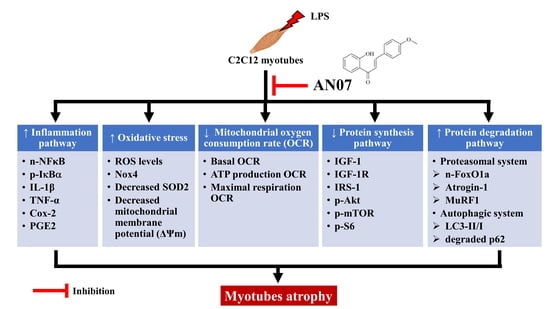
Protective Effects of the Chalcone-Based Derivative AN07 on Inflammation-Associated Myotube Atrophy Induced by Lipopolysaccharide
Abstract
1. Introduction
2. Results
2.1. AN07 Attenuated LPS-Induced C2C12 Myotube Atrophy
2.2. AN07 Attenuated LPS-Induced Oxidative Stress in C2C12 Myotubes
2.3. AN07 Improved Mitochondrial Respiration Function in LPS-Treated C2C12 Myotubes
2.4. AN07 Attenuated LPS-Induced Inflammatory Signaling in C2C12 Myotubes
2.5. AN07 Activated IGF/IGF-1R-Related Protein Synthesis Pathway in LPS-Treated C2C12 Myotubes
2.6. AN07 Suppressed LPS-Induced Protein Degradation Pathway in C2C12 Myotubes
2.7. Effects of PPARγ, IGF-1R, and PI3K/Akt Antagonists on AN07-Induced Protective Effects in LPS-Treated C2C12 Myotubes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Drug Treatment
4.3. Cell Viability Assay
4.4. Quantitative RT-PCR (qRT-PCR)
4.5. Measurement of Intracellular Reactive Oxygen Species (ROS) and Mitochondrial Membrane Potential (ΔΨm)
4.6. Western Blot Analysis
4.7. Measurement of PGE2 Production
4.8. Immunofluorescence Staining
4.9. Mitochondrial Bioenergetics
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, M.N.; Eggelbusch, M.; Naddaf, E.; Gerrits, K.H.L.; van der Schaaf, M.; van den Borst, B.; Wiersinga, W.J.; van Vugt, M.; Weijs, P.J.M.; Murray, A.J.; et al. Skeletal muscle alterations in patients with acute COVID-19 and post-acute sequelae of COVID-19. J. Cachex. Sarcopenia Muscle 2022, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.; Radu, B.M.; Radu, M.; Cretoiu, S.M. Muscle Changes During Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Macedo, T.G.; Valentova, M.; Anker, M.S.; Ebner, N.; Bekfani, T.; Haarmann, H.; Schefold, J.C.; Lainscak, M.; Cleland, J.G.F.; et al. Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J. Cachex- Sarcopenia Muscle 2020, 11, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Villanueva, R.; Westbury, L.D.; Syddall, H.E.; Sanchez-Santos, M.T.; Dennison, E.M.; Robinson, S.M.; Cooper, C. Health Care Costs Associated With Muscle Weakness: A UK Population-Based Estimate. Calcif. Tissue Res. 2018, 104, 137–144. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Fang, W.; Tseng, Y.; Lee, T.; Fu, Y.; Chang, W.; Lo, W.; Lin, C.; Lo, Y. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. J. Cereb. Blood Flow Metab. 2021, 178, 2998–3016. [Google Scholar] [CrossRef]
- Baker, L.A.; Martin, N.R.W.; Kimber, M.C.; Pritchard, G.J.; Lindley, M.R.; Lewis, M.P. Resolvin E1 (Rv E1) attenuates LPS induced inflammation and subsequent atrophy in C2C12 myotubes. J. Cell. Biochem. 2018, 119, 6094–6103. [Google Scholar] [CrossRef]
- Wang, D.-T.; Yin, Y.; Yang, Y.-J.; Lv, P.-J.; Shi, Y.; Lu, L.; Wei, L.-B. Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014, 19, 206–213. [Google Scholar] [CrossRef]
- Gómez-SanMiguel, A.B.; Villanúa, M.; Martín, A.I.; López-Calderón, A. D-TRP(8)-γMSH Prevents the Effects of Endotoxin in Rat Skeletal Muscle Cells through TNFα/NF-KB Signalling Pathway. PLoS ONE 2016, 11, e0155645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, P.; Wang, X.; Wu, H.; Zhu, H.; Hou, Y.; Wang, L.; Liu, Y. Glutamate alleviates muscle protein loss by modulating TLR4, NODs, Akt/FOXO and mTOR signaling pathways in LPS-challenged piglets. PLoS ONE 2017, 12, e0182246. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Dobrowolny, G.; Sica, G.; Musaro, A. Molecular Insights into Muscle Homeostasis, Atrophy and Wasting. Curr. Genom. 2018, 19, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.-H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin–proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Pomiès, P.; Blaquière, M.; Maury, J.; Mercier, J.; Gouzi, F.; Hayot, M. Involvement of the FoxO1/MuRF1/Atrogin-1 Signaling Pathway in the Oxidative Stress-Induced Atrophy of Cultured Chronic Obstructive Pulmonary Disease Myotubes. PLoS ONE 2016, 11, e0160092. [Google Scholar] [CrossRef]
- Aedo, J.; Reyes, A.; Avendaño-Herrera, R.; Molina, A.; Valdés, J. Bacterial lipopolysaccharide induces rainbow trout myotube atrophy via Akt/FoxO1/Atrogin-1 signaling pathway. Acta Biochim. et Biophys. Sin. 2015, 47, 932–937. [Google Scholar] [CrossRef]
- Valentine, R.J.; Jefferson, M.A.; Kohut, M.L.; Eo, H. Imoxin attenuates LPS-induced inflammation and MuRF1 expression in mouse skeletal muscle. Physiol. Rep. 2018, 6, e13941. [Google Scholar] [CrossRef]
- Liu, J.; Bi, X.; Chen, T.; Zhang, Q.; Wang, S.X.; Chiu, J.-J.; Liu, G.-S.; Zhang, Y.; Bu, P.; Jiang, F. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis. 2015, 6, e1827. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Frøsig, C.; Jeppesen, J.; Jensen, T.E.; Lundsgaard, A.-M.; Serup, A.K.; Schjerling, P.; Proud, C.G.; Richter, E.A.; Kiens, B. Role of AMPK in regulation of LC3 lipidation as a marker of autophagy in skeletal muscle. Cell. Signal. 2016, 28, 663–674. [Google Scholar] [CrossRef]
- Doyle, A.; Zhang, G.; Fattah, E.A.A.; Eissa, N.T.; Li, Y. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2010, 25, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.S.; Chauhan, P.; Maurya, P.; Saini, D.; Yadav, P.P.; Barthwal, M.K. Coagulin-L ameliorates TLR4 induced oxidative damage and immune response by regulating mitochondria and NOX-derived ROS. Toxicol. Appl. Pharmacol. 2016, 309, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Koduru, B.; Carlisle, C.; Akhter, H.; Liu, R.-M.; Schroder, K.; Brandes, R.P.; Ojcius, D.M. NADPH oxidase 4 modulates hepatic responses to lipopolysaccharide mediated by Toll-like receptor-4. Sci. Rep. 2017, 7, 14346. [Google Scholar] [CrossRef]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Song, Y.; Pinniger, G.J.; Bakker, A.J.; Moss, T.J.M.; Noble, P.B.; Berry, C.A.; Pillow, J.J. Lipopolysaccharide-Induced Weakness in the Preterm Diaphragm Is Associated with Mitochondrial Electron Transport Chain Dysfunction and Oxidative Stress. PLoS ONE 2013, 8, e73457. [Google Scholar] [CrossRef]
- Jo, A.L.; Han, J.W.; An, J.I.; Cho, K.-H.; Jeoung, N.H. Cuban Policosanol Prevents the Apoptosis and the Mitochondrial Dysfunction Induced by Lipopolysaccharide in C2C12 Myoblast via Activation of Akt and Erk Pathways. J. Nutr. Sci. Vitaminol. 2022, 68, 79–86. [Google Scholar] [CrossRef]
- Hernandez-Saavedra, D.; Swain, K.; Tuder, R.; Petersen, S.V.; Nozik-Grayck, E. Redox Regulation of the Superoxide Dismutases SOD3 and SOD2 in the Pulmonary Circulation. Adv. Exp. Med. Biol. 2017, 967, 57–70. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorganic Chem. 2021, 108, 104681. [Google Scholar] [CrossRef]
- Kar Mahapatra, D.; Asati, V.; Bharti, S.K. An updated patent review of therapeutic applications of chalcone derivatives (2014–present). Expert Opin. Ther. Pat. 2019, 29, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-S.; Chang, C.-C.; Du, Y.-C.; Chang, F.-R.; Wu, Y.-C.; Chang, W.-C.; Hsieh, T.-J. 2-Hydroxy-4ʼ-Methoxychalcone Inhibits Proliferation and Inflammation of Human Aortic Smooth Muscle Cells by Increasing the Expression of Peroxisome Proliferator–Activated Receptor Gamma. J. Cardiovasc. Pharmacol. 2012, 59, 339–351. [Google Scholar] [CrossRef]
- Seiri, P.; Abi, A.; Soukhtanloo, M. PPAR-γ: Its ligand and its regulation by microRNAs. J. Cell. Biochem. 2019, 120, 10893–10908. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARγ as a molecular target of EPA anti-inflammatory activity during TNF-α-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Kim, M.; Youn, B.-S.; Lee, N.S.; Park, J.W.; Lee, I.K.; Lee, Y.S.; Kim, J.B.; Cho, Y.M.; Lee, H.K.; et al. Glutathione Peroxidase 3 Mediates the Antioxidant Effect of Peroxisome Proliferator-Activated Receptor γ in Human Skeletal Muscle Cells. Mol. Cell. Biol. 2009, 29, 20–30. [Google Scholar] [CrossRef]
- Li, J.; Yang, T.; Sha, Z.; Tang, H.; Hua, X.; Wang, L.; Wang, Z.; Gao, Z.; Sluijter, J.P.; Rowe, G.C.; et al. Angiotensin II-induced muscle atrophy via PPARγ suppression is mediated by miR-29b. Mol. Ther.-Nucleic Acids 2020, 23, 743–756. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wu, S.-N.; Gao, J.-M.; Liao, Z.-Y.; Tseng, Y.-T.; Fülöp, F.; Chang, F.-R.; Lo, Y.-C. The Antioxidant, Anti-Inflammatory, and Neuroprotective Properties of the Synthetic Chalcone Derivative AN07. Molecules 2020, 25, 2907. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Hong, S.; Molagoda, I.M.N.; Jeong, J.-W.; Jin, C.-Y.; Kim, G.-Y.; Choi, S.H.; Hong, S.H. Inhibition of Lipopolysaccharide-Induced Inflammatory and Oxidative Responses by Trans-cinnamaldehyde in C2C12 Myoblasts. Int. J. Med Sci. 2021, 18, 2480–2492. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, M.; Zhou, F.; Cao, W.; Bi, L.; Xie, Y.; Yang, Q.; Wang, S. Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: The regulation of autophagy and ROS production. J. Mol. Cell. Cardiol. 2016, 101, 11–24. [Google Scholar] [CrossRef]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox Control of Skeletal Muscle Regeneration. Antioxidants Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Lynch, G.S. Current pharmacotherapies for sarcopenia. Expert Opin. Pharmacother. 2019, 20, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Memme, J.M.; Slavin, M.; Moradi, N.; Hood, D.A. Mitochondrial Bioenergetics and Turnover during Chronic Muscle Disuse. Int. J. Mol. Sci. 2021, 22, 5179. [Google Scholar] [CrossRef] [PubMed]
- Ábrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef]
- Frisard, M.I.; Wu, Y.; McMillan, R.P.; Voelker, K.A.; Wahlberg, K.A.; Anderson, A.; Boutagy, N.; Resendes, K.; Ravussin, E.; Hulver, M.W. Low Levels of Lipopolysaccharide Modulate Mitochondrial Oxygen Consumption in Skeletal Muscle. Metabolism 2014, 64, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Bloise, F.F.; van der Spek, A.H.; Surovtseva, O.V.; Ortiga-Carvalho, T.M.; Fliers, E.; Boelen, A. Differential Effects of Sepsis and Chronic Inflammation on Diaphragm Muscle Fiber Type, Thyroid Hormone Metabolism, and Mitochondrial Function. Thyroid 2016, 26, 600–609. [Google Scholar] [CrossRef]
- Frost, R.A.; Pereyra, E.; Lang, C.H. Ethyl Pyruvate Preserves IGF-I Sensitivity toward mTOR Substrates and Protein Synthesis in C2C12 Myotubes. Endocrinology 2011, 152, 151–163. [Google Scholar] [CrossRef][Green Version]
- François, A.; Terro, F.; Janet, T.; Bilan, A.R.; Paccalin, M.; Page, G. Involvement of interleukin-1β in the autophagic process of microglia: Relevance to Alzheimer’s disease. J. Neuroinflammation 2013, 10, 151. [Google Scholar] [CrossRef]
- O’Neill, B.T.; Lee, K.Y.; Klaus, K.; Softic, S.; Krumpoch, M.T.; Fentz, J.; Stanford, K.I.; Robinson, M.M.; Cai, W.; Kleinridders, A.; et al. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J. Clin. Investig. 2016, 126, 3433–3446. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Hsieh, T.-J.; El-Shazly, M.; Chuang, D.-W.; Tsai, Y.-H.; Yen, C.-T.; Wu, S.-F.; Wu, Y.-C.; Chang, F.-R. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorganic Med. Chem. Lett. 2012, 22, 3912–3915. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, W.-Y.; Lin, C.-L.; Chang, W.-H.; Chang, C.-H.; Huang, Y.-C.; Tsai, Y.-H.; Chang, F.-R.; Lo, Y.-C. Protective Effects of the Chalcone-Based Derivative AN07 on Inflammation-Associated Myotube Atrophy Induced by Lipopolysaccharide. Int. J. Mol. Sci. 2022, 23, 12929. https://doi.org/10.3390/ijms232112929
Fang W-Y, Lin C-L, Chang W-H, Chang C-H, Huang Y-C, Tsai Y-H, Chang F-R, Lo Y-C. Protective Effects of the Chalcone-Based Derivative AN07 on Inflammation-Associated Myotube Atrophy Induced by Lipopolysaccharide. International Journal of Molecular Sciences. 2022; 23(21):12929. https://doi.org/10.3390/ijms232112929
Chicago/Turabian StyleFang, Wei-Yu, Chih-Lung Lin, Wan-Hsuan Chang, Chih-Hsiang Chang, Yun-Cian Huang, Yi-Hong Tsai, Fang-Rong Chang, and Yi-Ching Lo. 2022. "Protective Effects of the Chalcone-Based Derivative AN07 on Inflammation-Associated Myotube Atrophy Induced by Lipopolysaccharide" International Journal of Molecular Sciences 23, no. 21: 12929. https://doi.org/10.3390/ijms232112929
APA StyleFang, W.-Y., Lin, C.-L., Chang, W.-H., Chang, C.-H., Huang, Y.-C., Tsai, Y.-H., Chang, F.-R., & Lo, Y.-C. (2022). Protective Effects of the Chalcone-Based Derivative AN07 on Inflammation-Associated Myotube Atrophy Induced by Lipopolysaccharide. International Journal of Molecular Sciences, 23(21), 12929. https://doi.org/10.3390/ijms232112929