COL1A1, COL3A1 |
| Decellularization Biomarkers | |
|---|---|
| Nuclear | APTX, UIMC1, DMRT1, H3F3A |
| Golgi-specific | B3GALNT1, MAN1A1, FUT2 |
| Mitochondrial | ACO2, AKAP10, GOT2 |
| Non-Collagenous extracellular matrix proteins | ACAN |
| Source | Decellularization Method | Characterization | In Vivo | Observations | Ref. |
|---|---|---|---|---|---|
| Porcine super Flexor Tendon | Freeze–thaw cycle Hypotonic buffer 0.1% SDS Nuclease solution Hypertonic buffer | Good results in the uniaxial tensile testing | No | Optimized an irradiation of 25 kGy for sterilization | [70] |
| Porcine super flexor tendon | Freeze–thaw cycle Hypotonic buffer Proteinase inhibitor SDS Nuclease Hypertonic buffer | Presence of Col-I, III, and tenascin C, but no Col II Significant depletion of GAG content, <1% of dry weight | Subcutaneous mice model, no inflammatory or immune response was observed | Maintained strength between native and dECM groups (52.5 mPA vs. 62 mPA, respectively) Presence of a Gal epitope after decellularization | [69] |
| Aquiles Porcine tendon | 1% SDS 0.2% sodium azide 5 mM EDTA Protease cocktail 0.05% trypsin 0.053 mM EDTA 3%Triton X-100 | Important structure and GAG loss | On subcutaneous nude mice test, no inflammation was detected | Compared macroscopic appearance Lack of quantitative comparison Animal conditions after surgery does not represent real conditions | [68] |
| Extensor and flexor bovine tendon | Lyophilization Hypotonic buffer 0.05%trypsin Hanks Buffer Protease inhibitor Antibiotic solution | Conservation of collagen content and structure | No | Lower mechanical properties than control | [50] |
| SDF horse tendon | Freeze–thaw Hypotonic solution (Tris 1 M) 1%Triton X-100 | Evaluation of tenogenic extracellular proteins (collagen 1A2, collagen 3A1, decorin, and tenascin c) | No | Gene expression evaluation | [71] |
| Tendon Source | Decellularization Protocol | Sterilization | Conservation | Digestion Method | 3D Bioprinting Technique | Bioink Composition | Reference |
|---|---|---|---|---|---|---|---|
| Porcine | 1: 0.25% Trypsin-EDTA at 4 °C for 48 h 2: 0.5% SDS + 0.5% Triton X-100 for 48 h | 0.1% PAA + 4% ethanol for 4 h | Freeze-dried | 3 mg/mL pepsin in 0.1 M HCL | Extrusion | 2.5% dECM + SD-BMSCs | [78] |
| Porcine | 1: 0.25% Trypsin-EDTA overnight 2: 2% SDS for 96 h | 100% ethanol for 30 min + 70% ethanol at 4 °C overnight | Freeze-died | 3 mg/mL pepsin in 0.1 M HCL | Extrusion | 3% dECM + SD-BMSCs | [79] |
| Bovine Achilles tendon | 1: 5 F–T cycles with alternate hypo/hypertonic solution 2: 0.05%Trypsin EDTA for 30 min 3: 2%SDS for 4 days | Antibiotic solution | Freeze-dried | 1 mg/mL pepsin in 0.01 M HCL | Piston-driven aspiration-extrusion | 10 mg/mL dECM + NIH 3T3 cell | [73] |
| Porcine Achilles tendon | 1: 5 F–T cycles 2: 0.5% Trypsin + EDTA at 37 °C for 6 h 3: 2% Triton X-100 at 21 °C for 3 days 4.50 U/mL DNAse at 37 °C for 2 days | 0.1% PAA in 4% ethanol for 3 h | Freeze-dried | 10% pepsin in 0.5 M acetic acid | Extrusion | 20 mg/mL TdECM + 40 mg/mL BdECM + hBMSCs | [80] |
| Porcine Achilles tendon | 1: 6 F–T cycles 2: 0.5% Trypsin + EDTA at 37 °C for 6 h 3: 2% Triton X-100 at 21 °C for 3 days 4.50 U/mL DNAse at 37 °C for 2 days | 0.1% PAA in 4% ethanol for 3 h | Freeze-dried | 1 mg/mL in 0.5 M glacial acetic acid | Extrusion | 2% TdECM + 2% LidECM + hBMMSCs | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hakim Khalak, F.; García-Villén, F.; Ruiz-Alonso, S.; Pedraz, J.L.; Saenz-del-Burgo, L. Decellularized Extracellular Matrix-Based Bioinks for Tendon Regeneration in Three-Dimensional Bioprinting. Int. J. Mol. Sci. 2022, 23, 12930. https://doi.org/10.3390/ijms232112930
Al-Hakim Khalak F, García-Villén F, Ruiz-Alonso S, Pedraz JL, Saenz-del-Burgo L. Decellularized Extracellular Matrix-Based Bioinks for Tendon Regeneration in Three-Dimensional Bioprinting. International Journal of Molecular Sciences. 2022; 23(21):12930. https://doi.org/10.3390/ijms232112930
Chicago/Turabian StyleAl-Hakim Khalak, Fouad, Fátima García-Villén, Sandra Ruiz-Alonso, José Luis Pedraz, and Laura Saenz-del-Burgo. 2022. "Decellularized Extracellular Matrix-Based Bioinks for Tendon Regeneration in Three-Dimensional Bioprinting" International Journal of Molecular Sciences 23, no. 21: 12930. https://doi.org/10.3390/ijms232112930
APA StyleAl-Hakim Khalak, F., García-Villén, F., Ruiz-Alonso, S., Pedraz, J. L., & Saenz-del-Burgo, L. (2022). Decellularized Extracellular Matrix-Based Bioinks for Tendon Regeneration in Three-Dimensional Bioprinting. International Journal of Molecular Sciences, 23(21), 12930. https://doi.org/10.3390/ijms232112930








