Nna1, Essential for Purkinje Cell Survival, Is also Associated with Emotion and Memory
Abstract
1. Introduction
2. Results
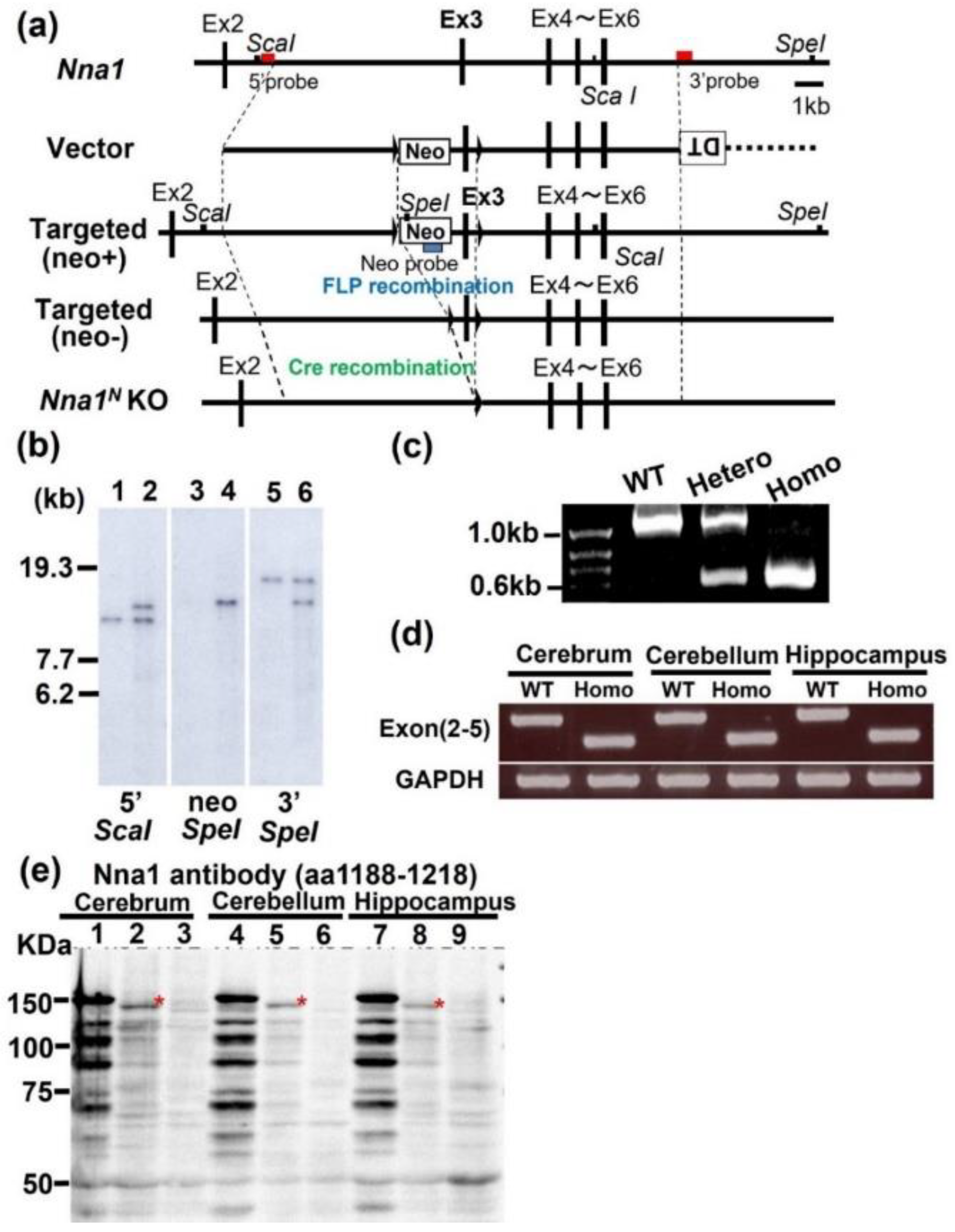
2.1. Generation of Nna1N (Nna1ΔEx3) KO Mice
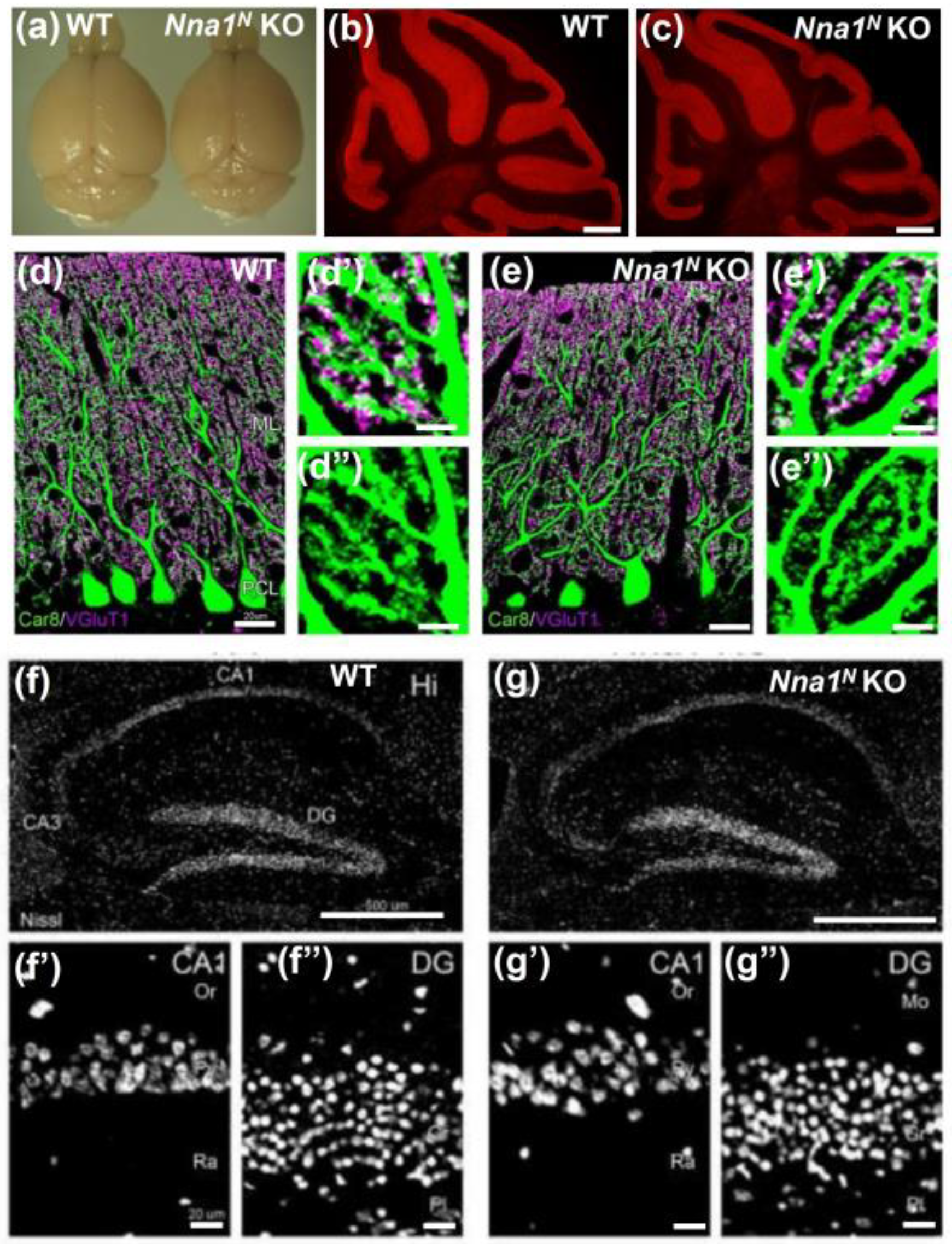
2.2. Normal Morphology in Nna1N KO Mice
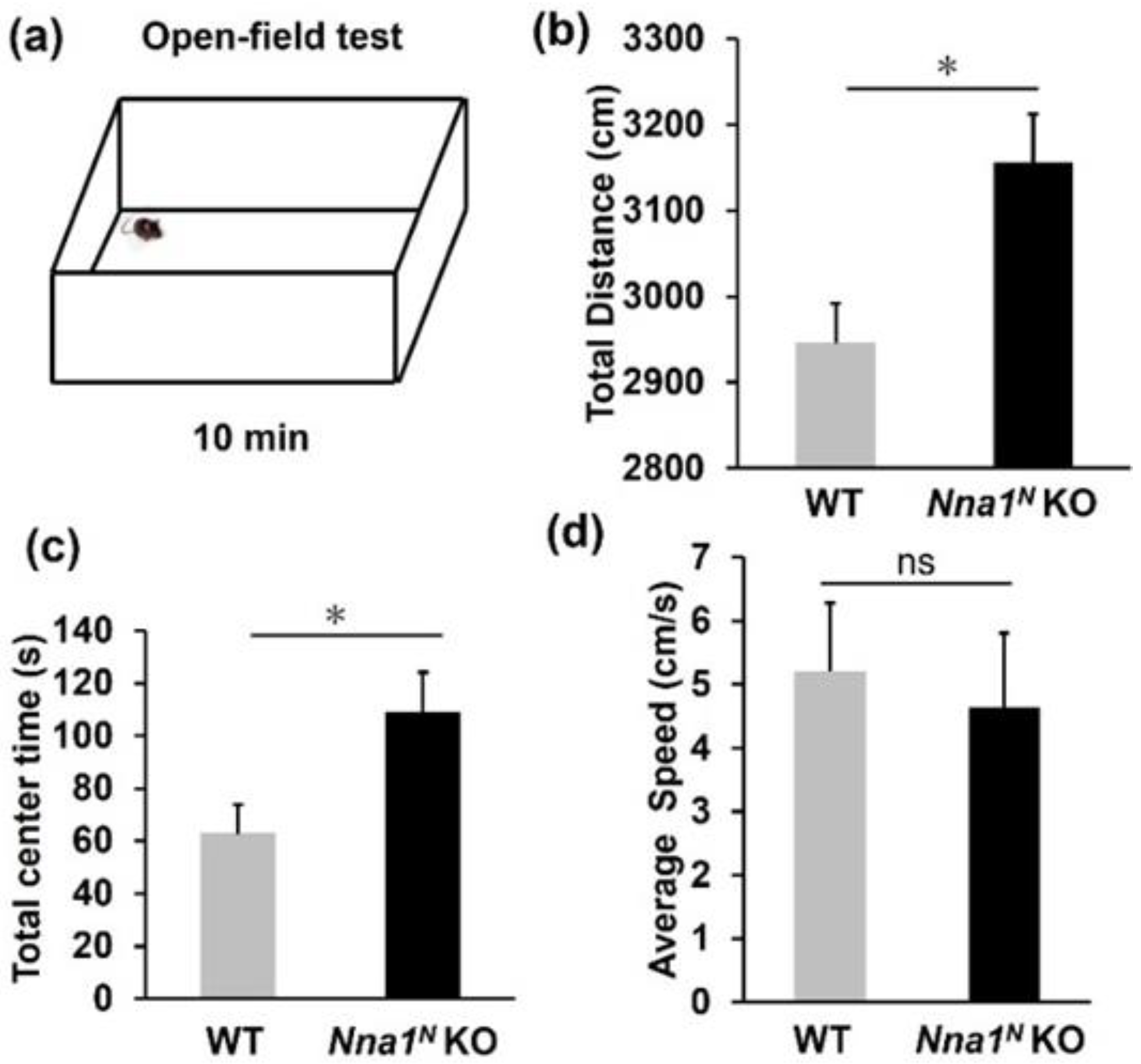
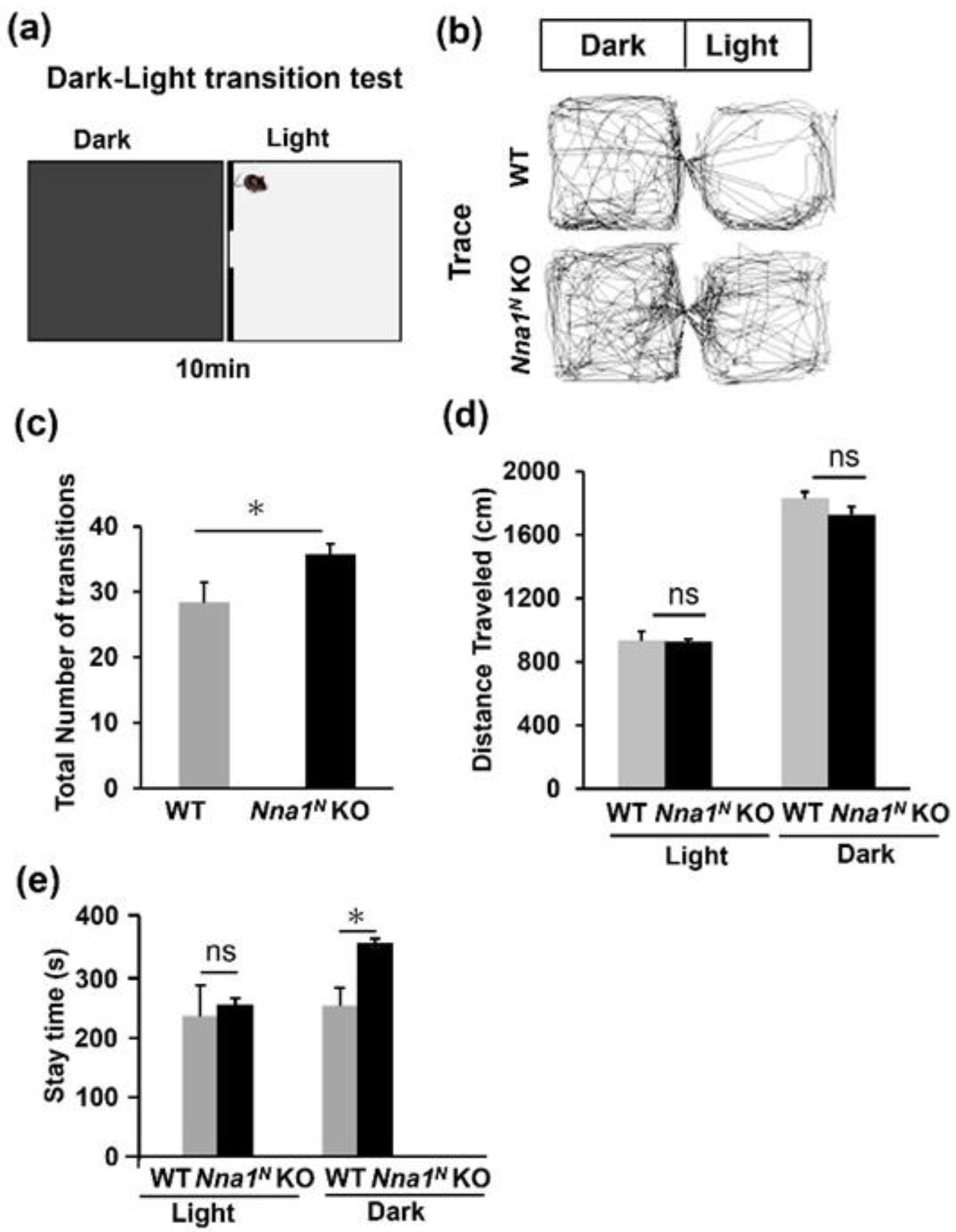
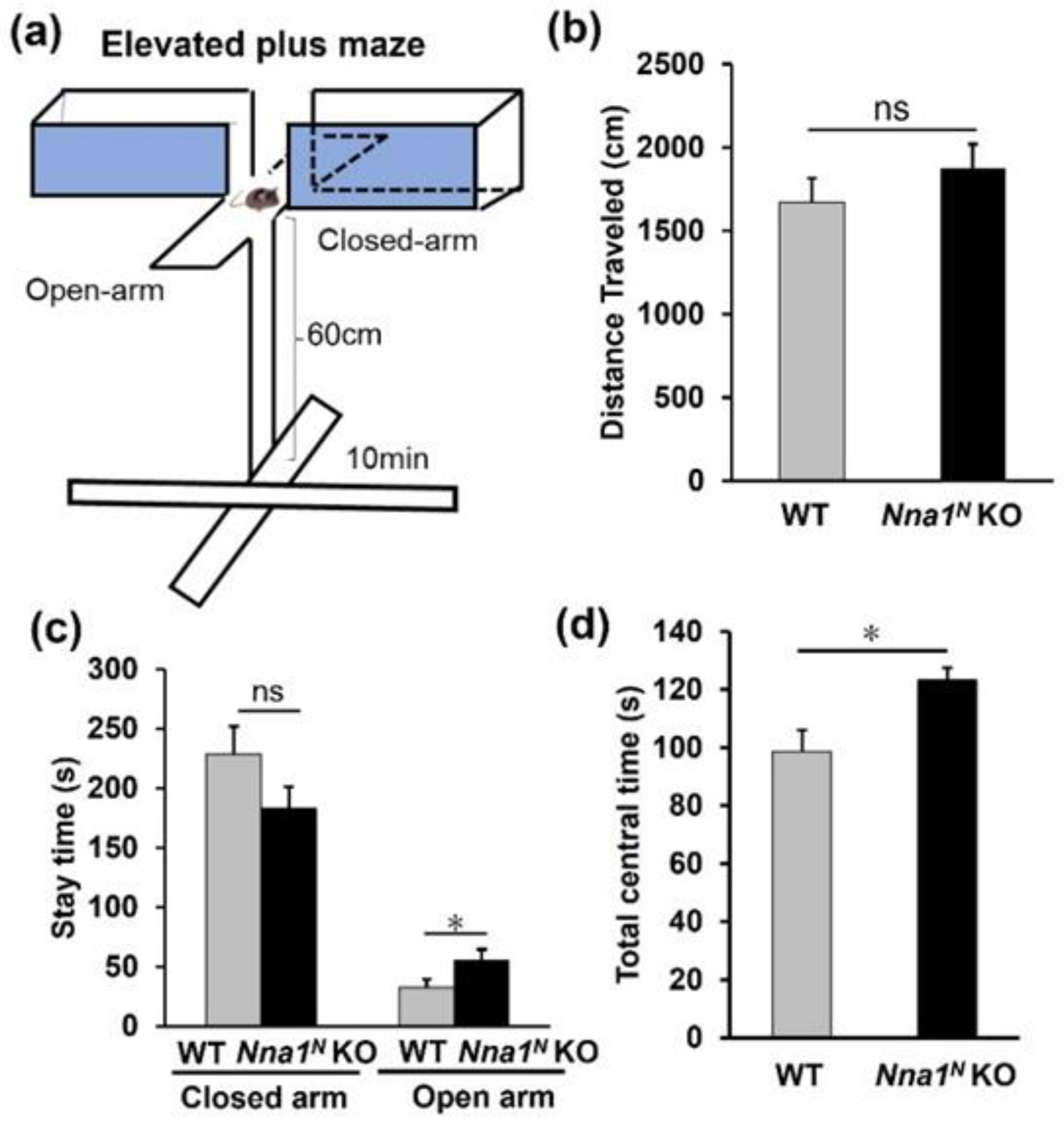
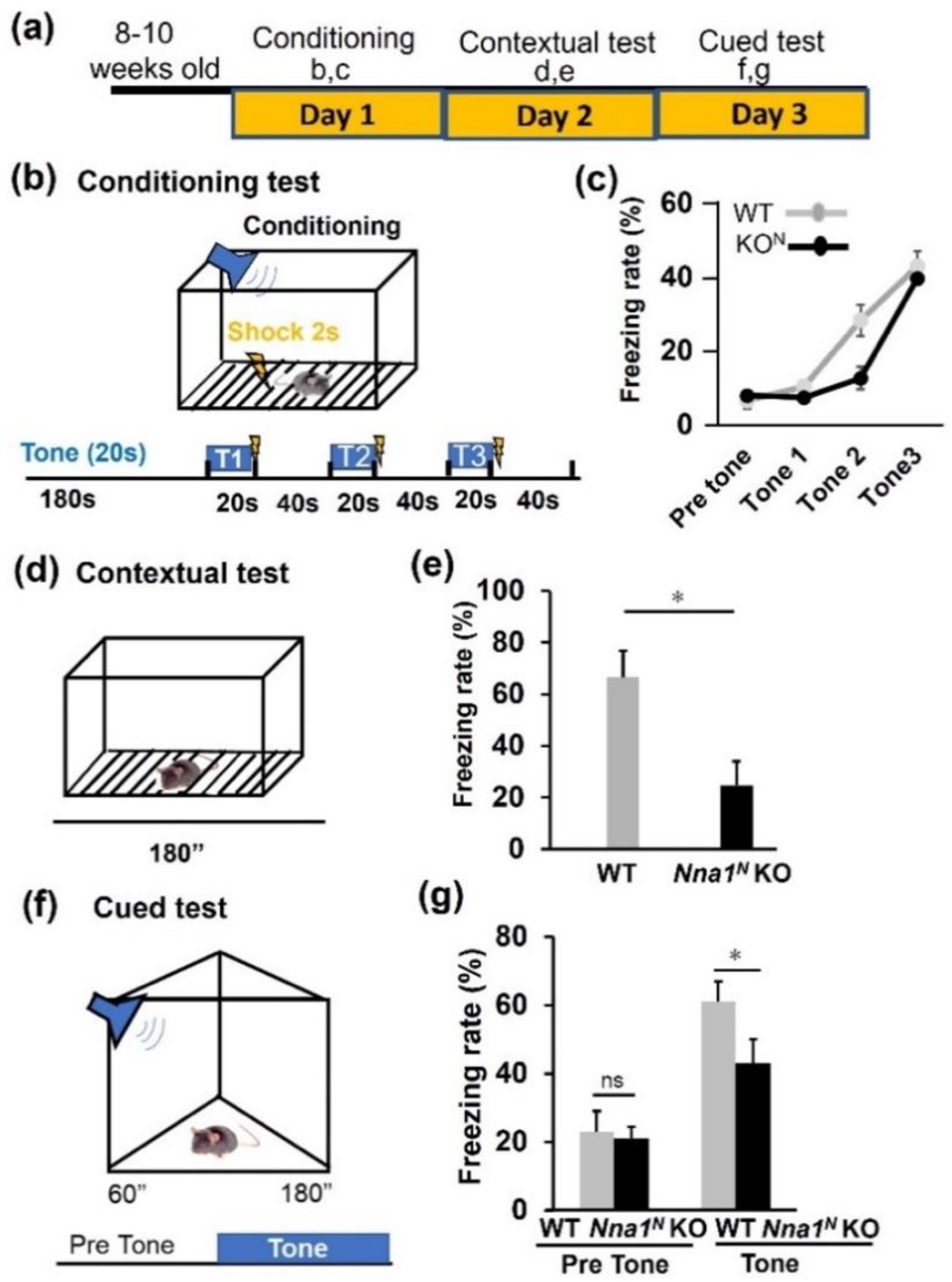
2.3. Nna1N KO Mice Are Impaired in Emotional and Memory Learning
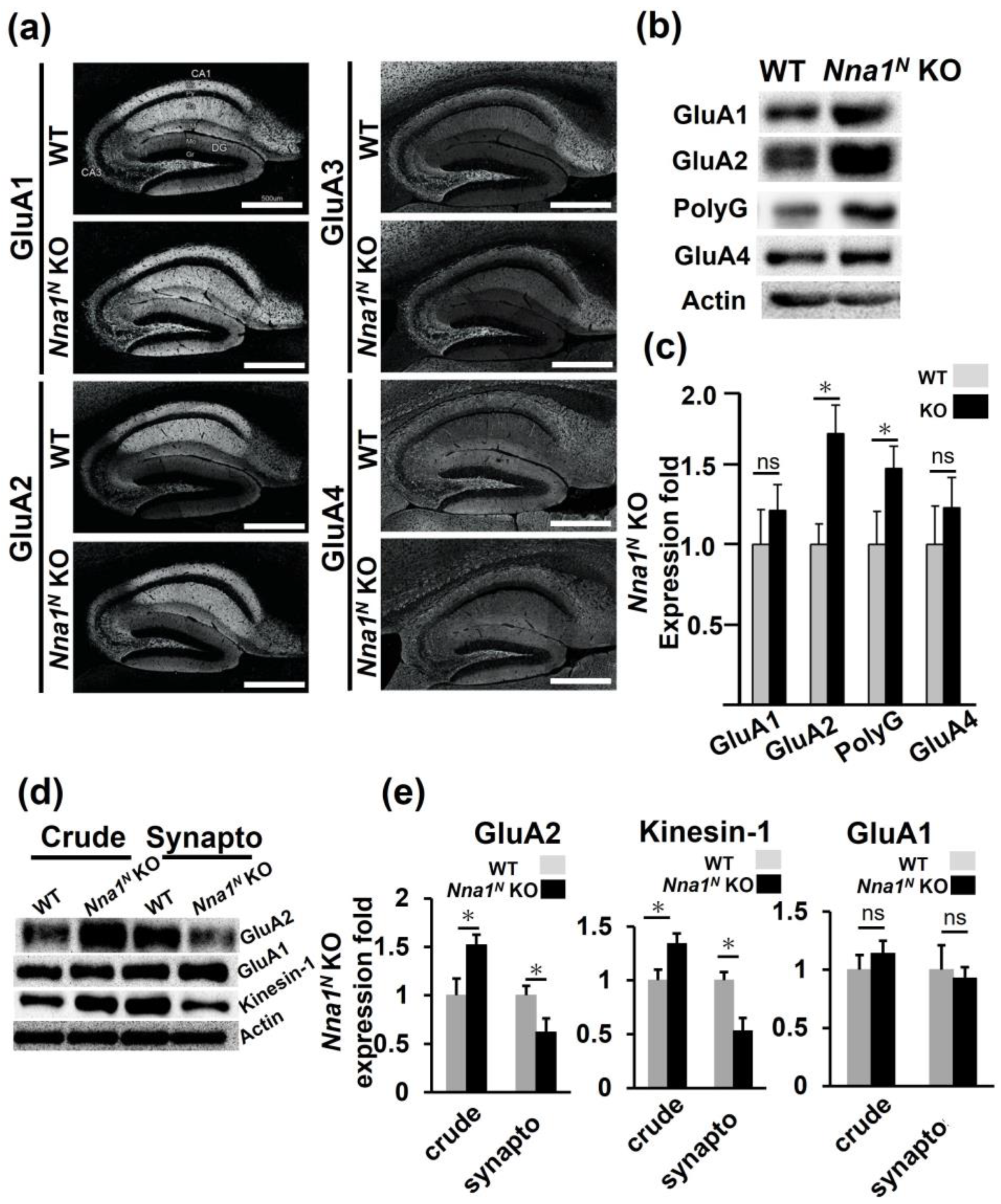
2.4. Nna1N KO Mice Have a Different Subunit Composition of Glutamate Receptors in the Hippocampus
3. Discussion
4. Materials and Methods
4.1. Construction of the Nna1 Conditional Allele
4.2. Generation of Nna1N Knockout (KO) Mice
4.3. Genotyping PCR
4.4. Northern Blot Analysis
4.5. Behavior Tests
4.6. Subcellular Fraction and Western-Blot Analysis
4.7. Immunohistochemistry
4.8. Nissl Staining
4.9. In Situ Hybridization
4.10. Image Analysis for Behavior Tests
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landis, S.C.; Mullen, R.J. The development and degeneration of Purkinje cells in pcd mutant mice. J. Comp. Neurol. 1978, 177, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Mullen, R.J.; Eicher, E.M.; Sidman, R.L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc. Natl. Acad. Sci. USA 1976, 73, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Greer, C.A.; Shepherd, G.M. Mitral cell degeneration and sensory function in the neurological mutant mouse Purkinje cell degeneration (PCD). Brain Res. 1987, 235, 156–161. [Google Scholar] [CrossRef]
- O’Gorman, S.; Sidman, R.L. Degeneration of thalamic neurons in “Purkinje cell degeneration” mutant mice. I. Distribution of neuron loss. J. Comp. Neurol. 1985, 234, 277–297. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, S. Degeneration of thalamic neurons in "Purkinje cell degeneration" mutant mice. II. Cytology of neuron loss. J Comp Neurol. 1985, 234, 298–316. [Google Scholar] [CrossRef]
- Blank, J.C.; Spee, C. Retinal degeneration in the pcd/pcd mutant mouse: Accumulation of spherules in the interphotoreceptor space. Exp. Eye Res. 1992, 54, 637–644. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, A.; La Spada, A.; Treadaway, J.; Higdon, J.; Harris, B.; Sidman, R.; Morgan, J.; Zuo, J. Purkinje cell degeneration (pcd) phenotypes caused by mutation in the axotomy-induced gene, Nna1. Science 2002, 295, 1904–1906. [Google Scholar] [CrossRef]
- Wang, T.; Morgan, J. The Purkinje cell degeneration (pcd) mouse: An unexpected molecular link between neuronal degeneration and regeneration. Brain Res. 2007, 1140, 26–40. [Google Scholar] [CrossRef]
- Wu, H.; Wang, T.; Li, L.; Correia, K.; Morgan, J. A structural and functional analysis of Nna1 in Purkinje cell degeneration (pcd) mice. FASEB J. 2012, 26, 1–13. [Google Scholar]
- Harris, A.; Morgan, J.; Pecot, M.; Soumare, A.; Osborne, A.; Soares, H. Regenerating motor neurons express Nna1, a novel ATP/GTP binding protein related to zinc carboxypeptidases. Mol. Cell Neurosci. 2000, 16, 578–596. [Google Scholar] [CrossRef]
- Harada, T.; Pineda, L.L.; Nakano, A.; Omura, K.; Zhou, L.; Iijima, M.; Yamasaki, Y.; Yokoyama, M. Ataxia and male sterility (AMS) mouse. A new genetic variant exhibiting degeneration and loss of cerebellar Purkinje cells and spermatic cells. Pathol. Int. 2003, 53, 382–389. [Google Scholar] [CrossRef]
- Zhou, L.; Araki, A.; Nakano, A.; Sezer, C.; Harada, T. Different types of neural cell death in the cerebellum of the ataxia and male sterility (AMS) mutant mouse. Pathol. Int. 2006, 56, 173–180. [Google Scholar] [CrossRef]
- Araki, A.; Maruyama, R.; Harada, Y.; Ishikawa, N.; Harada, T. Analysis of the light-sensitivity of the photoreceptor cells of the ataxia and male sterility (AMS) mouse, a Nna1 mutant. Pathol. Int. 2012, 62, 719–727. [Google Scholar] [CrossRef]
- Zhou, L.; Hossain, M.I.; Yamazaki, M.; Abe, M.; Natsume, R.; Konno, K.; Kageyama, S.; Komatsu, M.; Watanabe, M.; Sakimura, K.; et al. Deletion of exons encoding carboxypeptidase domain of Nna1 results in Purkinje cell degeneration (pcd) phenotype. J. Neurochem. 2018, 147, 557–572. [Google Scholar] [CrossRef]
- Brogna, S.; McLeod, T.; Petric, M. The meaning of NMD: Translate or perish. Trends Genet. 2016, 32, 395–407. [Google Scholar] [CrossRef]
- Reimer, A.E.; de Oliveira, A.R.; Brandão, M.L. Glutamatergic mechanisms of the dorsal periaqueductal gray matter modulate the expression of conditioned freezing and fear-potentiated startle. Neuroscience 2012, 219, 72–81. [Google Scholar] [CrossRef]
- Hoerndli, F.J.; Maxfield, D.A.; Brockie, P.J.; Mellem, J.E.; Jensen, E.; Wang, R.; Madsen, D.M.; Maric, A.V. Kinesin-1 regulates synaptic strength by mediating the delivery, removal, and redistribution of AMPA receptors. Neuron 2013, 80, 1421–1437. [Google Scholar] [CrossRef]
- Wu, H.; Rong, Y.; Correia, K.; Min, J.; Morgan, J. Comparison of the enzymatic and functional properties of three cytosolic carboxypeptidase family members. J. Biol. Chem. 2015, 290, 1222–1232. [Google Scholar] [CrossRef]
- Türay, S.; Eröz, R.; Başak, A.N. A novel pathogenic variant in the 3’ end of the AGTPBP1 gene gives rise to neurodegeneration without cerebellar atrophy: An expansion of the disease phenotype? Neurogenetics 2021, 22, 127–132. [Google Scholar] [CrossRef]
- Wloga, D.; Gaertig, J. Post-translational modifications of microtubules. J. Cell Sci. 2010, 123, 3447–3455. [Google Scholar] [CrossRef]
- Kalinina, E.; Biswas, R.; Berezniuk, I.; Hermoso, A.; Aviles, F.; Fricker, L. A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 2007, 21, 836–850. [Google Scholar] [CrossRef]
- Rodriguez de la Vega, M.; Sevilla, R.G.; Hermoso, A.; Lorenzo, J.; Tanco, S.; Diez, A.; Fricker, L.D.; Bautista, J.M.; Avilés, F.X. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 2007, 21, 851–865. [Google Scholar] [CrossRef]
- Rogowski, K.; Dijk, J.; Magiera, M.; Bosc, C.; Deloulme, J.; Bosson, A.; Peris, L.; Gold, N.; Lacroix, B.; Grau, M.; et al. A family of protein-deglutamating enzymes associated with neurodegeneration. Cell 2010, 143, 564–578. [Google Scholar] [CrossRef]
- Mishina, M.; Sakimura, K. Conditional gene targeting on the pure C57BL/6 genetic background. Neurosci. Res. 2007, 58, 105–112. [Google Scholar] [CrossRef]
- Miya, K.; Inoue, R.; Takata, Y.; Abe, M.; Natsume, R.; Sakimura, K.; Hongou, K.; Miyawaki, T.; Mori, H. Serine racemase is predominantly localized in neurons in mouse brain. J. Comp. Neurol. 2008, 510, 641–654. [Google Scholar] [CrossRef]
- Nakamura, K.; Manabe, T.; Watanabe, M.; Mamiya, T.; Ichikawa, R.; Kiyama, Y.; Sanbo, M.; Yagi, T.; Inoue, Y.; Nabeshima, T.; et al. Enhancement of hippocampal LTP, reference memory and sensorimotor gating in mutant mice lacking a telencephalon-specific cell adhesion molecule. Eur. J. Neurosci. 2001, 13, 179–189. [Google Scholar] [CrossRef]
- Fuse, T.; Kanai, Y.; Kanai-Azuma, M.; Suzuki, M.; Nakamura, K.; Mori, H.; Hayashi, Y.; Mishina, M. Conditional activation of RhoA suppresses the epithelial to mesenchymal transition at the primitive streak during mouse gastrulation. Biochem. Biophys. Res. Commun. 2004, 318, 665–672. [Google Scholar] [CrossRef]
- Lehrach, H.; Diamond, D.; Wozney, J.M.; Boedtker, H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 1977, 16, 4743–4751. [Google Scholar] [CrossRef]
- Wahl, G.M.; Stern, M.; Stark, G.R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc. Natl. Acad. Sci. USA 1979, 76, 3683–3687. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, C.; Kawamura, M.; Nakatsukasa, E.; Natsume, R.; Takao, K.; Watanabe, M.; Abe, M.; Takeuchi, T.; Sakimura, K. GluD1 knockout mice with a pure C57BL/6N background show impaired fear memory, social interaction, and enhanced depressive-like behavior. PLoS ONE 2020, 15, e0229288. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Miyakawa, T. Light/dark transition test for mice. J. Vis. Exp. 2006, 1, 104. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. 2008, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Tanda, K.; Nakamura, K.; Kasahara, J.; Nakao, K.; Katsuki, M.; Nakanishi, K.; Yamasaki, N.; Toyama, K.; Adachi, M.; et al. Comprehensive behavioral analysis of calcium/calmodulin-dependent protein kinase IV knockout mice. PLoS ONE 2010, 5, e9460. [Google Scholar] [CrossRef]
- Carlin, R.K. Isolation and characterization of postsynaptic densities from various brain regions: Enrichment of different types of postsynaptic densities. J. Cell Biol. 1980, 86, 831–845. [Google Scholar] [CrossRef]
- Yamasaki, M.; Matsui, M.; Watanabe, M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J. Neurosci. 2010, 30, 4408–4418. [Google Scholar] [CrossRef]
| Name | Forward | Reverse PCR Product |
|---|---|---|
| Nna1fl allele: | 5’-CACAGAATCCACACTAATG-3’ | 5’-GTAAGCACTCCAGACACAC-3’ 1161 bp |
| Nna1wt allele: | 5’-CACAGAATCCACACTAATG-3’ | 5’-GTAAGCACTCCAGACACAC-3’ 1050 bp |
| Nna1ΔEx3 allele: | 5’-CATGGACGGGTCCGGGGAGCA-3’ | 5’-TCAGCCCATCTTCTTCCAGA-3’ 560 bp |
| Nna1 (Ex17–23) probe: | 5’-AAGCGAGTACGACCTTATC-3’ | 5’-CCTGTATCCCATGTCTTCCA-3’ 1170 bp |
| Nna1 5’ probe: | 5’-CTAGCTCCTCGTTAGAAGTA-3’ | 5’-GATATAAGTCAACGGTAGA-3’ 373 bp |
| Nna1 3 ‘probe: | 5’-TGGTGATGTCCGACCTGTTC-3’ | 5’-CTTGCAGAAGACCAATCTGCG-3’ 491 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Konno, K.; Yamazaki, M.; Abe, M.; Natsume, R.; Watanabe, M.; Takebayashi, H.; Sakimura, K. Nna1, Essential for Purkinje Cell Survival, Is also Associated with Emotion and Memory. Int. J. Mol. Sci. 2022, 23, 12961. https://doi.org/10.3390/ijms232112961
Zhou L, Konno K, Yamazaki M, Abe M, Natsume R, Watanabe M, Takebayashi H, Sakimura K. Nna1, Essential for Purkinje Cell Survival, Is also Associated with Emotion and Memory. International Journal of Molecular Sciences. 2022; 23(21):12961. https://doi.org/10.3390/ijms232112961
Chicago/Turabian StyleZhou, Li, Kohtarou Konno, Maya Yamazaki, Manabu Abe, Rie Natsume, Masahiko Watanabe, Hirohide Takebayashi, and Kenji Sakimura. 2022. "Nna1, Essential for Purkinje Cell Survival, Is also Associated with Emotion and Memory" International Journal of Molecular Sciences 23, no. 21: 12961. https://doi.org/10.3390/ijms232112961
APA StyleZhou, L., Konno, K., Yamazaki, M., Abe, M., Natsume, R., Watanabe, M., Takebayashi, H., & Sakimura, K. (2022). Nna1, Essential for Purkinje Cell Survival, Is also Associated with Emotion and Memory. International Journal of Molecular Sciences, 23(21), 12961. https://doi.org/10.3390/ijms232112961





