Size-Based Effects of Anthropogenic Ultrafine Particles on Lysosomal TRPML1 Channel and Autophagy in Motoneuron-like Cells
Abstract
1. Introduction
2. Results
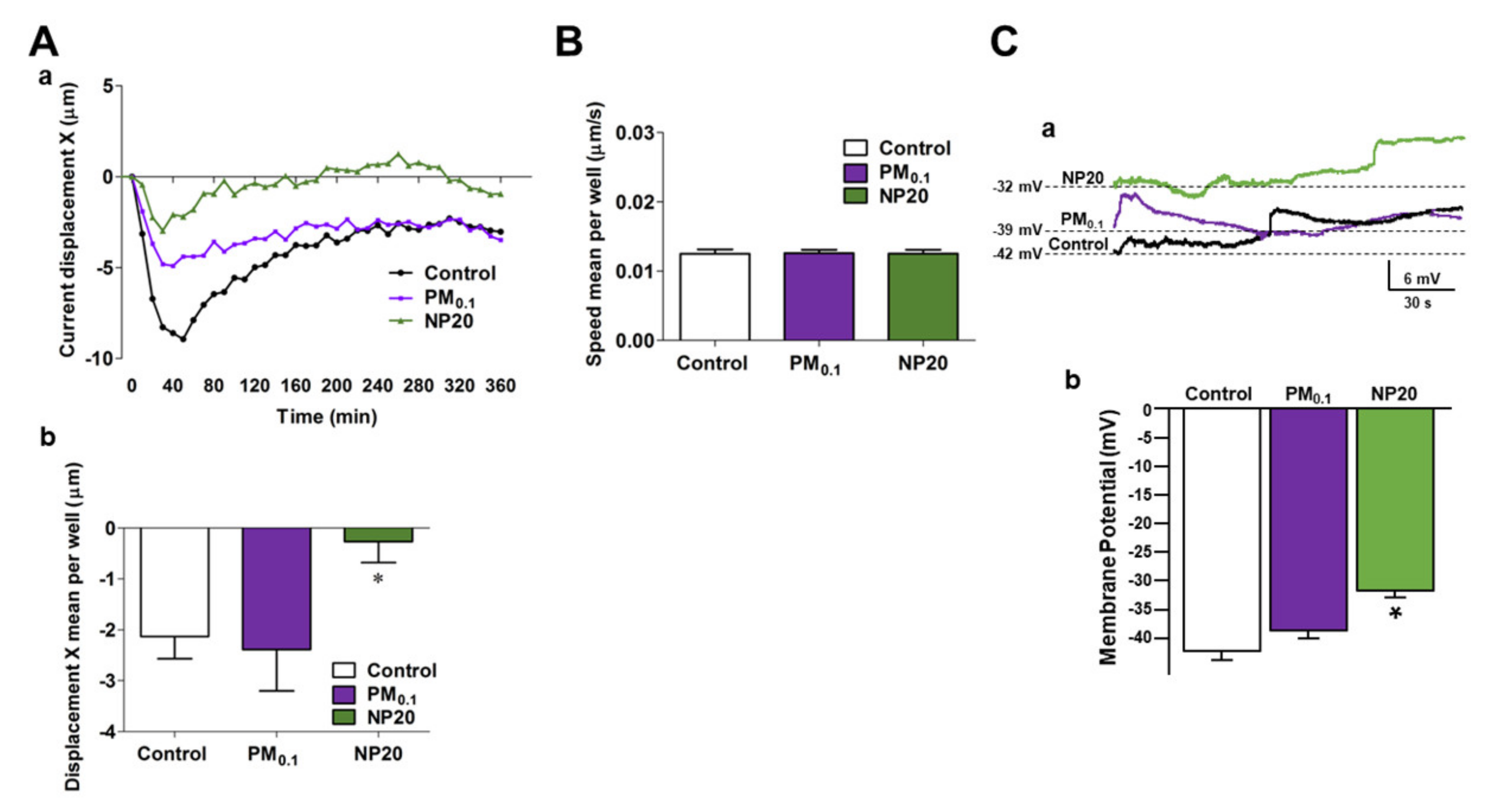
2.1. Effects of PM0.1 and NP20 on Cell Tracking and Membrane Potential of NSC-34 Motor Neurons
2.2. Effects of PM0.1 and NP20 on Endocytosis, Mitochondrial Viability and ROS Production in NSC-34 Motor Neurons
2.3. Effects of PM0.1 and NP20 on Lysosomal Channel TRPML1 and Autophagy in NSC-34 Motor Neurons
3. Discussion
4. Materials and Methods
4.1. Ultrafine Particulate Isolation
4.2. Drugs and Chemicals
4.3. Cell Cultures and Treatments
4.4. Time-Lapse and High-Content Microscopy
4.5. Endocytosis Assay
4.6. Western Blotting
4.7. Electrophysiological Recordings
4.8. [Ca2+]i Measurement by Single-Cell Video Imaging
| Antibody | Supplier | Catalog Number | Species | Type | Dilution Used | Application | Already Tested in: |
|---|---|---|---|---|---|---|---|
| β-actin-peroxidase | Sigma-Aldrich (Milan, Italy) | A3854 (RRID:AB_262011) | Mouse | Monoclonal | 1:10000 | WB | Tedeschi et al. [35] |
| Caspase 9 (cleaved Asp330) | GeneTex Inc. (Irvine, CA, USA) | GTX132331 (RRID:AB_2886615) | Rabbit | Polyclonal | 1:1000 | WB | Tedeschi et al. [37] |
| GRP78 (BiP) | Cell Signaling Technology, Inc. (Danvers, MA, USA) | 3183 (RRID:AB_10695864) | Rabbit | Polyclonal | 1:1000 | WB | Secondo et al. [56] Tedeschi et al. [37] |
| LAMP1 | Merck Millipore (Darmstadt, Germany) | AB2971 (RRID:AB_11212777) | Rabbit | Polyclonal | 1:1000 | WB | Tedeschi et al. [37] |
| 1:200 | IP | ||||||
| LAMP1 | Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) | sc-20011 (RRID:AB_626853) | Mouse | Monoclonal | 1:1000 | ICC | Tedeschi et al. [35] |
| LC3B | GeneTex Inc. (Irvine, CA, USA) | GTX127375 (RRID:AB_11176277) | Rabbit | Polyclonal | 1:1000 | WB | Tedeschi et al. [37] |
| p62/SQSTM1 | Novus Biologicals (Littleton, CO, USA) | NBP1-48320 (RRID:AB_10011069) | Rabbit | Polyclonal | 1:1000 | WB | Tedeschi et al. [37] |
| TPC2 | Alomone Labs (Jerusalem, Israel) | ACC-072 (RRID:AB_10918019) | Rabbit | Polyclonal | 1:1000 | WB | |
| 1:1000 | ICC | ||||||
| α-tubulin | Sigma-Aldrich (Milan, Italy) | T5168 (RRID:AB_477579) | Mouse | Monoclonal | 1:5000 | WB | Secondo et al. [56] Tedeschi et al. [35] |
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butt, E.W.; Turnock, S.T.; Rigby, R.; Reddington, C.L.; Yoshioka, M.; Johnson, J.S.; Regayre, L.A.; Pringle, K.J.; Mann, G.W.; Spracklen, D.V. Global and regional trends in particulate air pollution and attributable health burden over the past 50 years. Environ. Res. Lett. 2017, 12, 104017. [Google Scholar] [CrossRef]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 782462. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The outdoor air pollution and brain health workshop. Neurotoxicology 2012, 33, 972–984. [Google Scholar] [CrossRef]
- Madrigano, J.; Baccarelli, A.; Wright, R.O.; Suh, H.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Air pollution, obesity, genes and cellular adhesion molecules. Occup. Environ. Med. 2010, 67, 312–317. [Google Scholar] [CrossRef]
- Rückerl, R.; Ibald-Mulli, A.; Koenig, W.; Schneider, A.; Woelke, G.; Cyrys, J.; Heinrich, J.; Marder, V.; Frampton, M.; Wichmann, H.E.; et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am. J. Respir. Crit. Care Med. 2006, 173, 432–441. [Google Scholar] [CrossRef]
- Karagulian, F.; Van Dingenen, R.; Belis, C.A.; Janssens-Maenhout, G.; Crippa, M.; Guizzardi, D.; Dentener, F. Attribution of Anthropogenic PM2.5 to Emission Sources; Technical Report for Joint Research Centre (JRC); EUR 28510 EN; European Commission: Brussels, Belgium, 2017. [Google Scholar] [CrossRef]
- Russo, C.; Apicella, B.; Tregrossi, A.; Oliano, M.M.; Ciajolo, A. Thermophoretic sampling of large PAH (C ≥ 22–24) formed in flames. Fuel 2020, 263, 116722. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; García, R.; et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Mühlfeld, C.; Rothen-Rutishauser, B.; Blank, F.; Vanhecke, D.; Ochs, M.; Gehr, P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L817–L829. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Veronesi, B.; Calderón-Garcidueñas, L.; Gehr, P.; Chen, L.C.; Geiser, M.; Reed, W.; Rothen-Rutishauser, B.; Schürch, S.; Schulz, H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, W.Y.; Wang, M.; Shi, J.W.; Zhang, F.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F.; Huang, Y.Y.; Xie, Y.N.; et al. Transport of intranasally instilled fine Fe2O3 particles into the brain: Micro-distribution, chemical states, and histopathological observation. Biol. Trace Elem. Res. 2007, 118, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Casanova, R.; Wang, X.; Reyes, J.; Akita, Y.; Serre, M.L.; Vizuete, W.; Chui, H.C.; Driscoll, I.; Resnick, S.M.; Espeland, M.A.; et al. A Voxel-Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women. Front. Hum. Neurosci. 2016, 10, 495. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Engle, R.; Mora-Tiscareño, A.; Styner, M.; Gómez-Garza, G.; Zhu, H.; Jewells, V.; Torres-Jardón, R.; Romero, L.; Monroy-Acosta, M.E.; et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn. 2011, 77, 345–355. [Google Scholar] [CrossRef]
- Campbell, A.; Oldham, M.; Becaria, A.; Bondy, S.C.; Meacher, D.; Sioutas, C.; Misra, C.; Mendez, L.B.; Kleinman, M. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 2005, 26, 133–140. [Google Scholar] [CrossRef]
- Kleinman, M.T.; Araujo, J.A.; Nel, A.; Sioutas, C.; Campbell, A.; Cong, P.Q.; Li, H.; Bondy, S.C. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol. Lett. 2008, 178, 127–130. [Google Scholar] [CrossRef]
- Chen, J.C.; Wang, X.; Wellenius, G.A.; Serre, M.L.; Driscoll, I.; Casanova, R.; McArdle, J.J.; Manson, J.E.; Chui, H.C.; Espeland, M.A. Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Ann. Neurol. 2015, 78, 466–476. [Google Scholar] [CrossRef]
- Sirivelu, M.P.; MohanKumar, S.M.J.; Wagner, J.G.; Harkema, J.R.; MohanKumar, P.S. Activation of the stress axis and neurochemical alterations in specific brain areas by concentrated ambient particle exposure with concomitant allergic airway disease. Environ. Health Perspect. 2006, 114, 870–874. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Maronpot, R.R.; Torres-Jardon, R.; Henríquez-Roldán, C.; Schoonhoven, R.; Acuña-Ayala, H.; Villarreal-Calderón, A.; Nakamura, J.; Fernando, R.; Reed, W.; et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol. Pathol. 2003, 31, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.M.; Kumarathasan, P.; Calderón-Garcidueñas, L.; Vincent, R. Air pollution alters brain and pituitary endothelin-1 and inducible nitric oxide synthase gene expression. Environ. Res. 2007, 105, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Azzarelli, B.; Acuna, H.; Garcia, R.; Gambling, T.M.; Osnaya, N.; Monroy, S.; DEL Tizapantzi, M.R.; Carson, J.L.; Villarreal-Calderon, A.; et al. Air pollution and brain damage. Toxicol. Pathol. 2002, 30, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Reed, W.; Maronpot, R.R.; Henríquez-Roldán, C.; Delgado-Chavez, R.; Calderón-Garcidueñas, A.; Dragustinovis, I.; Franco-Lira, M.; Aragón-Flores, M.; Solt, A.C.; et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol. Pathol. 2004, 32, 650–658. [Google Scholar] [CrossRef]
- Hartz, A.M.S.; Bauer, B.; Block, M.L.; Hong, J.S.; Miller, D.S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008, 22, 2723–2733. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO(2) nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar] [CrossRef]
- Mantecca, P.; Farina, F.; Moschini, E.; Gallinotti, D.; Gualtieri, M.; Rohr, A.; Sancini, G.; Palestini, P.; Camatini, M. Comparative acute lung inflammation induced by atmospheric PM and size-fractionated tire particles. Toxicol. Lett. 2010, 198, 244–254. [Google Scholar] [CrossRef]
- Farina, F.; Sancini, G.; Mantecca, P.; Gallinotti, D.; Camatini, M.; Palestini, P. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol. Lett. 2011, 202, 209–217. [Google Scholar] [CrossRef]
- Apicella, B.; Russo, C.; Carpentieri, A.; Tregrossi, A.; Ciajolo, A. PAHs and fullerenes as structural and compositional motifs tracing and distinguishing organic carbon from soot. Fuel 2022, 309, 122356. [Google Scholar] [CrossRef]
- Russo, C.; Apicella, B.; Lighty, J.S.; Ciajolo, A.; Tregrossi, A. Optical properties of organic carbon and soot produced in an inverse diffusion flame. Carbon 2017, 124, 372–379. [Google Scholar] [CrossRef]
- Patel, S.; Muallem, S. Acidic Ca(2+) stores come to the fore. Cell Calcium 2011, 50, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ren, D. Lysosomal physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, V.; Petrozziello, T.; Secondo, A. Calcium Dyshomeostasis and Lysosomal Ca2+ Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2019, 8, 1216. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, V.; Sisalli, M.J.; Petrozziello, T.; Canzoniero, L.; Secondo, A. Lysosomal calcium is modulated by STIM1/TRPML1 interaction which participates to neuronal survival during ischemic preconditioning. FASEB J. 2021, 35, e21277. [Google Scholar] [CrossRef]
- Tedeschi, V.; Petrozziello, T.; Secondo, A. Ca2+ dysregulation in the pathogenesis of amyotrophic lateral sclerosis. Int. Rev. Cell. Mol. Biol. 2021, 363, 21–47. [Google Scholar]
- Tedeschi, V.; Petrozziello, T.; Sisalli, M.J.; Boscia, F.; Canzoniero, L.; Secondo, A. The activation of Mucolipin TRP channel 1 (TRPML1) protects motor neurons from L-BMAA neurotoxicity by promoting autophagic clearance. Sci. Rep. 2019, 9, 10743. [Google Scholar] [CrossRef]
- Tedeschi, V.; Secondo, A. Emerging role of lysosomal calcium store as a hub of neuroprotection. Neural Regen. Res. 2022, 17, 1259–1260. [Google Scholar]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef]
- Smith, Q.; Stukalin, E.; Kusuma, S.; Gerecht, S.; Sun, S.X. Stochasticity and Spatial Interaction Govern Stem Cell Differentiation Dynamics. Sci. Rep. 2015, 5, 12617–12627. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109–12121. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Q.; Yang, M.; Zhang, X.; Yu, L.; Lawas, M.; Li, X.; Bryant-Genevier, M.; Southall, N.T.; Marugan, J.; et al. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl. Acad. Sci. USA 2015, 112, E1373–E1381. [Google Scholar] [CrossRef] [PubMed]
- Marcella, S.; Apicella, B.; Secondo, A.; Palestra, F.; Opromolla, G.; Ciardi, R.; Tedeschi, V.; Ferrara, A.L.; Russo, C.; Galdiero, M.R.; et al. Size-based effects of anthropogenic ultrafine particles on activation of human lung macrophages. Environ. Int. 2022, 166, 107395. [Google Scholar] [CrossRef] [PubMed]
- Simkhovich, B.Z.; Kleinman, M.T.; Kloner, R.A. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J. Am. Coll. Cardiol. 2008, 52, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Gunes, Z.I.; Kan, V.W.Y.; Jiang, S.; Logunov, E.; Ye, X.; Liebscher, S. Cortical Hyperexcitability in the Driver’s Seat in ALS. Clin. Transl. Neurosci. 2022, 6, 5. [Google Scholar] [CrossRef]
- Orrenius, S.; Kaminskyy, V.O.; Zhivotovsky, B. Autophagy in toxicology: Cause or consequence? Annu. Rev. Pharmacol. Toxicol. 2013, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Qiang, L.; Joseph, J.; Kalyanaraman, B.; Viollet, B.; He, Y.Y. Mitochondrial dysfunction activates the AMPK signaling and autophagy to promote cell survival. Genes Dis. 2016, 3, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Apicella, B.; Barbella, R.; Ciajolo, A.; Tregrossi, A. Formation of low- and high-molecular-weight hydrocarbon species in sooting ethylene flames. Combust. Sci. Technol. 2002, 174, 309–324. [Google Scholar] [CrossRef]
- Cashman, N.R.; Durham, H.D.; Blusztajn, J.K.; Oda, K.; Tabira, T.; Shaw, I.T.; Dahrouge, S.; Antel, J.P. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. 1992, 194, 209–221. [Google Scholar] [CrossRef]
- Borriello, F.; Iannone, R.; Di Somma, S.; Loffredo, S.; Scamardella, E.; Galdiero, M.R.; Varricchi, G.; Granata, F.; Portella, G.; Marone, G. GM-CSF and IL-3 Modulate Human Monocyte TNF-α Production and Renewal in In Vitro Models of Trained Immunity. Front. Immunol. 2017, 7, 680. [Google Scholar] [CrossRef]
- Borriello, F.; Iannone, R.; Di Somma, S.; Vastolo, V.; Petrosino, G.; Visconte, F.; Raia, M.; Scalia, G.; Loffredo, S.; Varricchi, G.; et al. Lipopolysaccharide-Elicited TSLPR Expression Enriches a Functionally Discrete Subset of Human CD14+ CD1c+ Monocytes. J. Immunol. 2017, 198, 3426–3435. [Google Scholar] [CrossRef]
- Bedi, S.S.; Yang, Q.; Crook, R.J.; Du, J.; Wu, Z.; Fishman, H.M.; Grill, R.J.; Carlton, S.M.; Walters, E.T. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J. Neurosci. 2010, 30, 14870–14882. [Google Scholar] [CrossRef] [PubMed]
- Gunhanlar, N.; Shpak, G.; van der Kroeg, M.; Gouty-Colomer, L.A.; Munshi, S.T.; Lendemeijer, B.; Ghazvini, M.; Dupont, C.; Hoogendijk, W.J.G.; Gribnau, J.; et al. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol. Psychiatry 2018, 23, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, R.; Franco, C.; Piccialli, I.; Boscia, F.; Casamassa, A.; de Rosa, V.; Cepparulo, P.; Cataldi, M.; Annunziato, L.; Pannaccione, A. Amyloid β-Induced Upregulation of Nav1.6 Underlies Neuronal Hyperactivity in Tg2576 Alzheimer’s Disease Mouse Model. Sci. Rep. 2019, 9, 13592. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Secondo, A.; Petrozziello, T.; Tedeschi, V.; Boscia, F.; Vinciguerra, A.; Ciccone, R.; Pannaccione, A.; Molinaro, P.; Pignataro, G.; Annunziato, L. ORAI1/STIM1 Interaction Intervenes in Stroke and in Neuroprotection Induced by Ischemic Preconditioning Through Store-Operated Calcium Entry. Stroke. 2019, 50, 1240–1249. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapienza, S.; Tedeschi, V.; Apicella, B.; Palestra, F.; Russo, C.; Piccialli, I.; Pannaccione, A.; Loffredo, S.; Secondo, A. Size-Based Effects of Anthropogenic Ultrafine Particles on Lysosomal TRPML1 Channel and Autophagy in Motoneuron-like Cells. Int. J. Mol. Sci. 2022, 23, 13041. https://doi.org/10.3390/ijms232113041
Sapienza S, Tedeschi V, Apicella B, Palestra F, Russo C, Piccialli I, Pannaccione A, Loffredo S, Secondo A. Size-Based Effects of Anthropogenic Ultrafine Particles on Lysosomal TRPML1 Channel and Autophagy in Motoneuron-like Cells. International Journal of Molecular Sciences. 2022; 23(21):13041. https://doi.org/10.3390/ijms232113041
Chicago/Turabian StyleSapienza, Silvia, Valentina Tedeschi, Barbara Apicella, Francesco Palestra, Carmela Russo, Ilaria Piccialli, Anna Pannaccione, Stefania Loffredo, and Agnese Secondo. 2022. "Size-Based Effects of Anthropogenic Ultrafine Particles on Lysosomal TRPML1 Channel and Autophagy in Motoneuron-like Cells" International Journal of Molecular Sciences 23, no. 21: 13041. https://doi.org/10.3390/ijms232113041
APA StyleSapienza, S., Tedeschi, V., Apicella, B., Palestra, F., Russo, C., Piccialli, I., Pannaccione, A., Loffredo, S., & Secondo, A. (2022). Size-Based Effects of Anthropogenic Ultrafine Particles on Lysosomal TRPML1 Channel and Autophagy in Motoneuron-like Cells. International Journal of Molecular Sciences, 23(21), 13041. https://doi.org/10.3390/ijms232113041








